Back to Journals » Journal of Pain Research » Volume 15
Ketamine Assisted Psychotherapy: A Systematic Narrative Review of the Literature
Authors Drozdz SJ , Goel A, McGarr MW , Katz J , Ritvo P, Mattina GF , Bhat V, Diep C, Ladha KS
Received 1 February 2022
Accepted for publication 20 April 2022
Published 15 June 2022 Volume 2022:15 Pages 1691—1706
DOI https://doi.org/10.2147/JPR.S360733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Sandra J Drozdz,1 Akash Goel,1– 3 Matthew W McGarr,4 Joel Katz,3,5– 7 Paul Ritvo,5,7 Gabriella F Mattina,1 Venkat Bhat,8– 10 Calvin Diep,3 Karim S Ladha1,3,10
1Department of Anesthesia, St. Michael’s Hospital, Toronto, ON, Canada; 2Department of Anesthesiology, Pain and Perioperative Medicine, Stanford University, Stanford, CA, USA; 3Department of Anesthesiology and Pain Medicine, University of Toronto, Toronto, ON, Canada; 4Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 5Department of Psychology, York University, Toronto, ON, Canada; 6Department of Anesthesia and Pain Management, University Health Network, Toronto, ON, Canada; 7School of Kinesiology and Health Science, York University, Toronto, ON, Canada; 8Interventional Psychiatry Program, St. Michael’s Hospital, Toronto, ON, Canada; 9Department of Psychiatry, University of Toronto, Toronto, ON, Canada; 10Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
Correspondence: Karim S Ladha, St. Michael’s Hospital, 30 Bond St, 6th Floor Donnelly Wing, Toronto, ON, ON, Canada, Tel +1 416-864-6060 ext. 49308, Fax +1 416-864-6014, Email [email protected]
Abstract: Currently, ketamine is used in treating multiple pain, mental health, and substance abuse disorders due to rapid-acting analgesic and antidepressant effects. Its limited short-term durability has motivated research into the potential synergistic actions between ketamine and psychotherapy to sustain benefits. This systematic review on ketamine-assisted psychotherapy (KAP) summarizes existing evidence regarding present-day practices. Through rigorous review, seventeen articles that included 603 participants were identified. From available KAP publications, it is apparent that combined treatments can, in specific circumstances, initiate and prolong clinically significant reductions in pain, anxiety, and depressive symptoms, while encouraging rapport and treatment engagement, and promoting abstinence in patients addicted to other substances. Despite much variance in how KAP is applied (route of ketamine administration, ketamine dosage/frequency, psychotherapy modality, overall treatment length), these findings suggest psychotherapy, provided before, during, and following ketamine sessions, can maximize and prolong benefits. Additional large-scale randomized control trials are warranted to understand better the mutually influential relationships between psychotherapy and ketamine in optimizing responsiveness and sustaining long-term benefits in patients with chronic pain. Such investigations will assist in developing standardized practices and maintenance programs.
Keywords: addiction, alternative treatment, anesthesia, chronic pain, mental health, novel therapeutic
Introduction
There has been growing interest in using the drug ketamine to treat pain, psychological, addictive disorders in recent years. Along with the development of selective serotonin reuptake inhibitors, there has been a tremendous widening of the definition of depression and an impressive decrease in the difference in efficacy between placebo and drug and controlled studies.1 The Sequenced Treatment Alternatives to Relieve Depression Study found that response rates to new compounds after the failure of the first antidepressant are low.1–3 This delay in onset is a significant drawback to current antidepressant therapies, leaving a crucial need for the development of faster-acting antidepressants, especially in patients at risk of suicide. Furthermore, estimates from the National Institute of Mental Health indicate that the prevalence of major depressive disorder is 6.4% of the US adult population.4 Despite intense research efforts, the field has had little success in developing antidepressant treatments with fundamentally novel mechanisms of action over the past six decades, leaving the field wary and skeptical about any new developments.3 Nonetheless, several small proof-of-concept studies conducted over the past 15 years have gradually gained interest by displaying strong evidence that therapies associated with ketamine have provided a unique, rapid onset of sustained but temporally limited therapeutic effect.3
Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, synthesized initially as an alternative to the anesthetic, phencyclidine (PCP). Introduced in 1962,5 preliminary findings indicated potent anesthetic-analgesic properties and unique alterations of consciousness at variable dosages.6 Ketamine is now commonly used as an anesthetic in human medicine and an analgesic agent in multiple pain conditions.2,7 Although the mechanisms that contribute to symptom reductions are unclear,3,8,9 several neural mechanisms are proposed: a) the dampening of certain functional brain connections; b) enhanced synaptogenesis; c) glutamate modulation; and d) increases in neuroplasticity.4,10–13 In animal models, acute ketamine administration has been shown to activate several downstream signalling cascades that include the mammalian target of rapamycin (mTOR), glycogen synthase kinase-3 (GSK3), and elongation factor 2 (eEF2) kinase, which have been implicated in neuroplasticity mechanisms and the pathophysiology of neuropsychiatric disorders.4,10,11,13 The fundamental property of ketamine as a use-dependent blocker of NMDA receptor-mediated neurotransmission suggests that this compound’s cellular and behavioural effects are triggered by suppression of a tonically active form glutamatergic signalling in the brain.4 A prevailing hypothesis posits that ketamine-induced suppression of tonic NMDA receptor-mediated glutamatergic input onto GABA-ergic interneurons leads to a decrease in overall inhibition, also called disinhibition, tilting the balance of synaptic transmission toward excitation.4 Overall, it appears that on a microscopic scale, synaptic excitation and neuronal structural plasticity afforded from ketamine administration plays a significant role in the medication’s rapid and robust effectiveness in chronic mental health conditions, including substance use disorders and chronic pain.2,8,9,14 Unfortunately, symptom reductions are frequently transient, typically lasting 4–7 days. Thus, repeated administrations are required to maintain positive effects.15–17
One possible aid in prolonging ketamine’s effects is psychotherapy. Psychotherapy describes the treatment of psychological disorders or symptoms through the promotion of personal growth, symptom management, and well-being and is based on therapeutic structures, principles, and techniques.18 Separately, both psychotherapy and pharmacotherapy have shown equal efficacy, while the combination of both approaches has been found to be more effective in the treatment of psychological disorders than psychotherapy and pharmacotherapy alone; specifically, in certain populations including older adults and patients with comorbid medical disorders.18 The potential for ketamine to facilitate psychotherapy was first reported by Khorramzadeh and Lotfy (as an abreactive agent),19 and subsequently by Fontana as a pharmacological component of antidepressant psychotherapy.20 Ketamine-enhanced psychotherapy has since been trialed as a treatment for multiple conditions including chronic neuropathic pain,21 opioid tapering,22 major depressive disorder (MDD), generalized anxiety disorder (GAD), obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), and substance use disorders (SUD).23 While disagreement exists amongst researchers and clinicians about how ketamine facilitates psychotherapy, some theories about ketamine’s interactions with psychotherapy suggest a facilitation of emotional learning through enhanced neuroplasticity, evocations of emotionally arousing phenomenological experiences, reductions in defensiveness, and enhanced treatment adherence and engagement.24–28 Additionally, while some researchers and practitioners work to minimize the psychedelic effects of ketamine,22,29,30 some studies suggest that the presence of a psychedelic experiences fosters enhanced benefits, and may improve mental health and well-being. The transpersonal experience of ketamine may bring on new perspectives, stimulate critical reframing, and offer personal insights to clients.23,28,31,32 These findings parallel the relationship between psychedelic experience and treatment outcome in psilocybin studies.23 The overarching goal of linking psychotherapy with psychedelics such as MDMA and psilocybin, and more recently, ketamine, is to augment the effects of the psychotherapy through conscious awareness.23 The development of psychotherapy that uses ketamine as medicine for conscious awareness is new and ground-breaking.23 The theorized effectiveness of ketamine in psychotherapy is based on: (a) increased access to traumatic memory via enhanced synaptic connectivity; (b) decreased central sensitization via downregulation of the prefrontal cortex, and (c) enhanced extinction of previously paired pain-related memories.21 Therefore, KAP can utilize a dosage escalation strategy to achieve different levels of trance, increasing to full out-of-body experiences.23
While the published literature on Ketamine Assisted Psychotherapy (KAP) and the use of the intervention has grown substantially in recent years, there has yet to be a systematic review of the available evidence. This review of current KAP research aims to identify standard practices in pain, mental health, and addiction conditions, levels and patterns of effectiveness in symptom reduction, and the duration of treatment effects.
