Back to Journals » International Journal of General Medicine » Volume 16
KIF20A is a Prognostic Marker for Female Patients with Estrogen Receptor-Positive Breast Cancer and Receiving Tamoxifen as Adjuvant Endocrine Therapy
Authors Huang X, Li S, Gao W, Shi J, Cheng M, Mi Y, Liu Y, Sang M, Li Z, Geng C
Received 14 June 2023
Accepted for publication 31 July 2023
Published 21 August 2023 Volume 2023:16 Pages 3623—3635
DOI https://doi.org/10.2147/IJGM.S425918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xuchen Huang,1,2 Sainan Li,1,2 Wei Gao,1,2 Jiajie Shi,1,2 Meng Cheng,1,2 Yunzhe Mi,1,2 Yueping Liu,3 Meixiang Sang,4 Ziyi Li,4 Cuizhi Geng1,2
1Department of Breast Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Key Laboratory in Hebei Province for Molecular Medicine of Breast Cancer, Shijiazhuang, Hebei, People’s Republic of China; 3Department of Pathology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 4Research Center and Tumor Research Institute, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Cuizhi Geng, Department of Breast Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050000, People’s Republic of China, Tel/Fax +86-311-66696313, Email [email protected]
Purpose: Our aim was to verify whether KIF20A has the potential to serve as a prognostic marker for female patients with estrogen receptor (ER)-positive breast cancer (BC) and treated with tamoxifen (TAM).
Patients and Methods: Online tools were used to investigate the potential correlation between KIF20A gene expression and survival of patients with ER-positive BC and TAM treatment. Furthermore, immunohistochemistry (IHC) was conducted to assess the expression levels of KIF20A in patients included from our center. The prognostic value of KIF20A for disease-free survival (DFS) and overall survival (OS) was further evaluated using Cox regression analysis.
Results: According to the results obtained from online tools, it was found that patients with low KIF20A expression exhibited significantly better survival outcomes in terms of relapse-free survival (RFS), distant metastasis-free survival (DMFS), and OS compared to those with high KIF20A expression (P < 0.001, P < 0.001, and P = 0.008, respectively). Additionally, significantly lower gene expression of KIF20A was found in patients who responded to TAM than in those who did not respond to TAM (P < 0.001). We further included 203 patients with adjuvant TAM therapy, and IHC for KIF20A was performed on sections from paraffin-embedded blocks. Patients with low KIF20A expression had significantly better DFS and OS (P = 0.001 and 0.002, respectively, log rank test), and the expression of KIF20A was identified as an independent factor for predicting both DFS and OS (P = 0.001 and 0.008, respectively).
Conclusion: KIF20A expression is an independent prognostic factor for survival in patients with ER-positive BC who received adjuvant TAM therapy. In clinical practice, IHC evaluation of KIF20A expression in surgical samples before administering tamoxifen may assist in predicting the treatment outcomes of these patients.
Keywords: KIF20A, breast carcinoma, biomarker, tamoxifen, survival, prognosis
Introduction
Breast cancer (BC) account for 24.5% of total number of newly diagnosed cancers in female population in 2020.1 Estrogen receptor (ER)-positive BC is the most common subtype, which makes up about 80% of all kinds of BC.2 It is generally assumed that ER-positive BC is less sensitive to chemotherapy than ER-negative BC.3 Therefore, endocrine therapy plays a critical role in the treatments for ER-positive BC.
Tamoxifen (TAM), a selective ER modulator, is the most commonly used endocrine drug for ER-positive BC.4–6 TAM can be used to treat patients with BC, including both pre- and postmenopausal female patients as well as male patients, especially premenopausal female patients.7,8 TAM has been reported to reduce BC recurrence and mortality rates by 50% and 30%, respectively.9 Moreover, a longer treatment duration of TAM can yield better outcomes in reducing the recurrence and mortality of BC. Specifically, a 5-year treatment period is more effective than a 1-2-year treatment period,10 and continuing TAM for up to 10 years is superior to stopping at a 5-year treatment period.11 Despite its effectiveness, TAM therapy failure is still observed in some patients, and TAM resistance remains one of the primary causes.12 Some studies have been dedicated to discovering novel biomarkers or models for predicting the prognosis of patients with BC.13–17 However, in clinical practice, there is a lack of biological indicators that are cost-effective, easily implementable, and effectively predict the prognosis of patients with ER-positive BC and receiving TAM therapy. Therefore, the identification of novel markers for predicting TAM treatment outcomes is an urgent task that can inform clinical practice.
