Back to Journals » Drug Design, Development and Therapy » Volume 16
Knee Osteoarthritis Therapy: Recent Advances in Intra-Articular Drug Delivery Systems
Authors Ma L, Zheng X, Lin R, Sun AR, Song J, Ye Z, Liang D, Zhang M, Tian J, Zhou X, Cui L, Liu Y, Liu Y
Received 7 January 2022
Accepted for publication 17 April 2022
Published 4 May 2022 Volume 2022:16 Pages 1311—1347
DOI https://doi.org/10.2147/DDDT.S357386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Luoyang Ma,1,2 Xiaoyan Zheng,1,3 Rui Lin,1 Antonia RuJia Sun,4 Jintong Song,1 Zhiqiang Ye,1 Dahong Liang,1 Min Zhang,1 Jia Tian,1 Xin Zhou,2 Liao Cui,1 Yuyu Liu,1 Yanzhi Liu1,3,5
1Guangdong Provincial Key Laboratory for Research and Development of Natural Drug, School of Pharmacy, Guangdong Medical University, Zhanjiang City, Guangdong Province, 524023, People’s Republic of China; 2Marine Medical Research Institute of Zhanjiang, Zhanjiang City, Guangdong Province, 524023, People’s Republic of China; 3Zhanjiang Central Hospital, Guangdong Medical University, Zhanjiang city, Guangdong province, 524045, People’s Republic of China; 4Center for Translational Medicine Research and Development, Shenzhen Institute of Advanced Technology, Chinese Academy of Science, Shenzhen City, Guangdong Province, 518055, People’s Republic of China; 5Shenzhen Osteomore Biotechnology Co., Ltd., Shenzhen city, Guangdong Province, 518118, People’s Republic of China
Correspondence: Yanzhi Liu; Yuyu Liu, Tel +86-759-2388405 ; +86-759-2388588, Email [email protected]; [email protected]
Abstract: Drug delivery for osteoarthritis (OA) treatment is a continuous challenge because of their poor bioavailability and rapid clearance in joints. Intra-articular (IA) drug delivery is a common strategy and its therapeutic effects depend mainly on the efficacy of the drug-delivery system used for OA therapy. Different types of IA drug-delivery systems, such as microspheres, nanoparticles, and hydrogels, have been rapidly developed over the past decade to improve their therapeutic effects. With the continuous advancement in OA mechanism research, new drugs targeting specific cell/signaling pathways in OA are rapidly evolving and effective drug delivery is critical for treating OA. In this review, recent advances in various IA drug-delivery systems for OA treatment, OA targeted strategies, and related signaling pathways in OA treatment are summarized and analyzed based on current publications.
Keywords: osteoarthritis, knee, drug delivery, intra-articular
Introduction
Osteoarthritis (OA) is the most common degenerative joint disease that affects approximately more than 300 million people worldwide.1,2 Knee OA(KOA) accounts for approximately 85% of all OA cases worldwide.3 KOA represents a major public health challenge for the coming decades. The disease is ranked as the 10th largest cause of global years lived with disabilities,4 and its prevalence has more than doubled in the last 10 years.5,6 Medication management, hospitalizations, and joint surgery associated with OA treatment cost the healthcare system billions of dollars annually.4,6
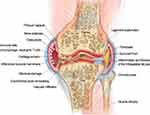
OA is a complex and multifactorial disease that involves various tissues lesion of the joint, including degenerative loss of articular cartilage, osteophyte formation, synovitis, subchondral bone remodeling and sclerosis, inflammation and fibrosis of the infrapatellar fat pad in the joints7 (Figure 1) Traditionally, OA has been defined as an abnormal biomechanic-induced disorder causing extensive changes in joint homeostasis; however, it is not merely a passive degenerative disease of “wear-and-tear” as commonly described, but an active dynamic alteration arising from an imbalance between the repair and destruction of joint tissues with complex inflammatory and metabolic factors involved.8,9 Over the last decade, OA has been increasingly recognized as a heterogeneous disease with the combined effects of aging, obesity, mechanical imbalance, gender, inflammation, metabolic imbalance, and genetic background.10,11 The degradation of the articular cartilage is the central feature of KOA.12 The local unbalanced biomechanical microenvironment and various biological factors in OA induce cartilage homeostasis dysregulation, resulting in collagen- and proteoglycan-rich extracellular matrix (ECM) degradation, articular surface fibrosis and erosion, cell death, matrix calcification, and vascular invasion. The progressive destruction of cartilage stimulated chondrocyte compensatory anabolism hypertrophy. Progressive destruction of cartilage stimulates chondrocytes to increase anabolism in the form of compensatory hypertrophy13 and this process simultaneously produce matrix degradation products and pro-inflammatory mediators to accelerate the development of KOA.14 With the further loss of cartilage and the exposure of subchondral bone, the increasing bone remodeling induces occurrence of osteophytosis, subchondral sclerosis, cyst formation, and abnormalities of bone contour in response to the rapid changes of the mechanical environment.15 Pro-inflammatory factors secreted by hypertrophic chondrocytes stimulate synovial cell proliferation and induce T, B lymphocytes and mast cell infiltration in synovial fluid.16 The triggered synovial tissue releases pro-inflammatory mediators to promote the cascade development of KOA.17 The progressive OA also affects the tissues in and around the joint. As the largest intraarticular adipose tissue, Hoffa’s infrapatellar fat pad (IFP) is a very sensitive tissue containing adipocytes, fibroblasts, leukocytes, macrophages and other immune cells.16,18 The IFP of KOA patients presented an increase in inflammatory infiltration, vascularization, fibrotic changes, and thickness of the interlobular septa.19,20 IFP also in turn to produce pro-inflammatory mediators and induce synovitis.20–23 Meniscal/ligament lesion often leads to subsequent cartilage loss, changes in subchondral bone, lesions of bone marrow and synovitis.24 Meniscal/ligament lesion induced biomechanical imbalance is a risk factor causing OA.25 Conversely, the progressive OA usually caused meniscal/ligament lesions. There appears to be a mutually reinforcing relationship between knee osteoarthritis and meniscal/ligament lesion.16,26 In addition, OA caused dysfunctions of the quadriceps muscle, hamstrings and hip muscles.16,27 The weakness of periarticular muscles of OA has a significant impact on knee biomechanics. However, it is not clear whether muscle weakness is associated with OA onset or OA progression.28,29
Current KOA treatments include surgery, exercise, weight management, training in self-efficacy and pain-coping skills, and medications.12 There is no cure for OA, even no drugs to stop the progress of the disease.30 Current treatments only rely on symptomatic interventions, especially focus on relieve pain and enhance physical function. Intra-articular (IA) injection is one of the pharmacological treatments recommended for KOA in patients who do not respond to oral or topical analgesics31 Compared with oral administration, IA circumvents systemic exposure and potential adverse side effects32 and strengthens the bioavailability of therapeutic drugs direct access to the joint, which reduces treatment costs. In addition, IA is an attractive strategy for delivering drugs with low oral and local bioavailability. Currently, corticosteroids and hyaluronic acid (HA) are the most common drugs administered by IA injection for pain management and joint lubrication. However, the consensus on IA corticosteroids injections among scientific societies have not yet unified and the efficacy and safety of corticosteroids are debated. The balance between GC benefits and potential safety issues have been highlighted among guidelines. (the 2019 American College of Rheumatology (ACR) guideline, the Osteoarthritis Research Society International (OARSI) guideline, the American Academy of Orthopedic Surgeons (AAOS) guideline)33–35 High molecular weight HA has been used to alleviate the symptoms of OA. HA injection has been shown to provide lubrication, protect the cartilage from mechanical degradation, anti-inflammation, increase proteoglycan and HA synthesis, and reduce nerve impulses and nerve sensitivity associated with OA pain.36
Considering long-term efficacy and safety concerns, current IA drug injection treatments (corticosteroids and HA) are only used for a short-term therapy and this strategy cannot reverse the progression of OA. There is an urgent need to develop new IA treatment strategies for OA. IA injection treatment have been rapidly developing with some promising progress in recent years. Several IA treatment small molecules are investigating in different clinical trial stages, of which some exhibited promising results (Table 1). For example, a single injection of CNTX-4975 (a trans-capsaicin that deactivates TRPV1-expressing nociceptive afferents) provided statistically significant improvements in pain with walking for subjects with moderate to severe knee pain associated with OA.37 However, most drug agents only remain in the joint for a few hours and rapidly clear after IA injection owing to the special physiological environment of the joint (Figure 2A).38–42 Multiple IA injections may result in less compliance and inflammation. Ensuring that drugs are consistently and effectively delivered into joint targets remains a significant challenge.43 Drug-delivery systems (DDSs) have evolved rapidly over the past 20 years, and substantial drug loading formulations have been developed to increase drug duration and provide controlled and/or sustained drug release. Some formulations, such as cationic nanoparticle IA formulations, have increased the drug duration from several hours to nearly one month (Figure 2B).44–49
 |
Table 1 Clinical Trials of Small Molecules Delivered by IA Injection as Currently Listed on www.clinicaltrials.gov |
 |
Figure 2 Representative half-lives or retention times of intra-articular (IA) drug (A)38–42/intra-articular drug (B)44–49 delivery system in a joint. |
In this review, we summarized the IA DDSs development over the past five years (Figure 3) and discuss the application prospects of different types of DDSs for OA treatment.
 |
Figure 3 A statistical chart of the research status of articular injection drug-delivery systems from the past five years. |
Methods
Studies on IA DDSs over the past five years were collected by using the PubMed/Google Scholar/Scopus/Web of Science databases. Search keywords were set as “injection” and “osteoarthritis”. “NoteExpress” software was used to remove the duplicated studies in the collected publications. Then, the publications were categorized into different categories for further analysis (for example: “hydrogel”, “microspheres”, and “nanoparticles”). In addition, the signaling pathway studies related to IA injection for OA treatment over the past five years were also collected by using the PubMed/Google Scholar/Scopus/Web of Science databases, Search keywords were as “Signaling pathway”, “injection” and “osteoarthritis”. Impact factor of the collected publications ≥ 5 were selected and categorized into signaling pathway categories for analysis.