Methods
Information Sources, Search Strategy and Study Selection
As summarized in Figure 1, a detailed search strategy was used to identify relevant studies. An information specialist from the Health Science Library at St. Michael’s Hospital in Toronto, Canada, in collaboration with the authors, designed and conducted a systematic search of Embase, Pubmed/Medline, CENTRAL (Cochrane Central Register of Controlled Trials), and Psychiatry Online to identify all English language articles published from 1973 to November, 2021.
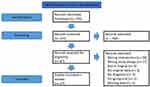 |
Figure 1 PRISMA flowchart of the search and selection processes for included studies. Notes: Adapted from: Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–b2700. doi:10.1136/bmj.b2700.33 Copyright 2009 Liberati et al. Creative Commons. |
Figure 1 summarizes the search strategy used for study selection. We followed the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA)33 guidelines for reporting our search. Studies were included if they reported on original data and/or described the short-term or long-term effects of ketamine-assisted psychotherapy on pain, mental health, and/or addiction. Articles were excluded if they involved animal subjects; and/or focused on recreational ketamine abuse/use.
The search used a combination of the following keywords: “Ketamine” OR “Ketamine assisted psychotherapy” OR “Ketamine-enhanced psychotherapy” AND “Chronic pain” OR “Treatment resistant” OR “Mood disorder” OR “Anxiety disorder” OR “Psychotherapy” OR “Cognitive behavioral therapy (CBT)” OR “Acceptance and Commitment Therapy” OR “Mindfulness-based intervention (MBI)” OR “Alcoholism” OR “Opioid withdrawal” OR “Addiction” OR “Post-traumatic stress disorder” OR “Depression” OR “Obsessive-compulsive disorder” OR “Neuropathic pain”.
Two-hundred and fifty-five articles were identified. Two researchers (MM and SD) independently screened the 255 titles and abstracts for relevance based on the inclusion and exclusion criteria. Abstracts were excluded if they did not include both ketamine and psychotherapy; did not contain original data; included preclinical animal models; did not have abstracts available in English; and did not specify a pain, substance abuse, or mental health condition. This resulted in 87 full-text articles that were retrieved for detailed examination. After review, a further 70 articles were deemed not relevant (Figure 1), leaving 17 articles for data extraction. The reference lists of the 17 full-text articles were reviewed for additional papers. Any discrepancies between reviewers were resolved by consultation with the senior author (KL).
For each included study, the following variables were extracted: (a) study design; (b) sample size; (c) participant demographics; (d) ketamine dosage and dose frequency; (f) ketamine delivery method; (g) psychotherapy type; (h) psychotherapy frequency; (i) temporal relationship between ketamine administration and psychotherapy; (j) primary outcomes; and (k) duration of outcomes. Given the heterogeneity of study designs and underlying diagnoses treated, a narrative synthesis was most appropriate. The results were subdivided by description of intervention and the effectiveness and duration of treatment (to encompass the methodology and results reported in the included studies). Risk of bias was not formally assessed given the purpose of this review was an exploration of standard practices and outcomes in KAP, rather than an evaluation of the quality of individual studies. For the purposes of this review, ketamine-assisted psychotherapy is defined as psychotherapy provided either before, concurrently, and/or after ketamine administration.
Results
Seventeen articles and abstracts involving 603 participants were identified and reviewed. Seven articles reported randomized controlled trials; five articles were case studies or case series; four were open-label trials; and one was a retrospective study. Table S1 contains a summary of included articles, along with a description of the relevant variables extracted for the present review.
The primary and co-morbid diagnoses investigated in the included articles are summarized in Figure 2. Six articles studied participants with PTSD and six studied participants with MDD, the two most frequent diagnoses in this review. The remaining articles recruited participants with: obsessive-compulsive disorder (OCD) (2 studies); chronic neuropathic pain (2 studies); two bipolar disorder (BD) studies; generalized anxiety disorder (GAD); attention-deficit hyperactivity disorder (ADHD); and one with an eating disorder not otherwise specified (EDNOS). Six studies involved participants with substance use disorders: two on heroin use disorder; one on cannabis use disorder (CUD); one on cocaine use disorder; one on alcohol use disorder; and one on opioid-induced hyperalgesia (OIH).
Description of Interventions
To investigate the nature, dosing, and frequency of KAP in the included studies, we extracted data relating to the method of ketamine administration, frequency, and dose, as well as common modalities of psychotherapy, treatment length, setting and procedure; and the temporal relationship between ketamine administration and provision of psychotherapy.
Ketamine Route of Administration, Dosage, and Frequency
Eleven of the 17 included articles involved intravenous (IV) ketamine administration, three investigated intramuscular (IM) administration, one sublingual (SL), one intranasal (IN), and one study, which used a combination of IM and SL. In relation to frequency of administration, six studies investigated a single session of ketamine administration, five involved multiple ketamine sessions (with one study administering five 10mg IN doses of ketamine in each ketamine session), three studies implemented a mixed method (single or multiple ketamine sessions), and two studies that administered a single continuous infusion over 5 days. These results are summarized in Figure 3 and Table 1.
 |
Table 1 Shows the Methods of Ketamine Administration |
 |
Figure 3 Frequency and route of ketamine administration. Abbreviations: IM, intramuscular; IN, intranasal; IV, intravenous; SL, sublingual. |
Across all 17 studies there was considerable variability in ketamine dosage. Of the 11 studies, which administered ketamine intravenously, seven administered ketamine at 0.5mg/kg. Of these 7 studies, four administered 0.5mg/kg of IV ketamine during a single ketamine session; one study had 3 ketamine sessions; one study had 4 ketamine sessions; and one study had 6 ketamine sessions. One IV ketamine study administered 0.71mg/kg infusion of ketamine during one or two ketamine sessions; one IV ketamine study administered a single 0.6mg/kg infusion; one IV ketamine study administered a single continuous dose of ketamine over 5 days, starting at 2μg/kg/min and increasing by 1–2μg/kg/min every 3–4 hours to a maximum of 11–15μg/kg/min; and one IV ketamine study administered two separate 5-day continuous infusions of ketamine, with each continuous session separated by 6-months; starting at 0.09mg/kg/h titrated up by 10mg/kg to a maximum of 0.77mg/kg/h. A total of 3 studies administered IM ketamine, one study delivering 0.2mg/kg or 2.0mg/kg of IM ketamine; one study delivering 2.0mg/kg of IM ketamine during a single or multiple (3) ketamine sessions; and one study delivering 25mg of IM ketamine during a single or multiple (2) ketamine sessions. One study delivered multiple (1–25) IM and SL ketamine sessions as part of clinical practice, administered an average of 80–90mg of IM ketamine and an average of 200–250mg of SL ketamine. One study delivered 150mg of SL ketamine over 4 ketamine sessions. One study delivered multiple doses (5) of ketamine during multiple sessions (8); during each session 10mg of IN ketamine was administered over 20 minutes to a total of 50mg.