Kinesin family member 20A (KIF20A) (also known as RAB6KIFL) consisting of 890 amino acids belongs to the kinesin family which is a superfamily of microtubule-based motor proteins playing important roles in mitosis and intracellular transport.18,19 KIF20A can bind to microtubules and generate mechanical force to accelerate the movement of organelles.20 It has been found to be highly expressed in almost all types of cancers,18 and a previous systematic review has confirmed the association between high KIF20A expression and poor prognosis in human cancer.21 KIF20A has been reported to be involved in cell growth and the cell cycle regulation in BC, and suppression of endogenous KIF20A expression can significantly inhibit the growth of BC cells.22 In colorectal cancer, KIF20A can activate the JAK/STAT3 signaling pathway, thereby promoting carcinogenesis, and suppressing the expression of KIF20A can inhibit the proliferation and migration of colorectal cancer cells.23 KIF20A peptides also have the ability to mediate the specific cytotoxic effect of killer T cells and it can play a role as a tumor-associated antigen in multiple cancers, including biliary tract cancer, gastric cancer, and pancreatic cancer.24–26 Besides, KIF20A can lead to resistance of multiple anticancer drugs, such as paclitaxel,27 docetaxel,28 oxaliplatin,29 and 5-FU.30 Hence, KIF20A may be a potential marker for predicting patients’ prognosis in patients with BC who are administered adjuvant TAM treatment. To evaluate the value of KIF20A in predicting the survival of patients with ER-positive BC and TAM treatment, we utilized web-based tools to evaluate the association between KIF20A expression and survival of this patient population, and further validated it through immunohistochemistry (IHC) experiments based on samples from our center. IHC was performed to assess the expression of KIF20A, and patients were grouped based on their expression levels. Furthermore, Cox regression analysis was applied to identify independent predictors of patient survival based on the expression level of KIF20A and clinicopathological factors.
Materials and Methods
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. Signed informed consent was obtained before sampling.
Exploration of Web Tools
The Kaplan-Meier Plotter online survival analysis tool (http://kmplot.com/analysis) was used to investigate the potential association between KIF20A expression and survival in patients with ER-positive BC and receiving TAM treatment.31 Moreover, the website tool of ROC Plotter (https://www.rocplot.org/) was used to assessed the difference in KIF20A expression levels between TAM therapy responders and non-responders.32 The visualization of the results and the statistical analysis were both carried out using the web tools.
Patients and Clinicopathological Variables
To further verify the prognostic value of KIF20A expression on the survival of patients with ER-positive BC and receiving TAM treatment, paraffin-embedded surgical samples, clinicopathological parameters, and follow-up data of 203 female patients with primary BC who underwent surgery for BC between January 2013 and December 2017 in our center were collected. Follow-up began at the time of surgery, and the final follow-up time was set as December 2022. At our center, patients initially diagnosed with BC would routinely undergo a series of imaging tests to rule out the possibility of distant metastasis. Patients were scheduled for routine screening every three months during the first two years after the operation, followed by every six months within the next three years. Subsequently, a routine annual screening was performed. Once the first site of recurrence or metastasis was identified, comprehensive screening was conducted to detect potential recurrent or metastatic events at other sites.
Patients were eligible for inclusion in our study if they met the following criteria: (1) Female patients aged ≥ 18 years; (2) Patients diagnosed with primary, unilateral, and operable invasive ductal carcinoma of the breast histologically, and ER-positive status was confirmed by IHC with a cut-off value of 1%; (3) Patients who underwent radical surgery for BC and had no residual cancer after surgery; (4) Patients who did not receive any neoadjuvant therapy prior to surgery; (5) Patients who could receive adjuvant chemoradiotherapy or not, but patients were included only if they received TAM monotherapy as adjuvant endocrine therapy before the onset of the first recurrence or distant metastasis; and (6) Patients who were not lost to follow-up before the final scheduled follow-up. The exclusion criteria were as follows: (1) Patients who were initially diagnosed with recurrent or metastatic BC, or pure carcinoma in situ; (2) Patients who only underwent cytoreductive surgeries for BC; and (3) Patients with contralateral BC following the first BC were also excluded because it is controversial whether it was another primary BC or a metastatic event.