Different Drug-Delivery Systems for Intra-Articular Injection of Knee Joint
At present, biodegradable and bio-eliminable materials have been widely utilized for IA DDSs with diverse benefits, including, but not limited to, enhancing the stability of encapsulated drugs, decreasing toxicity, reducing adverse effects, improving pharmacokinetics, and targeting specific sites. In this study, the current IA DDSs are divided into three categories according to their material characteristics; that is, nanoparticles, microspheres, and hydrogels. We collected 22,040 publications on IA DDSs from the past five years. Of these publications, hydrogels, nanoparticle carriers, and microspheres accounted for 41.2, 40.47, and 18.33%, respectively, of the IA DDSs used for OA treatment (Figure 3).
Hydrogels
Hydrogels are cross-linked 3D polymer networks with a high water content which expand in water-soluble environments and form polymerization systems with targeted substances such as proteins, nucleic acids, small organic molecules, etc. The high water content of hydrogels (typically 70–99%) is physically similar to tissues, providing excellent biocompatibility and the ability to easily encapsulate hydrophilic drugs. Hydrogel DDSs have been applied in different medicinal fields, including cardiology, oncology, immunology, wound healing, and pain management.50 Hydrogel gelation strategies are selected according to the characteristics of various materials and include Schiff-base cross-linking, enzyme-mediated cross-linking, photocrosslinking, or thermosensitive cross-linking.51 Hydrogels have a long application history as a joint lubricant, and were reported in animals as early as 2002.52
Hydrogels Used as a Drug-Delivery System
Hydrogels are generally composed of macromolecules with long retention times in the knee joint compared with small-molecule materials. Therefore, hydrogels are generally used as a DDS for prolonged drug effects in joints. Chondrogenic factors,53 chondroitin sulfate nanoparticles,54 and oligonucleotides55,56 have been commonly loaded into hydrogel delivery systems for therapeutic studies. Some traditional medicines (such as celecoxib,57 meloxicam,58 dexamethasone,59 and triamcinolone60 loaded in hydrogel delivery systems not only provided improved long-term drug release, but also enhanced anti-inflammatory effect than the original formulations (See Table 2 for details).
 |  |  |  |  |  |  |  |  |  |  |  |  |
Table 2 Summary of Hydrogel Drug Delivery System |
Hydrogels Used as Cell and Tissue Engineering Scaffolds
Currently, many tissue or cell transplantation techniques have been used for OA cartilage regeneration. However, the cumbersome in vitro cell manipulations and potential cancer risks limit their application.61 In the last few decades, cell and pro-growth factors combined with hydrogel scaffolds applied in tissue engineering has attracted significant attention. As typical representatives, natural hydrogels with high performances are ideal biomaterial scaffolds for cartilage repair because of their preferable reconstructions of cell growth, proliferation, differentiation, and new tissue formation. Hydrogels such as polyglucosamine,62 chitosan,63 hyaluronic acid,64 and acellular cartilage matrix65 showed promising applications in the field of cellular implantation for the treatment of OA.
Functional Hydrogels
Hydrogels made of natural polymer materials have their limited properties (including, rheological, stability, mechanical strength, et al.); therefore, modified hydrogels with different functional groups are rapidly evolving because of the modifications improve the properties of natural polymer. In addition, these modifications create different kinds of smart hydrogels, which could automatically sense and respond to changes in the external environment (include temperature sensitive, pH sensitive, photosensitive, magnetic sensitive and temperature/pH dual sensitivity hydrogels).66–71
Temperature-sensitive hydrogels have always been favored because they are easier to load with drugs or cells and possess more convenient in situ formation properties than other hydrogels.66,67 Thermosensitive hydrogels, such as chitosan with β-glycerol phosphate,72 glycol chitosan,73 PEG-grafted polyalanine or poly(lactide-co-glycolide),74–76 poly(N-isopropylacrylamide-co-acrylic acid) (p(NIPAAm-AA)),77,78 poly(ethyleneglycol)-(p(HPMAm-lac)-PEG),79 poloxamers,80 poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) (PLEL),81,82 hyaluronic acid-chitosan-poly(N-isopropylacrylamide),83 and amphiphilic poly(organophosphazene),84 have been increasingly applied in the treatment of OA. A previous study demonstrated that temperature-sensitive PNIPAM (poly(N-isopropylacrylamide)) hydrogels85 could regulate drug release according to the joint temperature. This design offers a novel concept for the hydrogel treatment of OA.
Nanoparticles
Nanoparticles (NPs) are also frequently used in OA treatment studies. NPs are submicron particles with dimensions ranging from 1 to 300 nm, which is approximately 1000 times smaller than that of chondrocytes. NPs as carriers can incorporate drugs on the surface or matrix to protect them from enzymatic degradation, improve their penetration across the cartilage matrix, and regulate drug pharmacokinetics, which is beneficial for improving the efficacy and reducing the toxicity of therapeutic compounds.86 By adjusting the physicochemical properties or decorating with moieties, NPs can be modified with functional groups to target the components and/or cells in the cartilage.87 This section reviews the current developments and novel applications of OA-related NP-based DDSs, including liposomes, micelles, dendrimers, polymeric nanoparticles (PNPs), and exosomes.51,88–92
Liposomes
Liposomes are spherical vesicles composed of phospholipids and steroids,93 and are both hydrophilic and lipophilic.94 Liposomes have been proven to prolong drug efficacy, improve bioavailability, and provide tissue/cell targeting.95–98 Some liposome formulations are already commercially available for treating OA. For example, Lipotalon® (Merckle, Germany) is the first used liposome formulation in Germany to clinically treat OA by IA delivery,43 containing dexamethasone palmitate as active ingredient, preferentially taken up by synovial macrophages after IA injection and transformed to its active form by intracellular esterases, thus preventing the side effects of free dexamethasone (synovitis and tissue irritation).99,100
However, the aqueous environment of the synovial fluid may lead to rapid drug release. These shortcomings have limited the application of liposomes.
1). Prolonged duration of drug efficacy
Liposomes can prolong drug retention because the drugs are encapsulated within the phospholipid bilayers and are more difficult to remove than small-molecule drugs. The efficacy of many drugs has reportedly improved through liposome formulations. For example, the liposome formulations for rapamycin,101 fish oil protein,102 diclofenac sodium,103 and d-glucosamine sulfate104 have been reported to enhance the inhibition of pro-inflammatory factors or promote cartilage-related genes expression (See Table 3 for details).
 |
Table 3 Summary of Nanoparticles Drug Loading System |
2). Improved bioavailability
The phospholipid bilayer structure of liposomes has a high affinity for the cell membrane and good stability, and the uptake of drugs can be improved by liposome formulations. In recent years, natural molecules, such as curcumin,105,106 lornoxicam,107 and rapamycin,101 with poor bioavailability were also loaded into liposomes for OA treatment. The results showed superior therapeutic effects and better bioavailability than the original forms.
3). Liposome tissue/cell targeting
Modified liposomes with functional groups for targeting are generally based on two strategies.
① Targeting agents loaded in liposome formulation
The targeting agents can be loaded into the liposomes. This strategy typically targets protein receptors or genes, such as CGS21680 (adenosine A2A receptor agonist),108,109 miR-15a (a microRNA that silences SMAD2 gene expression),110 and microRNA-143-3p (targeted regulation of BMPR2 expression).111 The stable lipid bilayer structure of liposomes can help transport sensitive cargo into cells and enhance the effect of the agents.112
② Liposome surface modification with functional targeting groups
Liposome surface modifications with functional targeting groups is another common strategy. The synovium and inflammatory microenvironment can be targeted by modifying with the HAP-1 (ALSQAFRHAFTS; sc-HAP-1) peptide,113 ART-2 (CKPFDRALC) peptide,114 or a specified number of PEG groups.115 Bone-targeted delivery of liposomes can be achieved by modifying with alendronate, pyrophosphate, and oligopeptides.116 Considering that cartilage is rich in type II collagen, liposomes modified with type II collagen antibodies117 reportedly provided cartilage targeting for targeted drug delivery.
Micelles
Micelles are nanoscale materials comprised of amphiphilic polymers that self-assemble in aqueous solvents, and their size range is generally 5–100 nm.118 Micelle formulation requires a critical polymer concentration known as the critical micelle concentration (CMC). The self-assembly process occurs when the amphiphile concentration in the aqueous solution reaches the CMC.119
Micelle formulations are commonly used as a DDS for hydrophobic agents. Wei et al.120 encapsulated the sPLA2 (secretory phospholipase A2) inhibitor into micelles for lesion tissue targeted drug delivery, and Wang et al.121 loaded siRNA in micelles to target p56 of the NF-κB pathway. Oxygen species (ROS)-responsive micelles were developed based on elevated ROS exposure in arthritic joints. For example, the diblock copolymer poly (ethylene glycol)-block-poly (propylene sulfide)122 and PLGA-SeSe-mPEG (poly (lactic-co-glycolic acid-SeSe-poly (ethylene glycol)) were used to modify micelles for ROS-sensitive drug delivery.123 Considering that the folic acid (FA) receptor is highly expressed on arthritic macrophage membranes, FA-modified PSA (polysialic acid)-CC (natural cholesterol) micelles were developed to target macrophages in the synovial inflammatory microenvironment.124 Conjugate polymers of heparin and d-α-tocopheryl succinate (LMWH-TOS) also reportedly target the inflammatory microenvironment.125
The acidic environment and overexpressed matrix metalloproteinases-13 (MMP-13) are typical OA markers, which enable the development of stimulus-responsive drug-delivery systems with high specificity for OA. pH-sensitive amphiphilic methoxy polyethylene glycol-polypropylene fumarate micelles,126 poly (β-amino ester) micelles,127–129 and multiple targeted micelles have been developed for OA drug delivery. For example, Lan et al.130 developed an MMP-13 enzyme and pH-responsive theranostic micelle for OA treatment and a specific collagen type II targeting peptide (WRYGRL) conjugate PPL was used to target the cartilage.
