Back to Journals » OncoTargets and Therapy » Volume 9
Isoorientin induces apoptosis, decreases invasiveness, and downregulates VEGF secretion by activating AMPK signaling in pancreatic cancer cells
Authors Ye T, Su J, Huang C, Yu D , Dai S, Huang X, Chen B, Zhou M
Received 18 September 2016
Accepted for publication 8 November 2016
Published 12 December 2016 Volume 2016:9 Pages 7481—7492
DOI https://doi.org/10.2147/OTT.S122653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Tingting Ye,1 Jiadong Su,1 Chaohao Huang,1 Dinglai Yu,1 Shengjie Dai,1 Xince Huang,1 Bicheng Chen,1,2 Mengtao Zhou1
1Department of Surgery, The First Affiliated Hospital, Wenzhou Medical University, 2Zhejiang Provincial Top Key Discipline in Surgery, Wenzhou Key Laboratory of Surgery, Wenzhou, Zhejiang Province, People’s Republic of China
Abstract: Isoorientin (or homoorientin) is a flavone, which is a chemical flavonoid-like compound, and a 6-C-glucoside of luteolin. Isoorientin has been demonstrated to have anti-cancer activities against various tumors, but its effects on pancreatic cancer (PC) have not been studied in detail. In this study, we aim to investigate whether isoorientin has potential anti-PC effects and its underlying mechanism. In PC, isoorientin strongly inhibited the survival of the cells, induced cell apoptosis, and decreased its malignancy by reversing the expression of epithelial–mesenchymal transition and matrix metalloproteinase and decreased vascular endothelial growth factor expression. Meanwhile, we investigated the activity of the AMP-activated protein kinase (AMPK) signaling pathway after isoorientin treatment, which was forcefully activated by isoorientin, as expected. In addition, in the PC cells that were transfected with lentivirus to interfere with the expression of the gene PRKAA1, there were no differences in the apoptosis rate and the expression of malignancy biomarkers in the tumors of the isoorientin-treated and untreated groups. Thus, we demonstrated that isoorientin has potential antitumor effects via the AMPK signaling pathway, and isoorientin merits further investigation.
Keywords: pancreatic cancer, AMPK, isoorientin, apoptosis, invasiveness, VEGF
Corrigendum for this paper has been published
Introduction
Pancreatic cancer (PC) is one of the most common malignancies of the digestive system worldwide, and its incidence has increased over the last several decades. It is the fourth leading cause of death by cancer in the world, and its 5-year relative survival is currently 8%.1,2 The high mortality rate is caused by its aggressive biological properties, late symptom onset, and lack of specific treatments.3,4 Therefore, it is necessary to learn more about PC at the molecular level and identify a new potential therapeutic target for anticancer drugs.
In recent years, with the universal upsurge in the study of anticancer drugs, herbal medicine has generated much attention. A significant number of studies have indicated that many herbal medicines that have antitumor effects can be separated into several compounds, including isoorientin.5–7 Isoorientin (or homoorientin) is a flavone, which is a chemical flavonoid-like compound, and a 6-C-glucoside of luteolin. Bioassay-directed fractionation techniques led to the isolation of isoorientin as the main hypoglycemic component in Gentiana olivieri.8 Our team confirmed that luteolin has a strong anti-cancer effect in PC.9 The effects of isoorientin, as a 6-C-glucoside of luteolin, on cancer need to be identified. With many biological activities and therapeutic effects, including anti-inflammatory, antidiabetic, antioxidant, proapoptotic, and autophagy-inducing effects, we believe that isoorientin has the potential to treat inflammatory and neoplastic diseases.10–14 Thus, we investigated whether isoorientin has potential antitumor effects on PC and its underlying mechanism.
AMP-activated protein kinase (AMPK) is a sensor of the cellular energy status and can be found in all types of eukaryotes, even in very primitive ones, such as Giardia lamblia.15 AMPK is a heterotrimeric complex, including a catalytic α subunit and regulatory β and γ subunits. Every subunit has 2–3 types (α1, α2, β1, β2, γ1, γ2, and γ3); hence, there are at least 12 types of AMPKs.16 Under specific circumstances, AMPK appears to have different capacities as a tumor suppressor or a tumor promoter.17–24 AMPK activators, such as metformin, phenformin, and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), exhibit antineoplastic effects on many cancers (acute myelocytic leukemia, renal cell carcinoma, breast cancer, malignant melanoma, PC, thyroid cancer, glioblastoma, colon cancer, etc.) in vivo or in vitro.25–29 It has been noted that activated AMPK inhibits cell proliferation and kills cancer cells via the induction of apoptosis. Furthermore, AMPK is required for the induction of the epithelial–mesenchymal transition (EMT), which is an important process that contributes to cancer metastasis.30 Moreover, metformin can reverse multidrug resistance in human breast cancer cells by activating AMPK.31
Although several aspects of the mechanisms of AMPK in cancer have been studied, the pathophysiological role of AMPK in PC has not been fully elucidated. In this study, our aim is to explore the medicinal benefits of isoorientin on PC and its relationship with AMPK, and our study focused on its effects on the cell proliferation, apoptosis, cell migration, and invasion of the human PC cell lines PANC-1 and PATU-8988.