Psychotherapy Modality and Length
Three studies did not report the type of psychotherapy administered. Six studies used cognitive behavioral therapy (CBT) and 4 used mindfulness-based interventions (MBI). In the remaining studies; three employed motivational enhancement therapy (MET); three used exposure therapies; one focused on existentially oriented therapy; and one employed functional analytic psychotherapy (FAP). We were unable to determine the average frequency and duration of psychotherapeutic activities due to the heterogeneity across studies. Fourteen of 17 studies reported on the total number of psychotherapy sessions, ranging from as few as 4 psychotherapy sessions to as many as 60 psychotherapy sessions, with a median of 11 (IQR = 5). One study was a retrospective study and included data from 3 clinical practices, and found the average length of psychotherapy was between 1 and 25 sessions. Details about the type and duration of psychotherapy applied in each individual study can be found in Table S1.
Setting and Procedure
The context in which ketamine is provided is known to impact the effects of the drug. Many of the studies listed did not outline in detail the environment in which ketamine and psychotherapy were administered. Nonetheless, we have extracted this information from each study and present it in Table S1. The data shows a considerable variability in the setting including university and hospital outpatient clinics, inpatient psychiatric wards, an MRI facility, and a combination of these (ie, ketamine administered in an inpatient setting and psychotherapy in an outpatient setting).
Temporal Relationship Between Ketamine Administration and Psychotherapy Provision
The timing of psychotherapy in relation to ketamine administration is shown in Table 2. In 5 studies, psychotherapy session(s) were provided separately, before and after the ketamine administration(s). Two studies provided psychotherapy concurrently with ketamine administration, and one provided psychotherapy concurrently and again after ketamine infusion. Two studies provided psychotherapy after ketamine administration(s) only, and 7 studies provided psychotherapy before, during, and after ketamine administration(s).
 |
Table 2 Timing of Psychotherapy in Relation to Ketamine Administration |
Efficacy and Duration of KAP on Alleviating Distressing Symptoms
Participant diagnoses are summarized in Figure 2. The present section highlights the primary findings and duration of effect when applicable according to the participants’ diagnosis or substance of abuse.
Mixed Mental Health Diagnoses and Substance Use Disorders
Dore et al23 collected data from 235 patients with a wide range of psychological and substance use related disorders (MDD, PTSD, ADHD, GAD, BPD, SUD, OCD) in three private psychiatric practices in Northern California. SL and/or IM ketamine was administered to patients, with doses titrated in office and then adjusted for home-use. Patients were started on SL ketamine in office to induce a trance state with respect to dosage so patients could replicate the effect at home if needed. Afterwards, IM ketamine was administered in-office with the clinician providing a safe and warm environment and guided psychotherapy during KAP sessions. A minimum of 2 therapists were present during the in-office KAP session(s) and subsequent monitoring, with each session lasting up to 3 hours. Home-use dosing was SL, lower dosing, and often unsupervised. On average, KAP sessions were held in-office roughly 2 weeks apart, where psychotherapy was offered concurrently with IM ketamine. Patients were sometimes prescribed SL ketamine for home use and were encouraged to replicate the setting and procedure as demonstrated by the clinician. Patients were given instructions for SL ketamine dosing, and told not to exceed more than 6 at home sessions over a two-week period. The frequency of psychotherapy was determined based on patient diagnosis and the severity of presenting difficulties. The results showed significant decreases in anxiety and depression scores as measured by the Hamilton Anxiety Scale (HAM-A) and the Beck Depression Inventory (BDI). The most significant improvements were seen in those with developmental trauma (complex PTSD), severe depression, and in those who received more ketamine-assisted psychotherapy sessions.
Major Depressive Disorder (MDD)
In an open-label trial in 2017, Wilkinson et al34 administered a total of 4 ketamine infusions, twice a week for two weeks, as an adjunct to a 12-session 10-week course of CBT in 16 patients with treatment-resistant depression. Participants received an initial CBT session 24–48 hours following the first IV ketamine (0.05mg/kg) session. Ketamine and CBT was provided twice weekly on different days over 2 weeks, with additional weekly sessions of CBT administered for another 8 weeks. Of the eight (50%) participants who responded to the ketamine infusions, seven achieved a remission of symptoms. Among the remitters, four of seven did so after the initial infusion, whereas two did so after the second infusion, and one patient did so after the fourth infusion. By the end of the study (10 weeks post-infusion), five of the eight responders relapsed with a median relapse time of 12 weeks from remission.
In their subsequent 2021 randomized controlled trial, Wilkinson et al35 administered 6 IV ketamine (0.5mg/kg over 40 min) sessions over the course of 3 weeks to 42 severely depressed patients. Of the 42 subjects, 28 (66.7%) achieved a response to ketamine as defined as a >50% improvement in depression severity by the end of the last ketamine session. The 28 ketamine responders were randomized into either a CBT or treatment as usual (TAU) groups for an additional 14 weeks. Those in the TAU group (n=14) attended a visit with the study physician every 1–2 weeks, focusing on medication management and management of adverse events. Those in the CBT group (n=14) received CBT twice weekly for 2 weeks, then once weekly for the remaining 12 weeks. They found a significant difference in Montgomery-Asberg Depression Rating Sale (MADRS) scores, and a significant interaction effect of time-by-treatment group as measured by the Quick Inventory of Depressive Symptomatology (QIDS) scores from baseline to week 17 (fourteen weeks after the last ketamine infusion), finding greater sustained improvements in the CBT group.
Becker28 presented case reports of two female participants. Case 1 was diagnosed with MDD comorbid with an Eating Disorder Not Otherwise Specified (EDNOS), and received two concurrent ketamine and psychotherapy sessions resulting in normalized caloric intake and improvements in treatment engagement and adherence. Case 2 had a diagnosis of BPD-I with a current depressive episode and received a single concurrent ketamine and psychotherapy session that resulted in immediate (next day) improvements in depressive symptoms that were sustained at the 2-month follow-up. Though the duration of effect was not reported for Case 1, both women reported an increased capacity to make effective use of psychological insight facilitated by the KAP experience.
Obsessive-Compulsive Disorder (OCD)
Adams et al36 reported on a case of a single male inpatient being treated with multiple (8) intranasal ketamine-assisted CBT sessions for OCD (with comorbid MDD). The patient received 8 weeks of inpatient care followed by an additional 8-weeks of CBT. During the inpatient stay, the subject received CBT on every week day with 1–2 hours of CBT homework. CBT during weeks 3–6 was supplemented with twice weekly administrations of 50mg of IN ketamine, administered in 10mg doses over a 20-minute period. The patient was then discharged 2 weeks after the final ketamine administration, and continued receiving CBT twice weekly for 4 weeks, followed by weekly CBT sessions over another 4 weeks. Patient reported worsening in OCD and MDD symptoms at 2 weeks following discharge, however these symptoms were attributed to leaving the clinical environment, and were reduced with continued CBT. At study conclusion (ten weeks following the initial ketamine administration), the patient reported less distress associated with OCD symptoms and reduced OCD-related functional impairment as measured by Yale Brown Obsessive Compulsive Scale (YBOCS). The patient also reported reduced suicidality as measured by item 10 on the Montgomery–Åsberg Depression Rating Scale (MADRS). While depression symptoms did improve following treatment, the symptoms were still reported as severe.