To date, few studies have reported micelle applications for clinical OA treatment,131 which may be related to the common limitations of micelles, including their inability to encapsulate hydrophilic drugs, CMC dependency, and toxicity concerns.
Dendrimers
Dendrimers are highly branched polymers with demonstrated therapeutic potential for drug delivery. Their structure can be divided into three main components: (1) the core or nucleus, (2) inner layers consisting of repetitive molecular units called dendrons, and (3) terminal groups on the surface.132–135 Poly(lysine) (PLL), polypropyleneimine (PPI), polyethylenimine (PEI), poly(arylether), and poly(amidoamine) (PAMAM) are the most popular dendrimers used in oral delivery.136 Dendrimers can steadily incorporate many active compounds and/or ligands that improve their solubility.137 Hu et al.138 conjugated kartogenin (KGN), PAMAM, and PEG to obtain PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) conjugates. This strategy improved KGN release and enhanced chondrogenic effects in OA joints. In addition, the dendrimer formed by PAMAM is a dense cationic macromolecule that binds anionic cartilage and easy to penetrates anionic cartilage tissues. Geiger et al.49 conjugated insulin-like growth factor 1 and PEG to PAMAM and improved cartilage-targeted drug delivery. Chondrocyte-affinity peptides139 or chondroitin sulfate (CS)-modified PAMAM polymers140 further enhanced polymer cartilage targeting, and azabisphosphonate (ABP)-capped dendrimers selectively targeted monocytes and directed them toward anti-inflammatory activation in the RA joint.141
Dendrimers possess various advantages, such as increased solubility of hydrophobic drugs and tunable physicochemical properties. Currently, there are many pre-clinical studies related to dendrimer applications;135,142–145 however, their application in OA treatment is limited. Disadvantages such as non-entrapment of hydrophilic drugs, cellular toxicity, and rapid drug release (up to 70% of the encapsulated drugs are released within a few hours146) limit their application in OA treatment.
Polymer Nanoparticles (PNPs)
Polymer nanoparticles (PNPs) are solid particles that can be prepared in the range of nanometers to microns.147 There are two structural types: nanocapsules, which consist of a hydrophilic drug reservoir and a polymer shell, and nanospheres, which contain a homogeneous polymer matrix that can load dispersed/intercepted drugs. The release kinetics of both types can be adjusted according to the formulation strategy, chemical composition, and molecular weight of polymers and drugs.148 PNPs have many promising advantages for drug delivery, including:
- Improved drug release
Several studies report significantly improved and sustained drug release using PNPs for drug delivery. PLGA,149,150 polylactic acid (PLA),151 polyurethane,152 different polymer combinations,153 silk fibroin,154 and polymer-grafted mesoporous silica155 used to prepare PNPs for drug delivery. These formulations reportedly provide improved and sustained drug-release profiles for celecoxib, curcumin, and KGN by IA drug delivery.
Microenvironmental-sensitive drug-release strategies have been attractive for PNP drug delivery. pH and temperature sensitive PNPs are rapidly evolving, including hollow dextran/poly (N-isopropyl acrylamide) NPs,156 dual-functional poly[N-isopropylacrylamide-2-methacryloyloxyethyl phosphorylcholine] (PNIPAM-PMPC) nanospheres,157 hyaluronic acid-poly(N-isopropylacrylamide) (HA-pNiPAM) nanospheres,158 and chitosan oligosaccharide-conjugated pluronic F127 grafting carboxyl group nanospheres159 which possess temperature-responsive release properties. Previous studies showed that NH4HCO3 laden poly (lactic-co-glycolic acid) (PLGA) NPs demonstrate pH-responsive drug release.160,161
In recent years, more and more PNPs targeted drug-delivery systems have been developed for OA therapy. Cartilage targeting is the most popular strategy that can be achieved by modifying the PNPs with cartilage affinity groups.162 Collagen II-binding peptide-modified formononetin (FMN)-poly (ethylene glycol) (PEG) NPs,163,164 xanthan gum-poly (sulfobetaine methacrylate) (XG-PSBMA) NPs,165 WYRGRL (a short cartilage-targeting peptide sequence)-modified PLGA NPs,164,166 polyethylene glycol (PEG) chains (PEG-SWCNTs) modified with single-walled carbon nanotube (SWCNT) NPs,44 and quaternary ammonium cation modified PLGA NPs167 have been developed for cartilage targeting. In some instances, cartilage targeting originates in the basic material of the PNPs.163–166In addition, dextran sulfate used to target macrophages,168 HAP-1 peptide used to target synovial,169 O-HTCC (O-(2-hydroxyl) propyl-3trimethyl ammonium chitosan chloride) and polyphenol–poloxamer used to target ROS,170,171 C11 peptide (the C-terminal region of rh174) used to target bone,172 PNPs have also been developed to target the specific microenvironment of OA joint.
Over the past decade, significant progress has been made in the functional modification of PNPs. However, the side effects, toxicity, and complicated preparation processes of PNPs continue to challenge their application in OA treatment.
Exosomes
Exosomes are extracellular vesicles (loading content including lipids, nucleic acids, and proteins) secreted by cells for cell-to-cell communication.92 The size of exosomes is generally between 30–150 nm.173 Previous studies demonstrated the utility of exosomes as drug-delivery systems. Compared with other nanoparticles, exosomes possess higher stability, biocompatibility, biological barrier permeability, and lower immunogenicity,174 and can overcome some challenges, such as toxicity and a high clearance rate.175 Current studies on exosomes for OA treatment are mainly focused on two aspects: 1) to investigate the diagnostic significance and biological effects of endogenous exosomes in OA patients; 2) to investigate stem cell-derived exosomes for OA treatment.92
On the other hand, in recent years, the utility of exosomes as drug-delivery systems has become increasingly attractive for OA treatment. Tao et al.82 prepared circRNA3503 loaded exosomes from synovial mesenchymal stem cells and further incorporated the exosomes into an injectable thermosensitive hydrogel. This composite gel strategy successfully delivered small-molecule RNAs, improved drug targeting, and prevented OA progression. Liang et al.176 reported chondrocyte-affinity peptides (CAP) and lysosome-associated membrane glycoprotein 2b (Lamp2b) protein-modified exosomes (CAP-exosomes) that can efficiently encapsulate miR-140 and target chondrocytes in vitro. The CAP-exosomes successfully delivered miR-140 to deep cartilage regions and alleviated OA progression, demonstrating a potential organelle-based, cell-free therapy for OA. Xu et al.177 reported KGN-loaded MSC-binding peptide E7 modified exosomes (E7-Exo) that targeted synovial fluid-derived mesenchymal stem cells. The E7-Exo induced a higher MSC cartilage differentiation than KGN alone or KGN-loaded unmodified exosomes.
Exosomes have shown promising potential as drug carriers in OA treatment. However, studies of using exosome as drug-delivery systems are still limited. The greatest technical challenge is the difficulty in obtaining sufficient amounts of exosomes for in vivo studies. Table 3 demonstrated more details of the nanoparticle drug delivery systems mentioned in this review.
Microspheres
Microspheres are skeletal spherical drug-delivery systems formulated by dispersing or dissolving a drug in a polymer, with a particle size of approximately 1–200 μm. Microspheres are commonly used in OA treatment to prolong the retention time of drugs and lubricate joints.178,179
Liang et al.180 reported that mometasone furoate-loaded PLGA microspheres increased the drug retention time in joints by up to 35 days. Stefani et al.181 developed an acellular agarose hydrogel incorporated with dexamethasone-loaded PLGA microspheres for OA treatment. Combining microspheres and hydrogels improved the sustained drug-release properties for OA treatment for up to 99 days. Li et al.182 demonstrated that super-activated platelet lysate (sPL)-loaded PLGA/chitosan/gelatin microspheres improved the sustained drug release, inhibited osteoarthritis, and promoted cartilaginous repairs. Park et al.183 reported the utility of gelatin microspheres that are responsive to proteolytic enzymes typically expressed in arthritic flares, resulting in the on-demand and spatiotemporally controlled release of anti-inflammatory cytokines for cartilage preservation and repair.
On the other hand, IA DDS are not only committed to improving drug effectiveness, but also focus on improving joint lubrication properties. Bio-lubricants have been designed for the treatment of early OA by improving the lubrication performance of synovial joints. Therefore, some people have also improved the lubricating properties of hydrogels by modifying polymer materials. Sulfone-modified HA,184 lactose modified chitosan185 and gellan gum186 could modulate viscoelastic properties of osteoarthritis synovial fluids and improve OA joint lubrication. Han et al.179 reported photocrosslinked methyl methacrylate gelatin (GelMA) hydrogel microspheres incorporated into a self-adhesive polymer (DMA-MPC) to prepare a new composite microsphere (GelMA@DMA-MPC). This biomimetic injectable hydrogel microsphere enhanced lubrication and controlled the drug release for OA treatment. Zhang et al.187 demonstrated that YAP (Yes-associated protein)-selective inhibitor-loaded chitosan microspheres could target subcellular YAP activity and alleviate OA progression. Yang et al.188 reported ball-bearing-inspired polyampholyte-modified microspheres with a super lubricated ability (MGS@DMA-SBMA). The MGS@DMA-SBMA microspheres significantly enhanced joint lubrication and improved the sustained drug release, which are highly desirable for IA OA treatment.
Several microsphere formulations have been commercially available or proved to be promising for OA treatment. A triamcinolone acetonide sustained-release microsphere formulation (Triamcinolone acetonide extended-release (ER), Zilretta®) was approved for OA treatment in the United States.189 In addition, several microsphere formulations, such as fluvastatin,190 celecoxib,191 and etoricoxib,192 are planning to tested in clinical trials.