Clinicopathological variables of interest for these patients were extracted: patient age, tumor size, lymph node (LN) metastasis, human epidermal growth factor receptor 2 (HER2) status, lymphovascular invasion (LVI), Ki-67 expression level, histological grades, chemotherapy, and radiotherapy. Disease-free survival (DFS) was defined as the interval from surgery to first recurrence and/or distant metastasis at any site. Overall survival (OS) was defined as the duration between diagnosis and death from any cause. Moreover, the sites involved were documented based on the results of the comprehensive screening when recurrent or metastatic events first occurred. Visceral metastasis was defined as the spread of BC to organs, such as the lungs, liver, and brain. Multiple-site involvement was defined as recurrence and/or metastasis of BC to more than one site at the first failure.
Age was stratified into two groups: ≤ 35 years and > 35 years. Tumor size was categorized as ≤ 2 or > 2 cm. Lymph node (LN) metastasis was classified as ≤ 3 or > 3 LN metastases. HER2 expression status was considered positive if membrane staining was scored as 3+ by IHC or if HER2 gene amplification was revealed by fluorescence in situ hybridization (FISH). Ki-67 expression was categorized as low or high, with a cut-off value of 30%.
Pathological Evaluation
Tissue sections (4 μm) were obtained from paraffin-embedded blocks for IHC analysis. IHC experiments for KIF20A expression were conducted following the manufacturer’s instructions (primary antibody: KIF20A monoclonal antibody, 67190-1-Ig, 1:400 dilution, Proteintech).
High-power field (HPF) images of five randomly selected invasive tumor fields were captured from each slide and processed using (Fiji Is Just) ImageJ (version 2.3.0/1.53f51) software. The color deconvolution plugin was used to extract positive staining, and the integrated optical density (IOD) values of immunostaining were calculated. The average of the five IOD values based on the images captured from the same slide represented the KIF20A expression values for each patient. The median of all patients’ KIF20A expression values was chosen as the cut-off point. Patients with KIF20A expression values above the median were assigned to the high KIF20A expression group while the other patients were assigned to the low KIF20A expression group.
Statistical Analysis
Categorical variables were expressed as frequencies (percentages). Differences in categorical variables between the groups were evaluated using Fisher’s exact test. Kaplan-Meier curves were plotted to depict patient survival stratified by KIF20A expression levels, with the log rank test to evaluate the difference in survival between patient groups. Univariate and multivariate Cox regression analyses were used to identify the independent prognostic variables for DFS and OS. Only variables with significant effects on DFS or OS in the univariate Cox regression were entered into the multivariate Cox regression analysis. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Statistical significance was defined as a two-tailed P value of < 0.05. All data were analyzed using the R software (version 4.1.3).
Results
A brief flowchart was presented to illustrate main steps of our study (Figure 1).
 |
Figure 1 The flowchart illustrating the main steps of our study. |
Prognostic Value of KIF20A Based on Web Tools
For the web tool of Kaplan-Meier Plotter, we specifically selected patients with ER-positive BC who only received TAM for endocrine therapy. A total of 760, 417, 150 patients were eligible for the evaluation of the association between KIF20A expression and relapse-free survival (RFS), distant metastasis-free survival (DMFS), OS, respectively. The median follow-up periods were 80 months for the RFS, 70.3 months for DMFS, and 66.6 months for OS. Each patient cohort was divided into high and low KIF20A expression groups, using the median KIF20A expression as the cutoff value. Kaplan-Meier curves depicting RFS, DMFS and OS of patients with ER-positive BC stratified by different KIF20A expression levels were shown in Figure 2A–C. The curves demonstrated that the survival of patients with low KIF20A expression was significantly better than those with high KIF20A expression for all prognostic outcomes, RFS, DMFS and OS (P < 0.001, P < 0.001 and P = 0.008, respectively).