Materials and methods
Reagents
Fetal bovine serum (FBS) was purchased from Sigma Chemical (St Louis, MO, USA). Roswell Park Memorial Institute (RPMI)-1640 (11875093), Dulbecco’s Modified Eagle’s Medium (DMEM), and trypsin were purchased from Gibco (Grand Island, NY, USA). The anti-AMPK (64 kDa, ab80039), anti-phospho-AMPK (64 kDa, ab133448), anti-E-cadherin (97 kDa, ab133597), anti-N-cadherin (125 kDa, ab18203), anti-VEGF (42 kDa, ab46154), anti-matrix metalloproteinase (MMP) 2 (75 kDa, ab86607), and anti-MMP9 (92 kDa, ab76003) antibodies were purchased from Abcam (Cambridge, UK). The anti-Bax (20 kDa, 5023s) and anti-Bcl-2 (26 kDa, 4223s) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The anti-GAPDH antibody (36 kDa, MB001) was purchased from Bioworld Technology (St Louis Park, MN, USA). Polyvinylidene difluoride (PVDF) membranes were purchased from Millipore (Billerica, MA, USA). Power SYBR Green PCR Master Mix was purchased from Applied Biosystems (Foster City, CA, USA), and a RevertAid First Strand cDNA Synthesis Kit was purchased from Thermo Fisher Scientific (Manassas, VA, USA). PCR primers were purchased from Generay Biotech (Shanghai, People’s Republic of China). Human VEGF enzyme-linked immunosorbent assay (ELISA) kit was purchased from NeoBioscience (Shenzhen, People’s Republic of China), and isoorientin (E-1060) was purchased from Tauto Biotech (Shanghai, People’s Republic of China).
Cell culture
The human PC cell line PANC-1 cell was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), and the human PC cell line PATU-8988 was provided by the American Type Culture Collection (Manassas, VA, USA). PANC-1 was cultured with DMEM and PATU-8988 was cultured with RPMI-1640 at 37°C with 5% CO2. Both media contained 100 U/mL penicillin, 10% FBS, and 100 μg/mL streptomycin, and it was changed every 2 days. When cells were ~80% confluent, we detached the cells with 0.25% trypsin–0.02% ethylenediaminetetraacetic acid for subculture or for the following experimental treatments.
Cell treatment
PANC-1 and PATU-8988 cells were plated into 6-cm culture dishes. When the cells reached 70%–90% confluence, isoorientin was added to the FBS-free medium for 24 hours. Then, the cells were prepared for subsequent experiments, such as extracting protein, isolating RNA, and transwell invasion assay.
Silencing the expression of the gene PRKAA1
Lentivirus vectors containing the short hairpin RNA (shRNA) of PRKAA1 (protein kinase AMP-activated catalytic subunit alpha 1, sequence: GCTTGATGCACACATGAAT) and their negative control were designed and produced by GeneChem (Shanghai, People’s Republic of China). First, PANC-1 and PATU-8988 cells were seeded in six-well plates (5×104 cells) and allowed to grow until nearly 30%–40% confluent. After the culture medium was discarded, the six-well plates were washed with phosphate-buffered saline (PBS) three times. Then, each of the plates was filled with a transfection-enhancing solution with lentivirus (2 multiplicity of infection for PANC-1 and 10 multiplicity of infection for PATU-8988) and 10 μg/mL polybrene. After 8–12 hours of incubation and transfection at 37°C with 5% CO2, we replaced the transfection medium with the common culture medium. When the cells reached 80% of the plate, the cells were harvested for passage or experiments. In all of the operations we mentioned above, the cells were processed using the proper biohazard safety equipment.