Generally, different polymers (such as polylactic acid, gelatin, and chitosan) can be developed into microspheres, which can also be modified with different functional groups to help improve drug delivery/targeting.193 Table 4 demonstrated more details of the microsphere drug delivery system mentioned in this review.
 |
Table 4 Summary of Microspheres Drug Delivery System |
Current Active and Passive Targeting Strategies for IA DDSs
Tissue/cell-specific active and passive targeting technologies have been developed to improve drug retention and efficacy in joints. We collected and analyzed 36 studies with clear target mechanisms. The results demonstrated that targeted synovium/synoviocytes, cartilage and chondrocytes, and joint microenvironments (synovial fluid) accounted for 61.11, 33.33, and 5.56%, respectively, of the IA DDSs used for OA treatment (Figure 4). The details are listed in Table 5.
 |
Table 5 Representative Research Targets of IA DDSs |
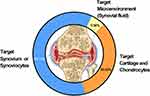 |
Figure 4 Representative research targets of IA DDSs. |
Different strategies have been used to target cartilages/chondrocytes, including the passively targeting anionic cartilage ECM/collagen type II.49,194–196 For example, a six-amino acid collagen II-binding peptide (WYRGRL)-conjugated drug-delivery system targets collagen and provides significantly longer joint retention than a normal control peptide.195,196 In addition, drug retention increased with the collagen II-binding peptide content in the drug-delivery system. Antibodies for collagen II197,198 have also been used as ligands in drug-delivery systems to target cartilage.117,199 A drug-delivery system incorporating a cell-penetrating peptide (YGRKKRRQRRR-C) significantly enhanced the drug uptake by chondrocytes by approximately 4-folds compared to the non-targeting formulation.200 A passive, electrostatic-based strategy was also developed for targeting cartilage.49,139,201,202 For example, drug-delivery systems with cationic surface charges (especially dendrimers136,139) significantly improved the joint retention of drugs, as the cartilage ECM accumulated anionic charges because of the dense sulfated proteoglycan networks.49,141,203
The OA targeting strategy not only focuses on the cartilage/chondrocytes, but also on other joint tissues/cells, such as the synovium/synoviocytes, owing to the complicated disease progression in OA. This strategy focused on targeting surface receptors in two major cells: 1) the minor type A synovial macrophages that significantly proliferate and activate under inflammatory conditions; 2) and the dominant type B fibroblast-like cells which produce ECM components of the synovial membrane and synovial fluid. Previous studies have demonstrated tuftsin peptide incorporated nanoparticles that target macrophages in the joint;204 copolymer nanoparticles incorporated with IL-1Ra proteins targeted synoviocytes through the IL-1 receptor and increased drug retention in the joint;45 RGD (Arg-Gly-Asp) peptide-modified liposomes targeted vascular endothelial cells of synovium though RGD peptide binding to αvβ3 integrins,205 which are strongly up-regulated on angiogenic endothelium at the inflammatory sites; αvβ3-targeted fumagillin nanoparticles significantly decreased inflammation and angiogenesis, and preserved proteoglycan integrity in synovial tissues.206
Overexpressed activated macrophages and acidic microenvironments are two significant features of RA joints. Multifunctional FA receptor-targeting and pH-responsive nanocarriers have been developed for RA IA treatment. Folate-incorporated nanoparticles promoted drug uptake of macrophages compared to untargeted nanoparticles,207 and drug-delivery systems incorporated with macrophage-derived micro-vesicles can target macrophages in the synovium or pannus of inflamed tissues.208 Drug deliveries were also designed to target synovial fluid by ionic cross-linking with endogenous/exogenous hyaluronic acid.48,209
Evidently, numerous targeted strategies have been developed to deliver and improve the efficacy of drugs in joints. Active and passive targeting strategies for OA joints have rapidly evolved over the past decades (Figure 4 and Table 5).
Current Signaling Pathways Targeted by IA Drug-Delivery Systems
Many pathways are involved in the pathogenesis of OA, and drug delivery system targeting different pathways have been used in IA drug-delivery systems. Articular drug-delivery systems provide significant support for the precise targeting of key sites in OA.210–221 We searched 14,160 studies on articular drug-delivery systems with clear signaling pathways from the 1st of January 2016 to the 13th of July 2021. The results demonstrated that the current research on articular drug-delivery systems mainly focus on five signaling pathways. TGF-β is the leading signaling pathway accounting for up to 26.35% of the studies (3730), followed by the NF-κB, PI3K, Wnt, and p38 MAPK (1790) signaling pathways which account for 21.97 (3109), 19.64 (2786), 19.39 (2745), and 12.65% (1790), respectively, of the studies (3109) (Figure 5, Table 6). None of the signaling pathways were proven to play the dominant role in OA initiation and progression. The mechanism of action of OA has yet to be fully elucidated.
 |
Table 6 Summary of Previous Studies on Signaling Pathway in IA DDSs |
 |
Figure 5 Statistics over the past five years on the current research status of IA DDSs target signaling pathways. |
Discussion
IA injections play an important role in the treatment of OA owing to their significant advantages. In this review, we summarized the current drug-delivery systems for IA DDSs. We collected and analyzed studies that reported the application of different nanoparticle-based IA DDSs in pre-clinical/clinical trials. Polymeric microspheres were the leading nanoparticles and accounted for 35.17% of IA DDSs. Nanoparticle and hydrogel/gel formulations accounted for 28.57 and 14.29%, respectively, of IA DDSs (Figure 6A). Although OA treatment remains a challenge in clinical practice, there are many IA drug delivery formulations for clinical application are ongoing, and several formulations including ZILRETTA, TIVORBEX, ZORVOLEX, and VIVLODEX have been approved222–225 (Figure 6B, Table 7).
 |
Table 7 Recent IA DDSs Reported in Preclinical and Clinical Trials |
Different IA drug delivery systems have their advantages and disadvantages. Hydrogel often used as lubricant as its high water content and rheological properties. The excellent biocompatibility of hydrogel is also attractive for loading live cells and drugs. However, the loose network and high water content of hydrogels makes it difficult to increase drug retention time in joint. Microspheres are widely used for controlled delivery of therapeutics via different routes of administration (mainly subcutaneous and intramuscular) for augmenting effectiveness and reducing toxicity of the incorporated agents to non-targeted cells and tissues. However, the stability of microspheres is a challenge issue and they are not easily to mass-produce. Nanoparticle is a promising drug delivery system as its unique ability to penetrate tissues and easy to modify functional groups. However, some challenge issues still hinder the application of these nanoparticles. For example, 1) the sustained release and retention properties of most nanoparticles are still weak; 2) the drug leakage issue of liposome formulations; 3) the entrapment efficiency issue of hydrophilic drug micelles; 4) drug release too fast issue of dendrimer. Although exosomes have unique advantages in biocompatibility and non-toxicity, it is difficult to address the large quantities production issue, which significant hinder its future application. At present, significant challenges remain regarding to the safety, efficacy duration, and targeting properties to all IA DDSs. There is another important issue that may raise significant concerns: how to sterilize DDs while maintaining their effectiveness? Liposomes and microspheres are more suitable for gamma ray sterilization.226 For unstable nanoparticles, aseptic filtration is recommended.227 High-pressure steam sterilization and ethylene oxide sterilization are not suitable for most drug delivery systems as the properties of DDSs and its loaded drugs would easy to be destroy.227 However, there still are some formulations had been proved that their properties maintained their properties after autoclave.228
A multifunctional drug-delivery system with excellent biosafety and drug-targeting properties is a promising direction of future IA drug delivery system development. Combined different strategies to prepare drug delivery systems for IA injection treatment are increasingly attractive. However, the more complex strategy of the drug delivery system, the more it is required to address synergies effects between different strategies.
Conclusions
Different IA drug delivery systems are developing rapidly with some significant progress. The ideal performance of an IA DDS depends on the nature of the drug delivery platform. Desirable properties of an IA DDS for OA included excellent continuous and controlled drug delivery, lubrication performance, and disease-targeting that could significantly improve the treatment of OA. At last, the most important issue is the benefits of new IA treatments must be carefully weighed against their cost and potential risks.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Funding
This research was funded by grants from the National Natural Science Foundation of China (No. 81703584); The regional joint fund of natural science foundation of Guangdong province (No. 2020B1515120052); Guangdong Province Natural Science Foundation of China (No. 2017A030310614, 2018A030313390, 2016ZC0178, 2021A1515010975); Discipline construction project of Guangdong Medical University (No. 4SG22002G, 4SG21156G); Special Funds for Scientific Technological Innovation of Undergraduates in Guangdong Province (No. pdjh2022a0214); Affiliated Hospital of Guangdong Medical University “Clinical Medicine+” CnTech Co-operation Project (No. CLP2021B012); Guangdong Medical University scientific research fund (No. B2017001); the Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (No. ZJW-2019-007).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review.
References
1. Boer CG, Hatzikotoulas K, Southam L, et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184(18):4784–4818 e4717. doi:10.1016/j.cell.2021.07.038
2. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi:10.1016/S0140-6736(18)32279-7
3. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi:10.1016/S0140-6736(16)31678-6
4. Dantas LO, Salvini TF, McAlindon TE. Knee osteoarthritis: key treatments and implications for physical therapy. Braz J Phys Ther. 2021;25(2):135–146. doi:10.1016/j.bjpt.2020.08.004
5. DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi:10.1016/S0140-6736(18)32335-3
6. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–138. doi:10.1016/j.rehab.2016.01.006
7. Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets. 2014;15(6):635–661. doi:10.2174/1389450115666140306153115
8. Prieto-Alhambra D, Arden N, Hunter DJ. Osteoarthritis: The Facts. OUP Oxford; 2014.
9. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5–6):333–339. doi:10.1016/j.rehab.2016.07.004
10. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2(1):16072. doi:10.1038/nrdp.2016.72
11. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. doi:10.1016/j.mcna.2019.10.007
12. Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
13. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–644. doi:10.1038/nrrheum.2016.148
14. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
15. Zhen G, Cao X. Targeting TGFbeta signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35(5):227–236. doi:10.1016/j.tips.2014.03.005
16. Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020;11(8):854. doi:10.3390/genes11080854
17. Hsia AW, Emami AJ, Tarke FD, et al. Osteophytes and fracture calluses share developmental milestones and are diminished by unloading. J Orthop Res. 2018;36(2):699–710. doi:10.1002/jor.23779
18. Paduszynski W, Jeskiewicz M, Uchanski P, Gackowski S, Radkowski M, Demkow U. Hoffa’s fat pad abnormality in the development of knee osteoarthritis. Adv Exp Med Biol. 2018;1039:95–102.