In a sample of 10 unmedicated OCD outpatients, Rodriguez et al37 administered a single dose of ketamine in addition to exposure-based CBT. Participants received a 90 minutes CBT session the day before a single 40-minutes IV ketamine (0.5mg/kg) infusion, followed by 10 hours of exposure sessions delivered over 2 weeks. A significant decrease in OCD symptoms as measured by the YBOCS was reported at 4 weeks post infusion among 8 of the 9 patients who completed the ketamine infusion.
Post-Traumatic Stress Disorder (PTSD)
In their randomized placebo-controlled trial, Pradhan et al38 investigated whether trauma interventions using Mindfulness-Based Extinction and Reconsolidation (TIMBER) therapy could extend the therapeutic response of ketamine in patients diagnosed with PTSD (n=20), with 10 PTSD patients assigned to each arm (ketamine + TIMBER vs saline + TIMBER). Both groups received a total of 12 TIMBER sessions, made up of 3 mini-TIMBER sessions administered during infusion, day 2, and day 8; and followed by 9 weekly 45-minute TIMBER sessions. All subjects experienced reductions in PTSD scores as measured by the Posttraumatic Stress Disorder Checklist (PLC) and the Clinically Administered PTSD Scale (CAPS) at 24 hours with no significant differences between groups. However, participants in the ketamine group had a greater duration of sustained response (34.44 ± 19.12 days) when compared to placebo (16.50 ± 11.39 days).
Similarly, Duek et al39 randomized 17 participants diagnosed with PTSD and undergoing prolonged exposure therapy to either receive a single ketamine or midazolam infusion over 40 minutes. Subjects underwent 7 daily exposure sessions, receiving the infusion after the first exposure session. Both groups experienced significantly reduced PTSD scores at the 30-day follow-up with no significant differences between groups; however, individuals in the ketamine group reported less severe PTSD symptoms than the midazolam group at 90-days. The significance values of the reduction in PTSD symptoms at the 90-day follow-up are missing from this conference abstract.
Halstead et al40 examined a case of a female patient with PTSD comorbid with persistent depressive disorder (PDD) related to racial discrimination. The patient initially received a preparatory psychotherapy session to develop goals and be taught self-regulation techniques to assist with the ketamine experience. During dosing sessions, the patient received ketamine lozenges (150mg) which were held in the mouth for 10 minutes before swallowing. Psychotherapy was provided concurrently during the dosing session. This was followed by an integration session where the psychotherapist worked with the patient to integrate ketamine-induced insights into daily life. Integration sessions also followed the second, third, and fourth dosing sessions. Following multiple sublingual doses of ketamine administered in combination with Mindfulness-Based Cognitive Therapy (MBCT) and Functional Analytic Psychotherapy (FAP) over a 13 days scores on the Posttraumatic Cognitions Inventory decreased at 2-weeks, which was maintained up to a 6-month follow-up. In addition to self-reported reductions in anxiety following treatment, severe depression scores on the BDI-II decreased from baseline to the 2-week time point, but returned to severe levels at the 6-month follow-up.
Shiroma et al41 investigated the feasibility of integrating multiple (3) ketamine infusions with standardized prolonged exposure therapy for 12 veterans with chronic PTSD. Participants received a IV ketamine (0.5mg/kg) infusion 24-hours before weekly prolonged exposure therapy sessions over 3 weeks. Up to 7 additional psychotherapy sessions were provided on a weekly basis. Ten veterans completed treatment, and the 4-month follow-up demonstrated a significant decrease in the severity of PTSD symptoms as measured by PLC-5 and the Clinical Global Impression-Severity (CDI-S) scale following treatment. After controlling for mean changes in the MADRS depression scores over time, changes in total PLC-5 scores and PLC-5 Avoidance remained significant. However, changes in PCL-5 Intrusions, Arousal, Negative Mood and Cognitions, as well as the CAPS-5, were no longer significant.
Chronic Neuropathic Pain
Keizer et al21 examined 11 chronic pain patients with comorbid PTSD receiving a 5-day continuous ketamine infusion concurrent with psychotherapy. Patients received a 5-day course of IV ketamine for chronic pain, beginning the infusion at 2μg/kg/min, increasing by 1–2μg/kg/min increments every 3–4 hours to a maximum dose of 11–15μg/kg/min. This maximum dose was maintained for 96 hours before being titrated down and discharged on day 5. Psychotherapy was administered daily at bedside for approximately 90 minutes. At treatment completion, 7 of the 11 (63.6%) patients reported clinically significant reductions in PTSD symptoms as measured by pre-post PCL scores. Reductions in pre-post pain as measured by the Numeric Pain Rating Scale (NPRS) was reported for 6 patients, whereas 2 showed no change and 3 reported increased pain intensity.
Ocker et al22 reported on a single male patient with Complex Regional Pain Syndrome (CRPS) with an NPRS score of 9 (maximum 10), undergoing rapid opioid tapering using a continuous 5-day ketamine infusion that was followed up with monthly CBT sessions. The patient started the continuous infusion at a dose of 0.09mg/kg/h and was titrated up in 10mg/h increments to a maximum of 0.77mg/kg/h. Ketamine dosage was reduced by 50% on the day of discharge, then discontinued. After discharge, the patient attended CBT sessions every 3–4weeks. The patient reported to be free of pain and opioids at a 30-day follow-up and reported mild pain at the 6-month follow-up (NPRS of 0 and 2, respectively). The patient then returned to the hospital and received another 5-day continuous infusion, and continued his CBT sessions for another 6 months, and subsequently reported continuous abstinence in opioids despite an increase in pain (NPRS = 4) at 1-year follow-up (post initial infusion). The patient was scheduled for a third continuous infusion at the time of publication.
Alcohol Use Disorder
Dakwar et al42 randomized 40 adults with alcohol use disorders to receive a single infusion of midazolam (n=23) or ketamine (n=17) plus 6 sessions of Motivational Enhancement Therapy (MET) over 5 weeks. During the second week of treatment, both groups received a 2-minute saline bolus (0.11mg/kg) followed by a 50-munite infusions of either ketamine (0.6mg/kg) or midazolam (0.025mg/kg). MET sessions were attended weekly, with an additional session provided 24 hours after the drug infusion in order to benefit from the hypothesized ketamine-induced enhanced motivation. At the 21-day post-infusion follow-up, more participants in the midazolam group (59.1%; n = 13/22) than the ketamine group (47.1%; n= 8/17) were using alcohol. Additionally, more individuals in the midazolam group (40.0%; n=9/22) had heavy drinking days compared to the ketamine group (17.6%; n=3/17). Of the 19 participants who completed the 6-month follow-up, 75% (6/8) of ketamine participants reported abstinence maintenance, compared to 27% (3/11) of participants in the midazolam group.