Moreover, In the web tool of ROC Plotter, patients with ER-positive BC who received TAM endocrine therapy were included. In the settings of ROC Plotter web tool, we identified TAM treatment responders and non-responders based on the RFS status at 5 years. In total, 870 patients were included in the analysis, including 126 TAM non-responders and 744 TAM responders. KIF20A expression was compared between patients who responded to TAM therapy and those who did not respond (Figure 3A). Significantly lower KIF20A gene expression was found in TAM responders (P < 0.001, Mann–Whitney test). Furthermore, the analysis demonstrated the potential of KIF20A as a predictor in determining the response to TAM therapy, with an area under the curve (AUC) value of 0.63 (P < 0.001) (Figure 3B). These findings suggest that KIF20A may be a promising marker for predicting TAM response and survival in patients receiving TAM endocrine therapy. We further validated the potential prognostic value of KIF20A through IHC testing.
Prognostic Value of KIF20A Based on Results of Our Center
Correlation Between Expression Levels of KIF20A and Clinicopathological Variables
A total of 203 patients were selected from our center and were included in the analysis. The median follow-up period was 79 months, during which 67 patients experienced recurrence and/or distant metastasis, and 39 deaths occurred during the follow-up period. Accordingly, in assessing the prognostic effect of KIF20A on DFS, a total of 67 patients experiencing recurrence and/or metastasis were included in the event cohort, while the other 136 disease-free patients were included in the non-event cohort. For the evaluation of KIF20A’s prognostic role on OS, the event cohort consisted of 39 deceased patients, with the other 164 surviving patients included in the non-event cohort. With reference to the sites of recurrence and metastasis identified during screening at first failure, 16 (23.88%) patients exhibited involvement at multiple sites. Bone metastasis, LN metastasis, and locoregional recurrence were observed in 30 (44.78%), 14 (20.90%), and 6 (8.96%) patients, respectively. Furthermore, there were 18 (26.87%) and 12 (17.91%) cases of metastasis in the lung and liver, respectively. Among all visceral organs affected by metastasis, these two organs exhibited the highest susceptibility to metastatic spread.
KIF20A staining was observed in both the cytoplasm and nucleus (Figure 4). Based on the IHC results for KIF20A, 101 patients were categorized as having the high expression level, while the remaining 102 patients had the low expression level. Among the 67 patients with recurrent and/or distant metastatic events, 45 (67.16%) were diagnosed with high KIF20A expression. We compared the KIF20A expression levels between patients with recurrent and/or metastatic events and disease-free patients. A significantly higher proportion of patients with a high expression level of KIF20A were identified in the patient cohort with recurrent and/or metastatic disease (45/67 vs 56/136, P = 0.001) (Table 1). Additionally, a significantly higher proportion of patients with a high expression of KIF20A were observed in the patient cohort with a deceased status compared to the patient cohort with an alive status at the final follow-up (28/39 vs 73/164, P = 0.002) (Table 1).
 |
Table 1 Relationship Between KIF20A Expression and Clinicopathological Features in Patients with ER-Positive BC |
Clinicopathological variables were compared between patient groups with different KIF20A expression levels (Table 1). Compared to low expression level of KIF20A, patients with high expression level of KIF20A exhibited higher proportions of negative HER2 status, high Ki-67 level, more involved lymph nodes, and more advanced TNM staging, despite not showing significant difference.
Prognostic Value of KIF20A Expression on Survival
The patients’ DFS and OS stratified by KIF20A expression levels was demonstrated by Kaplan–Meier curves in Figures 5 and 6. The difference in survival between patients with different KIF20A expression levels was compared, and patients with low KIF20A expression level had significantly better DFS and OS (P = 0.001 and 0.002, respectively, log rank test).
 |
Figure 5 The Kaplan-Meier curve depicting the DFS of patients, stratified by KIF20A expression levels (P = 0.001, log rank test). Abbreviation: DFS, disease-free survival. |
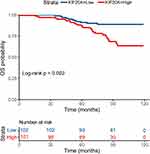 |
Figure 6 The Kaplan-Meier curve depicting the OS of patients, stratified by KIF20A expression levels (P = 0.002, log rank test). Abbreviation: OS, overall survival. |
To further verify the prognostic value of KIF20A expression in patient survival, Cox regression analysis was conducted. In univariate Cox analysis, variables including LVI, Tumor size, LN metastasis, and KIF20A expression level were significant predictors of DFS (Table 2) and OS (Table 3). These variables were entered into the multivariate Cox analysis. Multivariate Cox analysis identified LVI and KIF20A expression level as independent factors for predicting DFS (P = 0.041 and 0.001, respectively). Moreover, LVI, LN metastasis, and KIF20A expression level were found to be independent factors for predicting OS (P = 0.042, 0.035, and 0.008, respectively).