19. Zeng N, Yan ZP, Chen XY, Ni GX. Infrapatellar fat pad and knee osteoarthritis. Aging Dis. 2020;11(5):1317–1328. doi:10.14336/AD.2019.1116
20. Belluzzi E, Macchi V, Fontanella CG, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. doi:10.3390/ijms21176016
21. Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int. 2019;2019:6390182. doi:10.1155/2019/6390182
22. Macchi V, Stocco E, Stecco C, et al. The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J Anat. 2018;233(2):146–154. doi:10.1111/joa.12820
23. Belluzzi E, Olivotto E, Toso G, et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect Tissue Res. 2019;60(2):136–145. doi:10.1080/03008207.2018.1470167
24. Roemer FW, Guermazi A, Hunter DJ, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17(6):748–753. doi:10.1016/j.joca.2008.09.013
25. Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthritis Cartilage. 2020;28(5):544–554. doi:10.1016/j.joca.2019.12.007
26. Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–419. doi:10.1038/nrrheum.2012.69
27. Alnahdi AH, Zeni JA, Snyder-Mackler L. Muscle impairments in patients with knee osteoarthritis. Sports Health. 2012;4(4):284–292. doi:10.1177/1941738112445726
28. Kim JR, Yoo JJ, Kim HA. Therapeutics in osteoarthritis based on an understanding of its molecular pathogenesis. Int J Mol Sci. 2018;19(3). doi:10.3390/ijms19030674
29. Roos EM, Herzog W, Block JA, Bennell KL. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7(1):57–63. doi:10.1038/nrrheum.2010.195
30. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi:10.1186/s13075-017-1229-9
31. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701–712. doi:10.1016/j.semarthrit.2013.11.012
32. Nguyen C, Rannou F. The safety of intra-articular injections for the treatment of knee osteoarthritis: a critical narrative review. Expert Opin Drug Saf. 2017;16(8):897–902. doi:10.1080/14740338.2017.1344211
33. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):577–579. doi:10.5435/JAAOS-21-09-577
34. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–233. doi:10.1002/art.41142
35. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–1589. doi:10.1016/j.joca.2019.06.011
36. Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):168–175. doi:10.1016/j.semarthrit.2018.01.009
37. Stevens RM, Ervin J, Nezzer J, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019;71(9):1524–1533. doi:10.1002/art.40894
38. Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):226–242. doi:10.1016/j.addr.2006.01.018
39. Owen SG, Francis HW, Roberts MS. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol. 1994;38(4):349–355. doi:10.1111/j.1365-2125.1994.tb04365.x
40. Schumacher HR Jr. Aspiration and injection therapies for joints. Arthritis Rheum. 2003;49(3):413–420. doi:10.1002/art.11056
41. Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–352. doi:10.1002/art.24096
42. Vugmeyster Y, Wang Q, Xu X, et al. Disposition of human recombinant lubricin in naive rats and in a rat model of post-traumatic arthritis after intra-articular or intravenous administration. AAPS J. 2012;14(1):97–104. doi:10.1208/s12248-011-9315-4
43. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11–22. doi:10.1038/nrrheum.2013.159
44. Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8(12):12280–12291. doi:10.1021/nn504537b
45. Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, Garcia AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33(30):7665–7675. doi:10.1016/j.biomaterials.2012.06.101
46. Singh A, Agarwal R, Diaz-Ruiz CA, et al. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv Healthc Mater. 2014;3(10):1562–1567, 1525. doi:10.1002/adhm.201400051
47. Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi:10.1016/j.biomaterials.2014.08.042
48. Kim SR, Ho MJ, Lee E, Lee JW, Choi YW, Kang MJ. Cationic PLGA/Eudragit RL nanoparticles for increasing retention time in synovial cavity after intra-articular injection in knee joint. Int J Nanomedicine. 2015;10:5263–5271. doi:10.2147/IJN.S88363
49. Geiger BC, Wang S, Padera RF
50. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12). doi:10.1038/natrevmats.2016.71
51. Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021;123:31–50. doi:10.1016/j.actbio.2021.01.003
52. Barbucci R, Lamponi S, Borzacchiello A, et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23(23):4503–4513. doi:10.1016/S0142-9612(02)00194-1
53. Murphy MP, Koepke LS, Lopez MT, et al. Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 2020;26(10):1583–1592. doi:10.1038/s41591-020-1013-2
54. Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, Sethuraman S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials. 2018;162:82–98. doi:10.1016/j.biomaterials.2018.01.056
55. Lolli A, Sivasubramaniyan K, Vainieri ML, et al. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release. 2019;309:220–230. doi:10.1016/j.jconrel.2019.07.040
56. Garcia JP, Stein J, Cai Y, et al. Fibrin-hyaluronic acid hydrogel-based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co-delivered and resident joint cells in osteoarthritis. J Control Release. 2019;294:247–258. doi:10.1016/j.jconrel.2018.12.030
57. Cokelaere SM, Plomp SGM, de Boef E, et al. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur J Pharm Biopharm. 2018;128:327–336. doi:10.1016/j.ejpb.2018.05.001
58. Fattahpour S, Shamanian M, Tavakoli N, et al. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int J Biol Macromol. 2020;151:220–229. doi:10.1016/j.ijbiomac.2020.02.002
59. Wang QS, Xu BX, Fan KJ, Fan YS, Teng H, Wang TY. Dexamethasone-loaded thermo-sensitive hydrogel attenuates osteoarthritis by protecting cartilage and providing effective pain relief. Ann Transl Med. 2021;9(14):1120. doi:10.21037/atm-21-684
60. Chen K, Li S, Yuan F, Sun P, Zhang Y. GEL-MAN hydrogel loaded with triamcinolone acetonide for the treatment of osteoarthritis. Front Bioeng Biotechnol. 2020;8:872. doi:10.3389/fbioe.2020.00872
61. Bao W, Li M, Yang Y, et al. Advancements and frontiers in the high performance of natural hydrogels for cartilage tissue engineering. Front Chem. 2020;8:53. doi:10.3389/fchem.2020.00053
62. Pipino G, Risitano S, Alviano F, et al. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: a clinical and histological evaluation. J Clin Orthop Trauma. 2019;10(1):67–75. doi:10.1016/j.jcot.2018.03.001
63. Adali T, Kalkan R, Karimizarandi L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int J Biol Macromol. 2019;124:541–547. doi:10.1016/j.ijbiomac.2018.11.226
64. Cavalli E, Levinson C, Hertl M, et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci Rep. 2019;9(1):4275. doi:10.1038/s41598-019-40575-w
65. Lu J, Shen X, Sun X, et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics. 2018;8(18):5039–5058. doi:10.7150/thno.26981
66. Moon HJ, Ko du Y, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41(14):4860–4883. doi:10.1039/c2cs35078e
67. Zhang Y, Yu J, Ren K, Zuo J, Ding J, Chen X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules. 2019;20(4):1478–1492. doi:10.1021/acs.biomac.9b00043
68. Sattari S, Dadkhah Tehrani A, Adeli M. pH-Responsive hybrid hydrogels as antibacterial and drug delivery systems. Polymers. 2018;10(6):660. doi:10.3390/polym10060660
69. Tang Q, Chen C, Jiang Y, et al. Engineering an adhesive based on photosensitive polymer hydrogels and silver nanoparticles for wound healing. J Mater Chem B. 2020;8(26):5756–5764. doi:10.1039/D0TB00726A
70. Liu Z, Liu J, Cui X, Wang X, Zhang L, Tang P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front Chem. 2020;8:124. doi:10.3389/fchem.2020.00124
71. Lavanya K, Chandran SV, Balagangadharan K, Selvamurugan N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;111:110862. doi:10.1016/j.msec.2020.110862
72. Cheng YH, Chavez E, Tsai KL, et al. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr Polym. 2017;173:17–27. doi:10.1016/j.carbpol.2017.05.069
73. Lee EJ, Kang E, Kang SW, Huh KM. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr Polym. 2020;244:116432. doi:10.1016/j.carbpol.2020.116432
74. Petit A, Sandker M, Muller B, et al. Release behavior and intra-articular biocompatibility of celecoxib-loaded acetyl-capped PCLA-PEG-PCLA thermogels. Biomaterials. 2014;35(27):7919–7928. doi:10.1016/j.biomaterials.2014.05.064
75. Mok SW, Fu SC, Cheuk YC, et al. Intra-articular delivery of quercetin using thermosensitive hydrogel attenuate cartilage degradation in an osteoarthritis rat model. Cartilage. 2020;11(4):490–499. doi:10.1177/1947603518796550
76. Liu H, Cheng Y, Chen J, et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi:10.1016/j.actbio.2018.04.035
77. Tan J, Xie S, Wang G, et al. Fabrication and optimization of the thermo-sensitive hydrogel carboxymethyl cellulose/Poly(N-isopropylacrylamide-co-acrylic acid) for U(VI) removal from aqueous solution. Polymers. 2020;12(1):151. doi:10.3390/polym12010151
78. Zhang J, Yun S, Du Y, Zannettino ACW, Zhang H. Fabrication of a cartilage patch by fusing hydrogel-derived cell aggregates onto electrospun film. Tissue Eng Part A. 2020;26(15–16):863–871. doi:10.1089/ten.tea.2019.0318
79. Agas D, Laus F, Lacava G, et al. Thermosensitive hybrid hyaluronan/p(HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J Cell Physiol. 2019;234(11):20013–20027. doi:10.1002/jcp.28598
80. Zhang T, Chen S, Dou H, et al. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater Sci Eng C Mater Biol Appl. 2021;118:111352. doi:10.1016/j.msec.2020.111352
81. Tang Q, Lim T, Shen LY, et al. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials. 2021;268:120605. doi:10.1016/j.biomaterials.2020.120605
82. Tao SC, Huang JY, Gao Y, et al. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact Mater. 2021;6(12):4455–4469. doi:10.1016/j.bioactmat.2021.04.031
83. Chen CH, Chen SH, Mao SH, et al. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr Polym. 2017;173:721–731. doi:10.1016/j.carbpol.2017.06.019