Opioid Use Disorder
With the goal of studying whether higher doses of intramuscular ketamine in combination with psychotherapy resulted in heroin abstinence, Krupitsky et al43 randomly assigned 70 detoxified heroin-addicted inpatients into two groups where low-dose ketamine (0.2 mg/kg) was compared with a high-dose ketamine group (2.0 mg/kg). Both groups received 10 hours of psychotherapy prior to ketamine administration to prepare patients for a ketamine-induced psychedelic experience, emphasizing how the experience should allow them to realize the negative effects of heroin abuse and promote a life without drugs; implying that the process has the ability to modify their value systems, notions of self, and facilitate changes in personality. One 1.5–2-hour psychotherapy session was then administered concurrently with a single low-dose (0.2mg/kg) or high-dose (2.0mg/kg) of IM ketamine. Soothing instrumental music was played during the ketamine-induced psychotherapy session, and the content of the session was based on the individual subjects’ case history. An additional 5 hours of psychotherapy was administered within several days following the ketamine-psychotherapy session, with aim to integrate insights from the ketamine experience into daily life. Subjects were then discharged from inpatient care within 3–5 days following the ketamine-psychotherapy session. At the 24-month follow-up, participants were assessed for abstinence from heroin by reports from the subjects and their families, and confirmed by urine tests. Individuals assigned to the high-dose group reported higher rates of abstinence and lower rates of relapse when compared to the low-dose group. The differences between abstinence and relapse rates between groups were significant from the first month almost to the 24-month follow-up; with the exclusion of month 7 and 8. Both high and low-dose groups reported significantly reduced cravings for heroin as evaluated by the Visual Analog Scale of Cravings, with greater reductions experienced by the high-dose group immediately after the psychotherapy-ketamine sessions, and at the 1 and 3-month post psychotherapy-ketamine follow-up. The high-dose group reported reductions in cravings at the 24-month follow-up but not the low-dose group. Additional data collected on anxiety and depression, as measured by the Spielberger Anxiety Scale and the Zung Depression Scale, respectively, revealed that the high and low-dose groups both experienced reductions in anxiety and depression, but there were no significant between-group differences.
In a later study, Krupitsky et al44 investigated whether repeated ketamine dosing increased abstinence rates in heroin-addicted individuals. Fifty-nine detoxified heroin-dependent participants received 5 hours of psychotherapy to prepare for the ketamine experience prior to receiving the first intramuscular ketamine injection (2mg/kg). The ketamine session was 1.5–2hours in length and psychotherapy was provided concurrently. An additional 5 hours of psychotherapy was provided to patients following the ketamine-psychotherapy session to help integrate the experience. Six of the 59 participants dropped out during the initial ketamine sessions, and the remaining 53 were randomized into single dose (n=27) or multi-dose (n=26) groups. All patients were then discharged. Those in the multi-dose group returned for additional ketamine-psychotherapy sessions at 1 and 2 months. Addiction counselling was provided prior to the ketamine-psychotherapy sessions. Those in the single-dose group did not receive additional ketamine sessions, instead returning at 1 and 2 months for addiction counselling. Within the multi-dose group, 4 of 26 (15.4%) participants relapsed and dropped out of the study following the second KAP treatment, as compared to 7 of the 27 (25.9%) participants in the single-dose group who relapsed and dropped out after the first counseling session. The difference in treatment retention between the two groups was not significant. The 1-year follow-up, however, revealed a significant difference between abstinence rates, with 50% (13 of 26) of the multi-dose participants retaining abstinent, as compared to the 22.2% (6 of 27) of participants who only received a single dose. Reductions in depression and anxiety, as measured by the Zung Self-Rated Depression scale (ZDS) and the Spielberger Self-Rated State-Trait Anxiety Scale (SAS) were found amongst those who remained abstinent, but no significant differences were observed between groups.
Cocaine Use Disorder
Dakwar et al45 randomly assigned 55 cocaine-dependent participants to receive a single ketamine or midazolam infusion during a 5-day inpatient stay prior to initiating an additional 4-week course of Mindfulness-Based Relapse Prevention (MBRP). Participants were guided throughout the drug administration and received a 40-minute infusion on day 2 of either ketamine (0.5mg/kg) or midazolam (0.025mg/kg). Participants received daily MBRP sessions over the 5-day inpatient stay. An MBRP session was administered 2 hours after infusion. Following discharge, participants returned for twice weekly MBRP sessions for 4 weeks. Abstinence rates and relapse time were compared. Abstinence, as confirmed by urine tests, revealed that those in the ketamine group had a higher rate of abstinence (13/27) over the last 2 weeks of the trial than those in the midazolam group (3/28). Regarding time to relapse, more participants in the midazolam group (26/28) went on to use cocaine or drop out of the study compared to the ketamine group (15/27). These differences are also reflected in reductions in cocaine cravings, as those in the ketamine group were observed to have early improvements in craving scores such that their scores were 58.1% lower than those in the midazolam group. Moreover, a 6-month follow-up interview revealed that of the 27 individuals in the ketamine group, twelve (44%) remained abstinent compared to none in the midazolam group.
Cannabis Use Disorder
Azhari et al46 examined the efficacy of ketamine-assisted MET and MBRP in the treatment of 8 cannabis-dependent individuals. Participants were treated with a single (0.71 mg/kg) infusion or an additional higher dose (1.41 mg/kg) if they were identified as ketamine non-responders and or were observed to be struggling to maintain abstinence. All participants received 3 MET sessions, one during week 1, a second prior to the ketamine session in week 2, and a third on the same day but following the ketamine session. During the ketamine session(s), participants were guided through the infusion by the therapist. The second psychotherapist-guided ketamine session that was offered to non-responders and those experiencing difficulties was administered at week 4, and was also followed up by a same day MET session. MBRP was provided twice weekly to all participants in weeks 3 to 6. Of the eight participants enrolled in this study, three received the additional ketamine infusion. Participants self-reported cannabis use by the Timeline Follow-Back (TLFB) and were evaluated for their confidence in abstaining from further cannabis use by completion of Drug-Taking Confidence Questionnaire (DCQ) throughout the study. Cannabis use had decreased significantly following the initial infusion, and remained significant until study conclusion. Six of 8 (75%) participants maintained 3 or more weeks of post-study abstinence, confirmed by urine samples and reports on the TLFB. While no significant improvements were observed in pre-to-post assessments of craving, there was an increased capacity to abstain from cannabis use over time, with an average baseline DCQ of 44.7 increasing to an average of 87.5 by end of study. No significant differences between abstinence or confidence in abstaining were found between those who received single or multiple infusions.
Discussion
This systematic narrative review suggest that KAP may be effective in initiating rapid, significant benefits for a wide range of disorders. However, variability in study design, intervention structure, patient diagnoses, and outcome measurement, along with small sample sizes, limit firm conclusions. Nonetheless, some commonalities in the reviewed studies are identifiable and worthy of mention.
KAP’s capacity to facilitate rapid clinically significant reductions of anxiety and depression has been observed in multiple studies.23,28,34–36,40,41,43,44 Reported increases in treatment engagement28 have also been observed and attributed to such sudden gains, as rapid reductions in depression/anxiety have been associated with lower drop-out rates and increased participation in therapy.47
In the treatment of substance dependence, the integration of ketamine and psychotherapy appears to promote abstinence initiation, improve relapse prevention, improve craving reactivity management, and increase motivations to terminate drug-use in select individuals.42,43,45,46
Ketamine is currently being used in pain management;3,8,9 and additional psychotherapy appears to improve the duration of benefits in select patients.22
The psychoactive properties of higher ketamine doses have been hypothesized to facilitate psychotherapy, via linked “psychedelic” experiences that increase rapport, reduce defensiveness, and evoke transpersonal experiences that aid decision-making and support transformation, as observed in studies on depression, PTSD, and addiction.23,28,40,43 Ketamine has also been proposed to facilitate the neuroplasticity involved in new memory formation, fear extinction, and the restructuring of traumatic memories.14
Substantial variations in KAP administration were observed in the included studies, particularly in respect to the psychotherapeutic modalities applied to diverse clinical populations and settings. While the present results do not provide indications favoring any single KAP administration protocol, some common themes appear, including: (1) preparation of patients for KAP sessions as an important first step, as realistic goal setting and positive expectations promote therapeutic alliance and structure transformational experiences.23,28,40 (2) Supervision by qualified personnel during the administration of ketamine is recommended to: a) ensure patient safety, b) guide transformational experiences, c) provide comfort and assistance in navigating distress, and d) evoke psychological insight.23,28,40 (3) It has been proposed that ketamine administration should be followed by additional psychotherapy to facilitate the integration of ketamine-induced transpersonal experiences and promote patient acceptance of insights discovered during KAP.23,28,43,44 Longer-term psychotherapy may be helpful in sustaining these gains.23,36,40
While a single dose of ketamine can reduce distressing symptoms, some evidence supports the administration of multiple doses over multiple sessions. In a study of individuals with cannabis use disorder,46 additional infusions of ketamine (and higher doses), administered to non-responders and those experiencing difficulties in maintaining abstinence, were well tolerated and helped promote abstinence. Moreover, research into the efficacy of KAP in participants with heroin addiction indicated that those in the multi-dose group maintained greater abstinence rates than those in the single-dose group.44 While there were no reported differences in reductions of depression and anxiety scores between the single and multi-dose groups in the above studies, multiple administrations of ketamine seemed to prolong therapeutic effects and resulted in initial non-responders experiencing apparent ketamine-related benefits.34,46 As ketamine non-responders are identifiable,23,34,35,46 the frequency of ketamine administration during KAP treatments can be further investigated to maximize overall responsiveness.