 |
Table 2 Univariate and Multivariate Cox Analysis Results of Clinicopathological Variables and Expression Levels of KIF20A for DFS of ER-Positive BC Patients with TAM Therapy |
 |
Table 3 Univariate and Multivariate Cox Analysis Results of Clinicopathological Variables and Expression Levels of KIF20A for OS of ER-Positive BC Patients with TAM Therapy |
Discussion
Early identification of nonresponsive patients can aid in the implementation of alternative pharmacological interventions or more aggressive therapeutic strategies, thereby improving survival rates. In clinical practice, evaluating the therapeutic efficacy during neoadjuvant therapy can effectively assess a patient’s sensitivity to the medication. This is one of the purposes of neoadjuvant therapy – to evaluate the individual’s sensitivity to the drug. Although some advantages of neoadjuvant endocrine therapy (NET) have been observed, such as comparable breast conservation rates to neoadjuvant chemotherapy with fewer adverse events,33 the clinical application of NET is still limited.34 In addition, aromatase inhibitor was shown to be more effective in NET when compared to tamoxifen.33 Therefore, the current clinical use of tamoxifen primarily focuses on adjuvant therapy. In the adjuvant therapy stage, patients have undergone curative surgery and there are no residual lesions, making it impractical to utilize the evaluation methods employed in neoadjuvant therapy for assessing patients’ sensitivity to tamoxifen. However, the treatment outcomes of tamoxifen, such as DFS or OS, can provide some insights into its effectiveness. In a clinical setting, a shorter DFS can indicate an increased likelihood for patients to develop resistance to TAM and experience recurrence and/or metastasis. In this study, we found that KIF20A may be a novel marker for TAM response in patients receiving TAM therapy. However, we did not investigate the specific mechanisms by which KIF20A impacts the treatment outcomes of tamoxifen. Therefore, although KIF20A can serve as a prognostic indicator for ER-positive BC patients receiving adjuvant tamoxifen treatment, further exploration and validation of its underlying mechanisms are warranted.
KIF20A has been reported as a marker for multiple drug resistance previously. However, in the literature, no correlation has been established between KIF20A expression and the TAM response. KIF20A has recently been identified as a ferroptosis inhibitor, and knockdown of KIF20A can enhance the sensitivity of cancer cells to antitumor drugs by inducing ferroptosis.29,35 Inhibiting ferroptosis is a novel approach to induce drug resistance,36 and the inhibition of ferroptosis is also reported to be a possible cause of TAM resistance.37 Hence, KIF20A may induce TAM resistance by inhibiting ferroptosis.
In our study, we investigated the correlation between KIF20A gene expression and the survival of patients with ER-positive BC who received TAM treatment. We utilized online tools to explore this relationship. Furthermore, we conducted IHC to validate the prognostic value of KIF20A in terms of DFS and OS in ER-positive BC patients receiving adjuvant TAM therapy. In the comparison of clinicopathological parameters between patients with high and low expression levels of KIF20A, it was observed that individuals with high KIF20A expression were more likely to exhibit certain characteristics in BC. These characteristics included negative HER2 status, higher Ki-67 level, more involved LNs, and a more advanced TNM stage, when compared to patients with low expression level of KIF20A. In a previous study, the authors also reported that higher KIF20A expression was associated with a higher likelihood of lymph node metastasis.22 In addition, this study, a higher proportion of high KIF20A expression was observed in BC patients with positive HER2 status. The discrepancies in the association between KIF20A expression levels and HER2 status warrant further investigation. They found independent prognostic value of KIF20A expression for OS in patients with BC. The conclusion of their study was based on the total BC patient population they recruited, but it is unknown whether their conclusions remain valid for patient populations with specific molecular subtypes and under particular drug treatment regimens. This could potentially limit the generalizability of their findings to real-world clinical settings where patients with ER-positive BC may have different endocrine therapy regimens. Our study assessed the prognostic value of KIF20A expression using IHC in patients with ER-positive BC who received adjuvant TAM therapy. We only included patients with ER-positive BC who did not undergo neoadjuvant therapy, which led to a more homogeneous patient population, more reliable conclusions, and a clearer scope of clinical application.