84. Seo BB, Kwon Y, Kim J, et al. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact Mater. 2022;7:14–25. doi:10.1016/j.bioactmat.2021.05.028
85. Yang L, Liu Y, Shou X, Ni D, Kong T, Zhao Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale. 2020;12(32):17093–17102. doi:10.1039/D0NR04013D
86. Gu W, Wu C, Chen J, Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int J Nanomedicine. 2013;8:2305–2317. doi:10.2147/IJN.S44393
87. Li X, Dai B, Guo J, et al. Nanoparticle-cartilage interaction: pathology-based intra-articular drug delivery for osteoarthritis therapy. Nanomicro Lett. 2021;13(1):149. doi:10.3847/1538-4357/abeb18
88. Maudens P, Jordan O, Allemann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today. 2018;23(10):1761–1775. doi:10.1016/j.drudis.2018.05.023
89. Pullan JE, Confeld MI, Osborn JK, Kim J, Sarkar K, Mallik S. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16(5):1789–1798. doi:10.1021/acs.molpharmaceut.9b00104
90. Huyan T, Li H, Peng H, et al. Extracellular vesicles - advanced nanocarriers in cancer therapy: progress and achievements. Int J Nanomedicine. 2020;15:6485–6502. doi:10.2147/IJN.S238099
91. Pourakbari R, Khodadadi M, Aghebati-Maleki A, Aghebati-Maleki L, Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci. 2019;236:116861. doi:10.1016/j.lfs.2019.116861
92. Ni Z, Zhou S, Li S, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi:10.1038/s41413-020-0100-9
93. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi:10.2147/IJN.S68861
94. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
95. Fonseca-Santos B, Gremiao MP, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2015;10:4981–5003. doi:10.2147/IJN.S87148
96. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
97. Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi:10.1016/j.chemphyslip.2013.10.011
98. Cipollaro L, Trucillo P, Bragazzi NL, Della PG, Reverchon E, Maffulli N. Liposomes for intra-articular analgesic drug delivery in orthopedics: state-of-art and future perspectives. insights from a systematic mini-review of the literature. Medicina. 2020;56(9). doi:10.3390/medicina56090423
99. Chen YC, Gad SF, Chobisa D, Li Y, Yeo Y. Local drug delivery systems for inflammatory diseases: status quo, challenges, and opportunities. J Control Release. 2021;330:438–460. doi:10.1016/j.jconrel.2020.12.025
100. Lin W, Goldberg R, Klein J. Poly-phosphocholination of liposomes leads to highly-extended retention time in mice joints. J Mater Chem B. 2022;10(15):2820–2827. doi:10.1039/D1TB02346B
101. Chen CH, Kuo SM, Tien YC, Shen PC, Kuo YW, Huang HH. Steady augmentation of anti-osteoarthritic actions of rapamycin by liposome-encapsulation in collaboration with low-intensity pulsed ultrasound. Int J Nanomedicine. 2020;15:3771–3790. doi:10.2147/IJN.S252223
102. Sarkar A, Carvalho E, D’Souza AA, Banerjee R. Liposome-encapsulated fish oil protein-tagged gold nanoparticles for intra-articular therapy in osteoarthritis. Nanomedicine. 2019;14(7):871–887. doi:10.2217/nnm-2018-0221
103. Chang MC, Chiang PF, Kuo YJ, Peng CL, Chen KY, Chiang YC. Hyaluronan-loaded liposomal dexamethasone-diclofenac nanoparticles for local osteoarthritis treatment. Int J Mol Sci. 2021;22(2):665.
104. Ji X, Yan Y, Sun T, et al. Glucosamine sulphate-loaded distearoyl phosphocholine liposomes for osteoarthritis treatment: combination of sustained drug release and improved lubrication. Biomater Sci. 2019;7(7):2716–2728. doi:10.1039/C9BM00201D
105. Perkins K, Sahy W, Beckett RD. Efficacy of curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22(1):156–165. doi:10.1177/2156587216636747
106. Yeh CC, Su YH, Lin YJ, et al. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des Devel Ther. 2015;9:2285–2300. doi:10.2147/DDDT.S78277
107. He K, Huang X, Shan R, et al. Intra-articular injection of lornoxicam and MicroRNA-140 co-loaded cationic liposomes enhanced the therapeutic treatment of experimental osteoarthritis. AAPS PharmSciTech. 2021;23(1):9. doi:10.1208/s12249-021-02149-w
108. Corciulo C, Castro CM, Coughlin T, et al. Intraarticular injection of liposomal adenosine reduces cartilage damage in established murine and rat models of osteoarthritis. Sci Rep. 2020;10(1):13477. doi:10.1038/s41598-020-68302-w
109. Friedman B, Corciulo C, Castro CM, Cronstein BN. Adenosine A2A receptor signaling promotes FoxO associated autophagy in chondrocytes. Sci Rep. 2021;11(1):968. doi:10.1038/s41598-020-80244-x
110. Ma T, Cheng Y, Tan L. Mechanism of miR-15a regulating the growth and apoptosis of human knee joint chondrocytes by targeting SMAD2. Artif Cells Nanomed Biotechnol. 2019;47(1):3188–3193. doi:10.1080/21691401.2019.1613420
111. Tian J, Rui YJ, Xu YJ, Zhang SA. MiR-143-3p regulates early cartilage differentiation of BMSCs and promotes cartilage damage repair through targeting BMPR2. Eur Rev Med Pharmacol Sci. 2018;22(24):8814–8821. doi:10.26355/eurrev_201812_16649
112. Rahman M, Beg S, Anwar F, et al. Liposome-based nanomedicine therapeutics for rheumatoid arthritis. Crit Rev Ther Drug Carrier Syst. 2017;34(4):283–316. doi:10.1615/CritRevTherDrugCarrierSyst.2017016067
113. Vanniasinghe AS, Manolios N, Schibeci S, et al. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin Immunol. 2014;151(1):43–54. doi:10.1016/j.clim.2014.01.005
114. Meka RR, Venkatesha SH, Acharya B, Moudgil KD. Peptide-targeted liposomal delivery of dexamethasone for arthritis therapy. Nanomedicine. 2019;14(11):1455–1469. doi:10.2217/nnm-2018-0501
115. Ren H, He Y, Liang J, et al. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl Mater Interfaces. 2019;11(22):20304–20315. doi:10.1021/acsami.8b22693
116. Liu Y, Jia Z, Akhter MP, et al. Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomedicine. 2018;14(7):2271–2282. doi:10.1016/j.nano.2018.07.011
117. Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine. 2015;11(4):939–946. doi:10.1016/j.nano.2015.01.011
118. Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi:10.1016/S0169-409X(00)00124-1
119. Jones M, Leroux J. Polymeric micelles - A new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48(2):101–111. doi:10.1016/S0939-6411(99)00039-9
120. Wei Y, Yan L, Luo L, et al. Phospholipase A2 inhibitor-loaded micellar nanoparticles attenuate inflammation and mitigate osteoarthritis progression. Sci Adv. 2021;7:15. doi:10.1126/sciadv.abe6374
121. Wang Q, Jiang H, Li Y, et al. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. doi:10.1016/j.biomaterials.2017.01.008
122. An L, Li Z, Shi L, et al. Inflammation-targeted celastrol nanodrug attenuates collagen-induced arthritis through NF-kappaB and notch1 pathways. Nano Lett. 2020;20(10):7728–7736. doi:10.1021/acs.nanolett.0c03279
123. Wu X, Li P, Cheng J, et al. ROS-sensitive nanoparticles co-delivering dexamethasone and CDMP-1 for the treatment of osteoarthritis through chondrogenic differentiation induction and inflammation inhibition. Front Bioeng Biotechnol. 2021;9:608150. doi:10.3389/fbioe.2021.608150
124. Zhang N, Xu C, Li N, et al. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: in vitro and in vivo evaluation. Drug Deliv. 2018;25(1):1182–1191. doi:10.1080/10717544.2018.1472677
125. Li J, Long Y, Guo R, et al. Shield and sword nano-soldiers ameliorate rheumatoid arthritis by multi-stage manipulation of neutrophils. J Control Release. 2021;335:38–48. doi:10.1016/j.jconrel.2021.05.008
126. Seetharaman G, Kallar AR, Vijayan VM, Muthu J, Selvam S. Design, preparation and characterization of pH-responsive prodrug micelles with hydrolyzable anhydride linkages for controlled drug delivery. J Colloid Interface Sci. 2017;492:61–72. doi:10.1016/j.jcis.2016.12.070
127. Kang C, Jung E, Hyeon H, Seon S, Lee D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomedicine. 2020;23:102104. doi:10.1016/j.nano.2019.102104
128. Perni S, Prokopovich P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomedicine. 2017;13(2):539–548. doi:10.1016/j.nano.2016.10.001
129. Koo H, Lee H, Lee S, et al. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem Commun (Camb). 2010;46(31):5668–5670. doi:10.1039/c0cc01413c
130. Lan Q, Lu R, Chen H, et al. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J Nanobiotechnology. 2020;18(1):117. doi:10.1186/s12951-020-00666-7
131. Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci. 2016;83:184–202. doi:10.1016/j.ejps.2015.12.031
132. Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546(1–2):215–225. doi:10.1016/j.ijpharm.2018.05.045
133. Abedi-Gaballu F, Dehghan G, Ghaffari M, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–190. doi:10.1016/j.apmt.2018.05.002
134. Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv Transl Res. 2016;6(2):132–147. doi:10.1007/s13346-015-0234-2
135. Dias AP, da Silva Santos S, da Silva JV, et al. Dendrimers in the context of nanomedicine. Int J Pharm. 2020;573:118814. doi:10.1016/j.ijpharm.2019.118814
136. Yellepeddi VK, Ghandehari H. Pharmacokinetics of oral therapeutics delivered by dendrimer-based carriers. Expert Opin Drug Deliv. 2019;16(10):1051–1061. doi:10.1080/17425247.2019.1656607
137. Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23(12):1517–1526. doi:10.1038/nbt1171
138. Hu Q, Ding B, Yan X, et al. Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine. 2017;13(7):2189–2198. doi:10.1016/j.nano.2017.05.011
139. Hu Q, Chen Q, Yan X, Ding B, Chen D, Cheng L. Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage. Nanomedicine. 2018;13(7):749–767. doi:10.2217/nnm-2017-0335
140. Oliveira IM, Goncalves C, Oliveira EP, et al. PAMAM dendrimers functionalised with an anti-TNF alpha antibody and chondroitin sulphate for treatment of rheumatoid arthritis. Mater Sci Eng C Mater Biol Appl. 2021;121:111845. doi:10.1016/j.msec.2020.111845
141. Hayder M, Poupot M, Baron M, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med. 2011;3(81):81ra35. doi:10.1126/scitranslmed.3002212
142. Vergallo C, Hafeez MN, Iannotta D, et al. Conventional nanosized drug delivery systems for cancer applications. Adv Exp Med Biol. 2021;1295:3–27.