The antidepressant and anxiolytic effects of KAP have been reported in ketamine-responders across most studies regardless of dose or administration route. However, higher doses of ketamine in conjunction with psychotherapy may promote the durability of these gains as demonstrated by higher heroin abstinence rates in high-dose ketamine groups when compared to low-dose groups.43 Nonetheless, these findings are limited to a single RCT that compared the efficacy of high-dose vs low-dose ketamine. Future research should further examine whether higher doses of ketamine in KAP session may prolong beneficial effects within larger sample sizes.
Overall, it appears that higher-doses of ketamine, more frequent KAP sessions, and longer durations of psychotherapy increase the efficacy and durability of improvements within patients with a range of disorders.23,43,44,46 Based on the results of the present review and considering the heterogeneity of populations studied, it is not possible to recommend one psychotherapeutic modality over another or an ideal number of sessions, although it seems appropriate to tailor approaches to individuals and their presenting problems. Further work is required to make progress in this area.
KAP is now being used in private clinics for a variety of disorders.23 However, more work is needed to identify a consistent and effective protocol for KAP administration. While KAP has been reported to be effective in alleviating distressing symptomatology, many participants experience relapse and/or the reemergence of mental health and pain-related symptoms.22,34,36,42–46 This is compounded by the significant time and financial costs to patients involved in the uptake of KAP.15,48,49 Future research should build on our current understandings of the benefits of treatment and aim to improve efficacy and the duration of effects using larger sample sizes. These findings would serve to establish the feasibility of KAP interventions and could inform maintenance programs.
As mentioned above, some individuals appear not to respond to ketamine administration and have been called “non-responders”.23,28,34,35,46 It may be possible that those who do not achieve an immediate response to standard doses of ketamine may require either additional ketamine sessions, or perhaps require a higher dose, titrated to effect.34,44,46 More investigation is necessary to understand whether individuals are genuinely “non-responders” to ketamine, or if the dosage of ketamine can be tailored to the individual to elicit a response. While most participants tolerated KAP well, some were unable to complete ketamine administration and dropped out of treatment.23,37,41,44,45 Therefore, drop out in relation to administration patterns should be addressed in further investigations with the aims of increasing tolerability and response rates.
Although ketamine has been observed to be beneficial in reducing pain intensity in patients with chronic pain, the studies in which participants with chronic pain were recruited focused on other comorbid factors.21,22 The interaction between pain, mental health and addictive disorders, has been established in previous studies; indicating high levels of comorbidity, mutual exacerbation of symptoms, shared responses to similar treatments, and implicating similar biological pathways and neurotransmitters.50–53 Chronic pain patients are 2–3 times more likely to experience an anxiety disorder;53–55 with 20–50% of chronic pain patients reporting comorbid depression,56–58 and 36–56% experiencing a substance use disorder within their lifetime.59 Likewise, individuals with pain conditions are more likely to develop mental health and/or addictive disorders, and those with depression are more likely to develop addictive and/or pain conditions.52,55,59–61 Emotional distress has been demonstrated to increase pain intensity, fosters greater pain-related disability, and contributes to weaker responses to traditional pain treatments.53,54,62 For pain, treatment is usually based on the mechanisms involved (ie, neuropathic, nociceptive or a combination of both) and often includes the prescription of opioids.63 Long-term use of opioids has been associated increased tolerance and hyperalgesia, continued experiences of pain, gastrointestinal and CNS side effects, and opioid dependence/addiction.64,65 Non-steroidal anti-inflammatory drugs (NSAIDs) and antidepressants are also provided to treat pain symptoms, however NSAIDs have been found to be responsible for 30% of hospital admissions for adverse drug reactions,66 and the efficacy of antidepressants on pain management are inconclusive.67 Psychotherapy may be effective in the management of pain,68–70 however despite these findings, some patients do not benefit from this treatment alone.71 Traditional approaches to the treatment of mental health and addiction also includes the use of psychotropic medications and psychotherapy.72,73 Psychotropic medications elicit partial responses in 50–70% of depressed patients, with only 30% achieving remission of depressive symptoms; while psychotherapy alone has been found even less effective.74–76 Psychotropic medications show some depressive symptom relief for patients with comorbid mental health and substance abuse disorders, however they appear to have little effect on patients with substance abuse disorders without comorbid mental health issues.72 Likewise, relapse rates following common psychotherapeutic interventions such as cognitive-behavioral therapy have been estimated to be as high as 60%.77,78 The interaction between the experience of pain, mental health, and substance abuse is complex; however, research demonstrates better outcomes when all comorbid conditions are treated concurrently with pharmacology and psychotherapy rather than with medication or psychotherapy alone.79,80 Currently, there are no studies focusing on the effects of KAP on chronic pain without comorbid substance dependence or mental health disorders. KAP’s propensity to help with the management of pain should be specifically investigated in future studies and reviews. Both ketamine alone and psychotherapy alone have been shown to reduce pain in those with chronic pain conditions,2,7,81,82 however a gap in research exists regarding the efficacy of KAP for the management of chronic pain.
The findings from the present review should be interpreted in the light of the several methodological limitations. This review included studies with small sample sizes. There are five case studies,21,22,28,36,40 two pilot trials38,42 and two proof-of-concept trials.41,46 Due to the small number of participants in each study, their findings may not be generalizable to wider populations.
In addition, because of the relatively small number of published literature and our wish to be as inclusive as possible, the present review included conferences abstracts36,39 which lack important details, including adequate information on the long-term duration of KAP treatment, modalities of psychotherapy employed, significance of results, or populations studied (ie, patient demographics and/or group of allocation.)
Only 7 of the 17 studies included in this review were RCTs. Case series/case studies comprised 29.4% (n=5) of the studies highlighted in this review. While offering detailed descriptions of KAP interventions, these studies lack a concurrent control group, which makes drawing any conclusions relating to the efficacy of KAP difficult. Observed effects within these studies have little statistical validity, and may be influenced by outside factors, such as other concurrent treatments. Additionally, these studies lack blinding and randomization, which also makes assessing the efficacy of KAP a challenge, and introduces the possibility of selection bias.
Finally, the heterogeneity of the populations studied and the methods employed make generalizing findings difficult.