In the multivariate Cox analysis for DFS, KIF20A expression level and LVI were identified as independent prognostic factors. However, pathological staging variables, tumor size and LN metastasis, did not demonstrated the ability to predict DFS in patients with ER-positive BC receiving adjuvant TAM treatment. Similarly, a study conducted by Aogi et al aimed to identify definitive predictive factors for prognosis of patients with luminal-type BC using both conventional clinicopathological factors and maximum standardized uptake value (SUVmax) on 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography (FDG-PET/CT), and found that SUVmax was the only independent predictor for RFS (P = 0.055 in multivariate Cox analysis). None of the conventional factors were found to have significant prognostic value for RFS in their study.38 Besides, another study identified the marker CanAssist-Breast (CAB) as the only independent predictor of DMFS among the endocrine therapy cohort in their study when incorporating clinicopathological covariates in multivariate Cox analysis.15 These findings suggest that in specific patient populations with ER-positive BC, newly developed markers may be advantageous in predicting DFS over conventional pathological factors.
In addition to the expression level of KIF20A, the variables of LVI and LN metastasis were significant predictors of OS in multivariate Cox analysis. In previous studies, LN metastasis has been found to correlate with poor survival in patients with ER–positive BC. In a large-scale study including patients with ER–positive BC uniformly treated with adjuvant TAM, LN status was found to be an independent prognostic factor for disease-specific survival (DSS) using multivariate Cox analysis.39 Furthermore, LVI was reported to be significantly associated with LN metastasis and identified as an independent prognostic factor for poor OS in the entire cohort of 77,425 patients with ER–positive BC from the National Cancer Database (NCDB).40
Readers should note the following. First, we found that KIF20A is a prognostic marker for patients with ER-positive BC who received TAM treatment. However, the underlying mechanism by which high KIF20A expression negatively impacts treatment outcomes in patients receiving TAM therapy was not investigated in our study. Therefore, additional investigations are necessary to unravel this mechanism and provide further insights. Second, although the Cox analysis did not reveal a significant association between HER2 status and prognosis in the enrolled patients, the utilization of adjuvant targeted drugs might serve as an additional prognostic factor influencing patient outcomes. However, due to the cost associated with targeted drugs, a significant number of patients did not receive such medications during the adjuvant treatment stage. As a result, we did not gather data on the usage of adjuvant targeted drugs. Third, our study only included patients who did not receive neoadjuvant therapy, and we verified the significant prognostic value of KIF20A in this population. The reason for not including patients who received neoadjuvant therapy is because such treatment can lead to tumor tissue regression, which may potentially influence the results of IHC detection of KIF20A expression within the tumor tissue. Therefore, further confirmation is necessary to determine the generalizability of our findings to patient populations in other clinical scenarios, such as patient populations undergoing neoadjuvant chemotherapy and adjuvant tamoxifen treatment. Fourth, our study did not impose restrictions on the inclusion of other treatment modalities apart from endocrine therapy in the patient population. This lack of restriction could potentially introduce bias to our research findings, as other treatments, such as chemotherapy, can also impact patient prognosis. However, during the study design phase, we faced challenges in recruiting a sufficient number of patients who received tamoxifen monotherapy exclusively, especially those who received tamoxifen monotherapy and experienced clinical events such as recurrence or metastasis. This difficulty arises from the fact that early-stage patients who are suitable for tamoxifen monotherapy tend to have a very good prognosis.41 However, ER-positive BC patients generally exhibit lower sensitivity to chemotherapy compared to ER-negative BC patients,3 and the Cox analysis did not reveal a significant impact of chemotherapy and radiation therapy on the prognosis of the patients included in our study. Therefore, we believe that our research conclusions remain reliable. However, it would be necessary to conduct multi-center studies that include a sufficient number of patients receiving tamoxifen monotherapy alone in order to validate our findings.
Conclusion
In patients with ER-positive BC who receive adjuvant TAM therapy, the level of KIF20A expression serves as an independent predictor for both DFS and OS. Patients with high KIF20A expression level exhibit significantly inferior DFS and OS compared to those with low expression level. Hence, monitoring KIF20A expression levels through IHC may represent an effective and feasible clinical approach for prognosticating outcomes in this patient population.