143. Ortega MA, Guzman Merino A, Fraile-Martinez O, et al. Dendrimers and dendritic materials: from laboratory to medical practice in infectious diseases. Pharmaceutics. 2020;12(9):874. doi:10.3390/pharmaceutics12090874
144. Singh P. Dendrimers and their applications in immunoassays and clinical diagnostics. Biotechnol Appl Biochem. 2007;48(Pt 1):1–9. doi:10.1007/s12010-007-0004-9
145. Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2(4):561–566.
146. Patri AK, Kukowska-Latallo JF, Baker JR
147. Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20. doi:10.1016/S0168-3659(00)00339-4
148. Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi:10.1016/j.ejps.2012.12.006
149. Alarcin E, Demirbag C, Karsli-Ceppioglu S, Kerimoglu O, Bal-Ozturk A. Development and characterization of oxaceprol-loaded poly-lactide-co-glycolide nanoparticles for the treatment of osteoarthritis. Drug Dev Res. 2020;81(4):501–510. doi:10.1002/ddr.21642
150. Jung JH, Kim SE, Kim HJ, Park K, Song GG, Choi SJ. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int J Pharm. 2020;581:119249. doi:10.1016/j.ijpharm.2020.119249
151. Maudens P, Seemayer CA, Thauvin C, Gabay C, Jordan O, Allemann E. Nanocrystal-polymer particles: extended delivery carriers for osteoarthritis treatment. Small. 2018;14(8). doi:10.1002/smll.201703108
152. Fan W, Li J, Yuan L, et al. Intra-articular injection of kartogenin-conjugated polyurethane nanoparticles attenuates the progression of osteoarthritis. Drug Deliv. 2018;25(1):1004–1012. doi:10.1080/10717544.2018.1461279
153. Salama AH, Abdelkhalek AA, Elkasabgy NA. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int J Pharm. 2020;578:119081. doi:10.1016/j.ijpharm.2020.119081
154. Crivelli B, Bari E, Perteghella S, et al. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur J Pharm Biopharm. 2019;137:37–45. doi:10.1016/j.ejpb.2019.02.008
155. Wan L, Tan X, Sun T, Sun Y, Luo J, Zhang H. Lubrication and drug release behaviors of mesoporous silica nanoparticles grafted with sulfobetaine-based zwitterionic polymer. Mater Sci Eng C Mater Biol Appl. 2020;112:110886. doi:10.1016/j.msec.2020.110886
156. Song Y, Zhang T, Cheng H, et al. Imidazolium-based ionic liquid-assisted preparation of nano-spheres loaded with bio-active peptides to decrease inflammation in an osteoarthritis model: ex vivo evaluations. J Biomed Nanotechnol. 2021;17(5):859–872. doi:10.1166/jbn.2021.3069
157. Zhang K, Yang J, Sun Y, et al. Thermo-sensitive dual-functional nanospheres with enhanced lubrication and drug delivery for the treatment of osteoarthritis. Chemistry. 2020;26(46):10564–10574. doi:10.1002/chem.202001372
158. Maudens P, Meyer S, Seemayer CA, Jordan O, Allemann E. Self-assembled thermoresponsive nanostructures of hyaluronic acid conjugates for osteoarthritis therapy. Nanoscale. 2018;10(4):1845–1854. doi:10.1039/C7NR07614B
159. Kang ML, Kim JE, Im GI. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78. doi:10.1016/j.actbio.2016.05.005
160. Hu B, Gao F, Li C, et al. Rhein laden pH-responsive polymeric nanoparticles for treatment of osteoarthritis. AMB Express. 2020;10(1):158. doi:10.1186/s13568-020-01095-3
161. Zerrillo L, Que I, Vepris O, et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J Control Release. 2019;309:265–276. doi:10.1016/j.jconrel.2019.07.031
162. Faust HJ, Sommerfeld SD, Rathod S, et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials. 2018;183:93–101. doi:10.1016/j.biomaterials.2018.08.045
163. Xiong W, Lan Q, Liang X, et al. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J Nanobiotechnology. 2021;19(1):197. doi:10.1186/s12951-021-00945-x
164. Ai X, Duan Y, Zhang Q, et al. Cartilage-targeting ultrasmall lipid-polymer hybrid nanoparticles for the prevention of cartilage degradation. Bioeng Transl Med. 2021;6(1):e10187. doi:10.1002/btm2.10187
165. Ren K, Ke X, Chen Z, et al. Zwitterionic polymer modified xanthan gum with collagen II-binding capability for lubrication improvement and ROS scavenging. Carbohydr Polym. 2021;274:118672. doi:10.1016/j.carbpol.2021.118672
166. Jiang T, Kan HM, Rajpura K, Carbone EJ, Li Y, Lo KW. Development of targeted nanoscale drug delivery system for osteoarthritic cartilage tissue. J Nanosci Nanotechnol. 2018;18(4):2310–2317. doi:10.1166/jnn.2018.14311
167. Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake. Mol Pharm. 2019;16(2):469–479. doi:10.1021/acs.molpharmaceut.7b00484
168. She P, Bian S, Cheng Y, et al. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. Int J Biol Macromol. 2020;158:1082–1089. doi:10.1016/j.ijbiomac.2020.05.013
169. Mancipe CLM, Sequeira A, Garcia AJ, Guldberg RE. Articular cartilage- and synoviocyte-binding Poly(ethylene glycol) nanocomposite microgels as intra-articular drug delivery vehicles for the treatment of osteoarthritis. ACS Biomater Sci Eng. 2020;6(9):5084–5095. doi:10.1021/acsbiomaterials.0c00960
170. Gao X, Ma Y, Zhang G, et al. Targeted elimination of intracellular reactive oxygen species using nanoparticle-like chitosan- superoxide dismutase conjugate for treatment of monoiodoacetate-induced osteoarthritis. Int J Pharm. 2020;590:119947. doi:10.1016/j.ijpharm.2020.119947
171. Li X, Wang X, Liu Q, et al. ROS-responsive boronate-stabilized polyphenol-poloxamer 188 assembled dexamethasone nanodrug for macrophage repolarization in osteoarthritis treatment. Adv Healthc Mater. 2021;10(20):e2100883. doi:10.1002/adhm.202100883
172. Ren M, Li Y, Zhang H, et al. An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. J Mater Chem B. 2021;9(12):2831–2844. doi:10.1039/D0TB02926B
173. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3(2):22. doi:10.1002/0471143030.cb0322s30
174. Rasheed MH, E B, Helal GK, et al. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. 2017;18(3):538. doi:10.3390/ijms18030538
175. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
176. Liang Y, Xu X, Li X, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi:10.1021/acsami.0c10458
177. Xu X, Liang Y, Li X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. doi:10.1016/j.biomaterials.2020.120539
178. Edwards SH. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet J. 2011;190(1):15–21. doi:10.1016/j.tvjl.2010.09.019
179. Han Y, Yang J, Zhao W, et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater. 2021;6(10):3596–3607. doi:10.1016/j.bioactmat.2021.03.022
180. Liang Y, Zhang J, Zhao X, et al. Study on the slow-release mometasone furoate injection of PLGA for the treatment of knee arthritis. Curr Drug Deliv. 2021;18(3):357–368. doi:10.2174/1567201817666200917124759
181. Stefani RM, Lee AJ, Tan AR, et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020;102:326–340. doi:10.1016/j.actbio.2019.11.052
182. Li J, Liu N, Huang Z, Wang W, Hou D, Wang W. Intra-articular injection of loaded sPL sustained-release microspheres inhibits osteoarthritis and promotes cartilaginous repairs. J Orthop Surg Res. 2021;16(1):646. doi:10.1186/s13018-021-02777-9
183. Park E, Hart ML, Rolauffs B, Stegemann JP, TA R. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J Biomed Mater Res A. 2020;108(3):722–733. doi:10.1002/jbm.a.36852
184. Cai Z, Zhang H, Wei Y, Wu M, Fu A. Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater Sci. 2019;7(8):3143–3157. doi:10.1039/C9BM00298G
185. Scognamiglio F, Travan A, Donati I, Borgogna M, Marsich E. A hydrogel system based on a lactose-modified chitosan for viscosupplementation in osteoarthritis. Carbohydr Polym. 2020;248:116787. doi:10.1016/j.carbpol.2020.116787
186. Leone G, Consumi M, Pepi S, et al. Enriched Gellan Gum hydrogel as visco-supplement. Carbohydr Polym. 2020;227:115347. doi:10.1016/j.carbpol.2019.115347
187. Zhang X, Cai D, Zhou F, et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials. 2020;232:119724. doi:10.1016/j.biomaterials.2019.119724
188. Yang J, Han Y, Lin J, et al. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small. 2020;16(44):e2004519. doi:10.1002/smll.202004519
189. Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–462. doi:10.1007/s40265-019-01083-3
190. Goto N, Okazaki K, Akasaki Y, et al. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J Orthop Res. 2017;35(11):2465–2475. doi:10.1002/jor.23562
191. Janssen M, Timur UT, Woike N, et al. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244(Pt A):30–40. doi:10.1016/j.jconrel.2016.11.003
192. Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R. Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles–a potential intra-articular drug delivery system for the treatment of osteoarthritis. J Biomater Sci Polym Ed. 2016;27(4):303–316. doi:10.1080/09205063.2015.1125564
193. Sulaiman SB, Idrus RBH, Hwei NM. Gelatin microsphere for cartilage tissue engineering: current and future strategies. Polymers. 2020;12(10):2404. doi:10.3390/polym12102404
194. Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J Orthop Res. 2014;32(8):1044–1051. doi:10.1002/jor.22630
195. Hu HY, Lim NH, Ding-Pfennigdorff D, et al. DOTAM derivatives as active cartilage-targeting drug carriers for the treatment of osteoarthritis. Bioconjug Chem. 2015;26(3):383–388. doi:10.1021/bc500557s
196. Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7(3):248–254. doi:10.1038/nmat2116
197. Noyori K, Koshino T, Takagi T, Okamoto R, Jasin HE. Binding characteristics of antitype II collagen antibody to the surface of diseased human cartilage as a probe for tissue damage. J Rheumatol. 1994;21(2):293–296.
198. Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148(7):2103–2108.
199. Cho H, Stuart JM, Magid R, et al. Theranostic immunoliposomes for osteoarthritis. Nanomedicine. 2014;10(3):619–627. doi:10.1016/j.nano.2013.09.004
200. Elsaid KA, Ferreira L, Truong T, Liang A, Machan J, D’Souza GG. Pharmaceutical nanocarrier association with chondrocytes and cartilage explants: influence of surface modification and extracellular matrix depletion. Osteoarthritis Cartilage. 2013;21(2):377–384. doi:10.1016/j.joca.2012.11.011
201. Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi:10.1016/j.actbio.2019.03.010
202. Brown SB, Wang L, Jungels RR, Sharma B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2020;101:469–483. doi:10.1016/j.actbio.2019.10.003
203. Formica FA, Barreto G, Zenobi-Wong M. Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone. J Control Release. 2019;295:118–129. doi:10.1016/j.jconrel.2018.12.025
204. Jain S, Tran TH, Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi:10.1016/j.biomaterials.2015.05.028
205. Koning GA, Schiffelers RM, Wauben MH, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54(4):1198–1208. doi:10.1002/art.21719
206. Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. Alphavbeta3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23(9):2978–2985. doi:10.1096/fj.09-129874
207. Zhao J, Zhao M, Yu C, et al. Multifunctional folate receptor-targeting and pH-responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int J Nanomedicine. 2017;12:6735–6746. doi:10.2147/IJN.S140992
208. Li R, He Y, Zhu Y, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–134. doi:10.1021/acs.nanolett.8b03439
209. Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30(1):257–268. doi:10.1007/s11095-012-0870-x
210. Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):97. doi:10.1186/s12964-019-0411-x
211. Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28(4):400–409. doi:10.1016/j.joca.2020.02.027
212. Cherifi C, Monteagudo S, Lories RJ. Promising targets for therapy of osteoarthritis: a review on the Wnt and TGF-beta signalling pathways. Ther Adv Musculoskelet Dis. 2021;13:1759720X211006959. doi:10.1177/1759720X211006959
213. Lu Z, Liu S, Le Y, et al. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials. 2019;218:119190. doi:10.1016/j.biomaterials.2019.05.001
214. Ouyang Z, Tan T, Liu C, et al. Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd2(CO3)3@PDA nanoparticles via TLR-2/NF-kappaB/Akt signaling. Biomaterials. 2019;205:50–63. doi:10.1016/j.biomaterials.2019.03.018
215. Kang LJ, Yoon J, Rho JG, et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967. doi:10.1016/j.biomaterials.2021.120967
216. Chen P, Xia C, Mo J, Mei S, Lin X, Fan S. Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater Sci Eng C Mater Biol Appl. 2018;91:190–200. doi:10.1016/j.msec.2018.05.034
217. Bhattacharjee M, Ivirico JLE, Kan HM, et al. Preparation and characterization of amnion hydrogel and its synergistic effect with adipose derived stem cells towards IL1beta activated chondrocytes. Sci Rep. 2020;10(1):18751. doi:10.1038/s41598-020-75921-w
218. Maudens P, Seemayer CA, Pfefferle F, Jordan O, Allemann E. Nanocrystals of a potent p38 MAPK inhibitor embedded in microparticles: therapeutic effects in inflammatory and mechanistic murine models of osteoarthritis. J Control Release. 2018;276:102–112. doi:10.1016/j.jconrel.2018.03.007
219. Chu M, Wu P, Hong M, et al. Lingzhi and San-Miao-San with hyaluronic acid gel mitigate cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. J Orthop Translat. 2021;26:132–140. doi:10.1016/j.jot.2020.07.008
220. Li S, Stockl S, Lukas C, et al. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res Ther. 2021;12(1):252. doi:10.1186/s13287-021-02317-6
221. Zuo D, Tan B, Jia G, Wu D, Yu L, Jia L. A treatment combined prussian blue nanoparticles with low-intensity pulsed ultrasound alleviates cartilage damage in knee osteoarthritis by initiating PI3K/Akt/mTOR pathway. Am J Transl Res. 2021;13(5):3987–4006.
222. Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi:10.1016/j.jconrel.2014.03.053
223. Im GI, Kang ML. Kartogenin derivative-containing polymeric micelle, hyaluronic acid hydrogel, method for producing the same, and use thereof. Google Patents; 2019.
224. Bodick N, Jackson JD, Joshi U. Fluticasone extended-release formulations and methods of use thereof. Google Patents; 2020.
225. Ghandehari H, Oottamasathien S, Jensen MM, Cappello J, Jia W, Prestwich GD. Situ gelling compositions for the treatment or prevention of inflammation and tissue damage. Google Patents; 2019.
226. Sakar F, Ozer AY, Erdogan S, et al. Nano drug delivery systems and gamma radiation sterilization. Pharm Dev Technol. 2017;22(6):775–784. doi:10.3109/10837450.2016.1163393
227. Zielinska A, Soles BB, Lopes AR, et al. Nanopharmaceuticals for eye administration: sterilization, depyrogenation and clinical applications. Biology. 2020;9(10). doi:10.3390/biology9100336.
228. Lucke M, Winter G, Engert J. The effect of steam sterilization on recombinant spider silk particles. Int J Pharm. 2015;481(1–2):125–131. doi:10.1016/j.ijpharm.2015.01.024
229. Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35(1):538–549. doi:10.1016/j.biomaterials.2013.09.091
230. Yan H, Duan X, Pan H, et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A. 2016;113(41):E6199–E6208. doi:10.1073/pnas.1608245113
231. Cho H, Kim BJ, Park SH, Hasty KA, Min BH. Noninvasive visualization of early osteoarthritic cartilage using targeted nanosomes in a destabilization of the medial meniscus mouse model. Int J Nanomedicine. 2018;13:1215–1224. doi:10.2147/IJN.S149375
232. Chen Z, Chen J, Wu L, et al. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int J Nanomedicine. 2013;8:3843–3853. doi:10.2147/IJN.S50721
233. Laroui H, Grossin L, Leonard M, et al. Hyaluronate-covered nanoparticles for the therapeutic targeting of cartilage. Biomacromolecules. 2007;8(12):3879–3885. doi:10.1021/bm700836y
234. Labens R, Lascelles BD, Charlton AN, et al. Ex vivo effect of gold nanoparticles on porcine synovial membrane. Tissue Barriers. 2013;1(2):e24314. doi:10.4161/tisb.24314
235. Bhalekar MR, Upadhaya PG, Madgulkar AR. Fabrication and efficacy evaluation of chloroquine nanoparticles in CFA-induced arthritic rats using TNF-alpha ELISA. Eur J Pharm Sci. 2016;84:1–8. doi:10.1016/j.ejps.2016.01.009
236. Chen Z, Liu D, Wang J, et al. Development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Deliv. 2014;21(5):342–350. doi:10.3109/10717544.2013.848495
237. Schneider T, Welker P, Haag R, et al. Effects of dendritic polyglycerol sulfate on articular chondrocytes. Inflamm Res. 2015;64(11):917–928. doi:10.1007/s00011-015-0875-0
238. Kim MJ, Park JS, Lee SJ, et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J Control Release. 2015;216:140–148. doi:10.1016/j.jconrel.2015.08.025
239. Lu H, Dai Y, Lv L, Zhao H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS One. 2014;9(1):e84703. doi:10.1371/journal.pone.0084703
240. Zhou HF, Yan H, Pan H, et al. Peptide-siRNA nanocomplexes targeting NF-kappaB subunit p65 suppress nascent experimental arthritis. J Clin Invest. 2014;124(10):4363–4374. doi:10.1172/JCI75673
241. Nomura H, Takanashi S, Tanaka M, et al. Specific biological responses of the synovial membrane to carbon nanotubes. Sci Rep. 2015;5(1):14314. doi:10.1038/srep14314
242. Lee YK, Kim SW, Park JY, Kang WC, Kang YJ, Khang D. Suppression of human arthritis synovial fibroblasts inflammation using dexamethasone-carbon nanotubes via increasing caveolin-dependent endocytosis and recovering mitochondrial membrane potential. Int J Nanomedicine. 2017;12:5761–5779.
243. Wardwell PR, Forstner MB, Bader RA. Investigation of the cytokine response to NF-kappaB decoy oligonucleotide coated polysaccharide based nanoparticles in rheumatoid arthritis in vitro models. Arthritis Res Ther. 2015;17(1):310. doi:10.1186/s13075-015-0824-x
244. Barrera P, Blom A, van Lent PL, et al. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43(9):1951–1959. doi:10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K
245. Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. doi:10.1186/ar2106
246. Alam MM, Han HS, Sung S, et al. Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis. J Control Release. 2017;252:62–72. doi:10.1016/j.jconrel.2017.03.012
247. Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102. doi:10.1016/j.biomaterials.2015.05.018
248. Koo OM, Rubinstein I, Onyuksel H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm Res. 2011;28(4):776–787. doi:10.1007/s11095-010-0330-4
249. Schulze K, Koch A, Schöpf B, et al. Intraarticular application of superparamagnetic nanoparticles and their uptake by synovial membrane—an experimental study in sheep. J Magn Magn Mater. 2005;293(1):419–432. doi:10.1016/j.jmmm.2005.02.075
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.