Conclusion
The use of Ketamine Assisted Psychotherapy (KAP) can potentially fulfil the unmet clinical need for an effective treatment for multiple complex and often comorbid pain, psychological, and substance use disorders. Ketamine’s demonstrated ability to produce antidepressant and anxiolytic effects likely interacts with the processes involved in psychotherapy, ideally as a conduit for rapid-change, increasing treatment engagement and adherence, building the therapeutic alliance, and lowering defensiveness by providing reprieves from distressing symptomology while inducing transpersonal experiences at higher doses. Continued engagement in psychotherapy after ketamine administration may prolong the often-transient effects of ketamine and allow for the integration of psychological insights into everyday functioning. While at present there is no standard approach to the application of KAP, it is important to prepare and support the patient during ketamine administration and to offer follow-up psychotherapy sessions to maintain positive effects and delay or eliminate relapse. As KAP research continues to evolve, a focus on increasing the duration of positive effects may lead to effective interventions and maintenance programs, improving KAP such that it becomes an effective, long-lasting treatment for complex, resistant, and chronic conditions for people living with pain, mental health, and substance use disorders.
Acknowledgment
The authors would like to thank David Lightfoot, Information Specialist from St. Michael’s Hospital Health Sciences Library, for his assistance in designing and carrying out a systematic search and identifying articles for this review.
Disclosure
Karim S Ladha reports they are co-PI of an observational study on medical cannabis funded by Shoppers Drug Mart. The authors report no other potential conflicts of interest in relation to this work.
References
1. Belmaker RH. The future of depression psychopharmacology. CNS Spectr. 2008;13(8):682–687. doi:10.1017/S1092852900013766
2. Sigtermans MJ, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145(3):304–311. doi:10.1016/j.pain.2009.06.023
3. Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology. 2015;40(2):259–267. doi:10.1038/npp.2014.261
4. Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatr. 2012;169(11):1150–1156. doi:10.1176/appi.ajp.2012.12040531
5. Domino EF, Warner DS. Taming the ketamine tiger. Anesthesiology. 2010;113(3):678–684. doi:10.1097/ALN.0b013e3181ed09a2
6. Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45(1):29–40. doi:10.1213/00000539-196601000-00007
7. Dahan A, Olofsenl E, Sigtermans M, et al. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain. 2011;15(3):258–267. doi:10.1016/j.ejpain.2010.06.016
8. Iadarola ND, Niciu MJ, Richards EM, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97–114. doi:10.1177/2040622315579059
9. Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL. The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry. 2014;76(1):40–46. doi:10.1016/j.biopsych.2013.08.009
10. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi:10.1126/science.1190287
11. Maeng S, Zarate CA, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi:10.1016/j.biopsych.2007.05.028
12. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi:10.1038/nature17998
13. Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18(12):1236–1241. doi:10.1038/mp.2013.87
14. Collo G, Pich E. Ketamine enhances structural plasticity in human dopaminergic neurons: possible relevance for treatment-resistant depression. Neural Regener Res. 2018;13(4):645. doi:10.4103/1673-5374.230288
15. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039. doi:10.1001/jama.2019.10728
16. Feder A, Parides MK, Murrough JW, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder. JAMA Psychiatr. 2014;71(6):681. doi:10.1001/jamapsychiatry.2014.62
17. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatr. 2013;170(10):1134–1142. doi:10.1176/appi.ajp.2013.13030392
18. Cuijpers P. Four decades of outcome research on psychotherapies for adult depression: an overview of a series of meta-analyses. Can Psychol. 2017;58(1):7–19. doi:10.1037/cap0000096
19. Khorramzadeh E, Lotfy AO. The use of ketamine in psychiatry. Psychosomatics. 1973;14(6):344–346. doi:10.1016/S0033-3182(73)71306-2
20. Fontana AE, Loschi JA. [Antidepressive therapy with C1 581]. Acta Psiquiatr Psicol Am Lat. 1974;20(1):32–39. Catalan.
21. Keizer BM, Roache JD, Jones JR, Kalpinski RJ, Porcerelli JH, Krystal JH. Continuous ketamine infusion for pain as an opportunity for psychotherapy for PTSD: a case series of ketamine-enhanced psychotherapy for PTSD and pain (KEP-P2). Psychother Psychosom. 2020;89(5):326–329. doi:10.1159/000507095
22. Ocker AC, Shah NB, Schwenk ES, Witkowski TA, Cohen MJ, Viscusi ER. Ketamine and cognitive behavioral therapy for rapid opioid tapering with sustained opioid abstinence: a case report and 1‐year follow‐up. Pain Pract. 2020;20(1):95–100. doi:10.1111/papr.12829
23. Dore J, Turnipseed B, Dwyer S, et al. Ketamine Assisted Psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189–198. doi:10.1080/02791072.2019.1587556
24. Myers KM, Carlezon WA, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36(1):274–293. doi:10.1038/npp.2010.88
25. Dakwar E, Anerella C, Hart CL, Levin FR, Mathew SJ, Nunes EV. Therapeutic infusions of ketamine: do the psychoactive effects matter? Drug Alcohol Depend. 2014;136:153–157. doi:10.1016/j.drugalcdep.2013.12.019
26. Dakwar E, Nunes EV, Hart CL, Hu MC, Foltin RW, Levin FR. A sub-set of psychoactive effects may be critical to the behavioral impact of ketamine on cocaine use disorder: results from a randomized, controlled laboratory study. Neuropharmacology. 2018;142:270–276. doi:10.1016/j.neuropharm.2018.01.005
27. Luckenbaugh DA, Niciu MJ, Ionescu DF, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi:10.1016/j.jad.2014.02.017
28. Becker J. Regarding the transpersonal nature of ketamine therapy: an approach to the work. Int J Transpers Stud. 2014;33(2):151–159. doi:10.24972/ijts.2014.33.2.151
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi:10.1016/s0006-3223(99)00230-9
30. Krystal JH. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry. 1994;51(3):199. doi:10.1001/archpsyc.1994.03950030035004
31. Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, Morgan CJA. Ketamine for the treatment of addiction: evidence and potential mechanisms. Neuropharmacology. 2018;142:72–82. doi:10.1016/j.neuropharm.2018.01.017
32. Krupitsky EM, Grinenko AY. Ketamine Psychedelic Therapy (KPT): a review of the results of ten years of research. J Psychoactive Drugs. 1997;29(2):165–183. doi:10.1080/02791072.1997.10400185
33. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700–b2700. doi:10.1136/bmj.b2700
34. Wilkinson ST, Wright D, Fasula MK, et al. Cognitive behavior therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom. 2017;86(3):162–167. doi:10.1159/000457960
35. Wilkinson ST, Rhee TG, Joormann J, et al. Cognitive behavioral therapy to sustain the antidepressant effects of ketamine in treatment-resistant depression: a randomized clinical trial. Psychother Psychosom. 2021;90(5):318–327. doi:10.1159/000517074
36. Adams TG, Bloch MH, Pittenger C. Intranasal ketamine and cognitive-behavioral therapy for treatment-refractory obsessive-compulsive disorder. J Clin Psychopharmacol. 2017;37(2):269–271. doi:10.1097/JCP.0000000000000659
37. Rodriguez CI, Wheaton M, Zwerling J, et al. Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? J Clin Psychiatry. 2016;77(03):408–409. doi:10.4088/JCP.15l10138
38. Pradhan B, Mitrev L, Moaddell R, Wainer IW. d -Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R, S)-ketamine infusion and TIMBER psychotherapy: a pilot study. Biochimica et Biophysica Acta. 2018;1866(7):831–839. doi:10.1016/j.bbapap.2018.03.006
39. Duek O, Kelmendi B, Pietrzak RH, Harpaz-Rotem I. Augmenting the Treatment of PTSD with Ketamine—a Review. Curr Treat Options Psychiatr. 2019;6(2):143–153. doi:10.1007/s40501-019-00172-0
40. Halstead M, Reed S, Krause R, Williams MT. Ketamine-assisted psychotherapy for PTSD related to racial discrimination. Clin Case Stud. 2021;20(4):310–330. doi:10.1177/1534650121990894
41. Shiroma PR, Thuras P, Wels J, Erbes C, Kehle-Forbes S, Polusny M. A proof-of-concept study of subanesthetic intravenous ketamine combined with prolonged exposure therapy among veterans with posttraumatic stress disorder. J Clin Psychiatry. 2020;81(6). doi:10.4088/JCP.20l13406
42. Dakwar E, Levin F, Hart CL, et al. A single ketamine infusion combined with motivational enhancement therapy for alcohol use disorder: a randomized midazolam-controlled pilot trial. Am J Psychiatr. 2020;177(2):125–133. doi:10.1176/appi.ajp.2019.19070684
43. Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, Grinenko A. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abuse Treat. 2002;23(4):273–283. doi:10.1016/S0740-5472(02)00275-1
44. Krupitsky EM, Burakov AM, Dunaevsky IV, Romanova TN, Slavina TY, Grinenko AY. Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. J Psychoactive Drugs. 2007;39(1):13–19. doi:10.1080/02791072.2007.10399860
45. Dakwar E, Nunes EV, Hart CL, et al. A single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: a randomized clinical trial. Am J Psychiatr. 2019;176(11):923–930. doi:10.1176/appi.ajp.2019.18101123
46. Azhari N, Hu H, O’Malley KY, Blocker ME, Levin FR, Dakwar E. Ketamine-facilitated behavioral treatment for cannabis use disorder: a proof of concept study. Am J Drug Alcohol Abuse. 2021;47(1):92–97. doi:10.1080/00952990.2020.1808982
47. Aderka IM, Nickerson A, Bøe HJ, Hofmann SG. Sudden gains during psychological treatments of anxiety and depression: a meta-analysis. J Consult Clin Psychol. 2012;80(1):93–101. doi:10.1037/a0026455
48. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatr. 2019;76(9):893. doi:10.1001/jamapsychiatry.2019.1189