Acknowledgments
This study was supported by Key Program of Hebei Natural Science Foundation for Precision Medicine (H2020206199).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y. Relevance of spatial heterogeneity of immune infiltration for predicting risk of recurrence after endocrine therapy of ER+ breast cancer. J Natl Cancer Inst. 2018;110(2). doi:10.1093/jnci/djx137
3. Ohara AM, Naoi Y, Shimazu K, et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res Treat. 2019;173(3):533–543. doi:10.1007/s10549-018-5020-7
4. Emons G, Mustea A, Tempfer C. Tamoxifen and endometrial cancer: a janus-headed drug. Cancers. 2020;12(9):2535. doi:10.3390/cancers12092535
5. Cabling ML, Turner JW, Hurtado-de-Mendoza A, et al. Sentiment analysis of an online breast cancer support group: communicating about tamoxifen. Health Commun. 2018;33(9):1158–1165. doi:10.1080/10410236.2017.1339370
6. Heery M, Corbett P, Zelkowitz R. Precautions for patients taking tamoxifen. J Adv Pract Oncol. 2018;9(1):78–83.
7. Shagufta and Ahmad I. Tamoxifen a pioneering drug: an update on the therapeutic potential of tamoxifen derivatives. Eur J Med Chem. 2018;143:515–531. doi:10.1016/j.ejmech.2017.11.056
8. Wu HT, Liu YE, Hsu KW, et al. MLL3 induced by luteolin causes apoptosis in tamoxifen-resistant breast cancer cells through H3K4 monomethylation and suppression of the PI3K/AKT/mTOR pathway. Am J Chin Med. 2020;48(5):1221–1241. doi:10.1142/S0192415X20500603
9. Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE. PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics. 2013;23(11):643–647. doi:10.1097/FPC.0b013e3283656bc1
10. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi:10.1016/S0140-6736(05)66544-0
11. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi:10.1016/S0140-6736(12)61963-1
12. Zhou J, Li W, Ming J, et al. High expression of TRAF4 predicts poor prognosis in tamoxifen-treated breast cancer and promotes tamoxifen resistance. Anticancer Drugs. 2020;31(6):558–566. doi:10.1097/CAD.0000000000000943
13. Zhou L, Rueda M, Alkhateeb A. Classification of breast cancer nottingham prognostic index using high-dimensional embedding and residual neural network. Cancers. 2022;14(4):934. doi:10.3390/cancers14040934
14. Tabl AA, Alkhateeb A, ElMaraghy W, Rueda L, Ngom A. A machine learning approach for identifying gene biomarkers guiding the treatment of breast cancer. Front Genet. 2019;10:256. doi:10.3389/fgene.2019.00256
15. Bakre MM, Ramkumar C, Attuluri AK, et al. Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients. Cancer Med. 2019;8(4):1755–1764. doi:10.1002/cam4.2049
16. Loo CE, Rigter LS, Pengel KE, et al. Survival is associated with complete response on MRI after neoadjuvant chemotherapy in ER-positive HER2-negative breast cancer. Breast Cancer Res. 2016;18(1):82. doi:10.1186/s13058-016-0742-0
17. Wang K, Li J, Xiong YF, Zeng Z, Zhang X, Li HY. A potential prognostic long noncoding RNA signature to predict recurrence among ER-positive breast cancer patients treated with tamoxifen. Sci Rep. 2018;8(1):3179. doi:10.1038/s41598-018-21581-w
18. Jin Z, Peng F, Zhang C, Tao S, Xu D, Zhu Z. Expression, regulating mechanism and therapeutic target of KIF20A in multiple cancer. Heliyon. 2023;9(2):e13195. doi:10.1016/j.heliyon.2023.e13195
19. Liu H, Chen C, Fehm T, Cheng Z, Neubauer H. Identifying mitotic kinesins as potential prognostic biomarkers in ovarian cancer using bioinformatic analyses. Diagnostics. 2022;12(2):470. doi:10.3390/diagnostics12020470
20. Taniuchi K, Nakagawa H, Nakamura T, et al. Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with membrane trafficking of discs large homologue 5, can attenuate growth of pancreatic cancer cell. Cancer Res. 2005;65(1):105–112. doi:10.1158/0008-5472.105.65.1
21. Li X, Shu K, Wang Z, Ding D. Prognostic significance of KIF2A and KIF20A expression in human cancer: a systematic review and meta-analysis. Medicine. 2019;98(46):e18040. doi:10.1097/MD.0000000000018040
22. Nakamura M, Takano A, Thang PM, et al. Characterization of KIF20A as a prognostic biomarker and therapeutic target for different subtypes of breast cancer. Int J Oncol. 2020;57(1):277–288. doi:10.3892/ijo.2020.5060
23. Zhang Q, Di J, Ji Z, et al. KIF20A predicts poor survival of patients and promotes colorectal cancer tumor progression through the JAK/STAT3 signaling pathway. Dis Markers. 2020;2020:2032679. doi:10.1155/2020/2032679
24. Aruga A, Takeshita N, Kotera Y, et al. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014;12:61. doi:10.1186/1479-5876-12-61
25. Fujiwara Y, Okada K, Omori T, et al. Multiple therapeutic peptide vaccines for patients with advanced gastric cancer. Int J Oncol. 2017;50(5):1655–1662. doi:10.3892/ijo.2017.3955
26. Miyazawa M, Katsuda M, Maguchi H, et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int J Cancer. 2017;140(4):973–982. doi:10.1002/ijc.30510
27. Khongkow P, Gomes AR, Gong C, et al. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene. 2016;35(8):990–1002. doi:10.1038/onc.2015.152
28. Yu H, Xu Z, Guo M, et al. FOXM1 modulates docetaxel resistance in prostate cancer by regulating KIF20A. Cancer Cell Int. 2020;20(1):545. doi:10.1186/s12935-020-01631-y
29. Yang C, Zhang Y, Lin S, Liu Y, Li W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging. 2021;13(10):13515–13534. doi:10.18632/aging.202774
30. Xiong M, Zhuang K, Luo Y, et al. KIF20A promotes cellular malignant behavior and enhances resistance to chemotherapy in colorectal cancer through regulation of the JAK/STAT3 signaling pathway. Aging. 2019;11(24):11905–11921. doi:10.18632/aging.102505
31. Győrffy B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J. 2021;19:4101–4109. doi:10.1016/j.csbj.2021.07.014
32. Fekete JT, Győrffy B. ROCplot. org: validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3104 breast cancer patients. Int J Cancer. 2019;145(11):3140–3151. doi:10.1002/ijc.32369
33. Iwata H, Shien T. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020;50(3):225–229. doi:10.1093/jjco/hyz213
34. Sella T, Weiss A, Mittendorf EA, et al. Neoadjuvant endocrine therapy in clinical practice: a review. JAMA Oncol. 2021;7(11):1700–1708. doi:10.1001/jamaoncol.2021.2132
35. He H, Liang L, Huang J, et al. KIF20A is associated with clinical prognosis and synergistic effect of gemcitabine combined with ferroptosis inducer in lung adenocarcinoma. Front Pharmacol. 2022;13:1007429. doi:10.3389/fphar.2022.1007429
36. Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47. doi:10.1186/s12943-022-01530-y
37. Xu Z, Tang J. RelB silencing to reverse tamoxifen resistance by regulating GPx4 and ferroptosis in breast cancer. J Clin Oncol. 2020;38(15_suppl):e12513. doi:10.1200/JCO.2020.38.15_suppl.e12513
38. Aogi K, Kadoya T, Sugawara Y, et al. Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150(1):209–217. doi:10.1007/s10549-015-3303-9
39. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16(21):5222–5232. doi:10.1158/1078-0432.CCR-10-1282
40. Makower D, Lin J, Xue X, Sparano JA. Lymphovascular invasion, race, and the 21-gene recurrence score in early estrogen receptor-positive breast cancer. NPJ Breast Cancer. 2021;7(1):20. doi:10.1038/s41523-021-00231-x
41. Adachi Y, Oze I, Sawaki M, et al. Impact of adjuvant endocrine therapy on prognosis in small hormone receptor-positive, HER2-negative early breast cancer. Breast Cancer. 2021;28(5):1087–1095. doi:10.1007/s12282-021-01245-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