49. SPRAVATO® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc: 2020.
50. Gallagher RM, Verma S. Managing pain and comorbid depression: a public health challenge. Semin Clin Neuropsychiatry. 1999;4(3):203–220.
51. Blier P, Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J Psychiatry Neurosci. 2001;26(1):37–43.
52. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964–973. doi:10.1016/j.jpain.2011.03.003
53. Asmundson GJG, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26(10):888–901. doi:10.1002/da.20600
54. Katz J, Pagé MG, Fashler S, Rosenbloom BN, Asmundson GJG. Chronic pain and the anxiety disorders: epidemiology, mechanisms and models of comorbidity, and treatment. In: Mental Health and Pain. Paris: Springer; 2014:119–155. doi:10.1007/978-2-8178-0414-9_8
55. Demyttenaere K, Bruffaerts R, Lee S, et al. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129(3):332–342. doi:10.1016/j.pain.2007.01.022
56. Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–268. doi:10.1097/01.psy.0000204851.15499.fc
57. Kroenke K. Somatic symptoms and depression: a double hurt. Prim Care Companion J Clin Psychiatr. 2005;07(04):148–149. doi:10.4088/PCC.v07n0401
58. Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10(6):619–627. doi:10.1016/j.jpain.2008.12.007
59. Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116. doi:10.7326/0003-4819-146-2-200701160-00006
60. Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60(1):39–47. doi:10.1001/archpsyc.60.1.39
61. Croft PR, Papageorgiou AC, Ferry S, Thomas E, Jayson MI, Silman AJ. Psychologic distress and low back pain. Evidence from a prospective study in the general population. Spine. 1995;20(24):2731–2737. doi:10.1097/00007632-199512150-00015
62. IsHak WW, Wen RY, Naghdechi L, et al. Pain and depression: a systematic review. Harv Rev Psychiatry. 2018;26(6):352–363. doi:10.1097/HRP.0000000000000198
63. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi:10.1016/j.pain.2007.08.033
64. Bhamb B, Brown D, Hariharan J, Anderson J, Balousek S, Fleming MF. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin. 2006;22(9):1859–1865. doi:10.1185/030079906X132398
65. Jacobsen R, Sjøgren P, Møldrup C, Christrup L. Physician-related barriers to cancer pain management with opioid analgesics: a systematic review. J Opioid Manag. 2007;3(4):207–214. doi:10.5055/jom.2007.0006
66. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi:10.1136/bmj.329.7456.15
67. Patetsos E, Horjales-Araujo E. Treating chronic pain with SSRIs: what do we know? Pain Res Manage. 2016;2016:1–17. doi:10.1155/2016/2020915
68. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1):1–13. doi:10.1016/S0304-3959(98)00255-3
69. van Tulder MW, Ostelo R, Vlaeyen JWS, Linton SJ, Morley SJ, Assendelft WJJ. Behavioral treatment for chronic low back pain. Spine. 2001;26(3):270–281. doi:10.1097/00007632-200102010-00012
70. Bogaards MC, ter Kuile MM. Treatment of recurrent tension headache: a meta-analytic review. Clin J Pain. 1994;10(3):174–190. doi:10.1097/00002508-199409000-00003
71. Turk DC, Rudy TE. Neglected factors in chronic pain treatment outcome studies–referral patterns, failure to enter treatment, and attrition. Pain. 1990;43(1):7–25. doi:10.1016/0304-3959(90)90046-G
72. Torrens M, Fonseca F, Mateu G, Farré M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. Drug Alcohol Depend. 2005;78(1):1–22. doi:10.1016/j.drugalcdep.2004.09.004
73. Carroll KM. Integrating psychotherapy and pharmacotherapy to improve drug abuse outcomes. Addict Behav. 1997;22(2):233–245. doi:10.1016/S0306-4603(96)00038-X
74. Fawcett J. Antidepressants: partial response in chronic depression. Br J Psychiatry Suppl. 1994;165(26):37–41. doi:10.1192/S0007125000293276
75. Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–142. doi:10.1016/s0197-2456(03)00112-0
76. Cuijpers P, Geraedts AS, van Oppen P, Andersson G, Markowitz JC, van Straten A. Interpersonal psychotherapy for depression: a meta-analysis. Am J Psychiatr. 2011;168(6):581–592. doi:10.1176/appi.ajp.2010.10101411
77. Witkiewitz K, Masyn KE. Drinking trajectories following an initial lapse. Psychol Addict Behav. 2008;22(2):157–167. doi:10.1037/0893-164X.22.2.157
78. Maisto SA, Pollock NK, Cornelius JR, Lynch KG, Martin CS. Alcohol relapse as a function of relapse definition in a clinical sample of adolescents. Addict Behav. 2003;28(3):449–459. doi:10.1016/S0306-4603(01)00267-2
79. Castelnuovo G, Giusti EM, Manzoni GM, et al. Psychological treatments and psychotherapies in the neurorehabilitation of pain: evidences and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front Psychol. 2016;7:115. doi:10.3389/fpsyg.2016.00115
80. Samwel HJA, Kraaimaat FW, Crul BJP, van Dongen RD, Evers AWM. Multidisciplinary allocation of chronic pain treatment: effects and cognitive-behavioural predictors of outcome. Br J Health Psychol. 2009;14(Pt 3):405–421. doi:10.1348/135910708X337760
81. Noppers I, Niesters M, Aarts L, Smith T, Sarton E, Dahan A. Ketamine for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2010;11(14):2417–2429. doi:10.1517/14656566.2010.515978
82. Majeed MH, Ali AA, Sudak DM. Psychotherapeutic interventions for chronic pain: evidence, rationale, and advantages. Int J Psychiatr Med. 2019;54(2):140–149. doi:10.1177/0091217418791447
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