Cell viability detection using a Cell Counting Kit 8 assay
PANC-1 and PATU-8988 cells were plated onto 96-well plates. Each well contained ~5,000 cells and 200 μL of the medium with 10% FBS. When the cells of each well reached 70% confluency, the medium was changed, and FBS-free medium with different concentrations of isoorientin was added. After 24 hours, the cells were washed with PBS once, the medium containing isoorientin was discarded, and 100 μL of FBS-free medium with 10 μL of the Cell Counting Kit 8 (CCK8; Dojindo, Kumamoto, Japan) reagent were added. The cells were incubated for another 1–2 hours at 37°C, and the absorbance of each well was detected using an ELISA reader (BioTek, Winooski, VT, USA) at 490 nm based on the manufacturer’s instructions. Cell viability was expressed as the fold change of absorbance.
Apoptosis assay
Cells were plated in 6-cm culture dishes (5×105 cells per dish) and treated with isoorientin (0, 20, 40, 80, and 160 μM) for 24 hours. The cell apoptosis rate was measured using the Annexin V-FITC/PI Apoptosis Detection Kit (BD, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions. After the treatments, the fluorescence intensity was measured using a BD Accuri C6 (BD).
RNA extraction and quantitative real-time PCR analysis
After the cells were treated as described earlier, the total RNA was isolated by adding TRIzol (Ambion, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Then, the RevertAid First Strand cDNA Synthesis Kit was used to obtain cDNA from the RNA. Next, the quantitative real-time (qRT)-PCR was performed using the SYBR Green Master Mix in a 7500 Real-Time PCR System (Applied Biosystems). β-Actin was amplified as an internal standard. An analysis of the qRT-PCR data was performed using the ΔCt values. All the primer sequences are listed in Table 1.
  | Table 1 The primers used for real-time PCR |
Western blot analysis
After treatments, cells were lysed in radioimmunoprecipitation assay buffer (Beyotime, Shanghai, People’s Republic of China) containing 10% phosphatase inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) and 1% phenylmethylsulfonyl fluoride (Beyotime) for 30 minutes. Then, the cell lysate was centrifuged at 12,000×g, and the supernatant was collected. The protein concentration of each group was measured using a BCA Protein Assay Kit (Beyotime). After denaturation, 50 μg of protein for each group was then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to PVDF membranes and incubated with specific antibodies overnight at 4°C. Protein detection was performed using the enhanced chemiluminescence reagent obtained from Thermo Fisher Scientific, and the immunocomplexes were visualized using the AlphaEaseFC software.
Enzyme-linked immunosorbent assay
After the cells were treated properly, the culture medium was collected. VEGF protein involved in the culture media was directly measured by ELISA kit according to the manufacturer’s instructions (NeoBioscience). The absorbance was measured at 450 nm. The concentration of VEGF was calibrated with the VEGF standard curve.
Transwell migration and invasion assay
According to the manufacturer’s protocol, transwell inserts (Corning, Franklin Lakes, NJ, USA) with 8-μm pore membrane filters were used for the migration and invasion assay. Briefly, Matrigel Basement Membrane Matrix (Corning) was laid in the upper chambers to test for invasion, but not for migration. A volume of 500–600 μL RPMI-1640 (for PATU-8988) or DMEM (for PANC-1) with 10% FBS as a chemoattractant was added to the lower compartment. After the cells were starved in FBS-free medium overnight, the cells (migration: 5×103, invasion: 5×104) were placed in the upper compartment (FBS-free medium) and incubated for 24 hours. Then, we gently scrubbed off the cells on the upper surface with a cotton swab, washed the filters with PBS three times, and then fixed the cells with 4% paraformaldehyde for 20 minutes. Subsequently, the cells were stained with a 0.05% crystal violet solution, followed by three washes with PBS. Then, the inserts were allowed to dry at room temperature, and the membranes were photographed under an inverted microscope.
Statistical analysis
The data were analyzed using the SPSS 19.0 software (IBM, Armonk, New York, USA). The results are expressed as the mean ± SEM of at least three independent experiments. When P<0.05, differences were considered to be statistically significant.
Results
Isoorientin inhibits cell proliferation
At the beginning of our study, we performed a CCK8 assay to assess whether isoorientin affects the cell viability of PANC-1 and PATU-8988 cells. Both cell lines were grown for 24 hours in the presence of isoorientin (0, 20, 40, 80, and 160 μM), and a CCK8 solution was added. We found that the cell viability decreased significantly at the concentrations of 20, 40, 80, and 160 μM (Figure 1A). Then, we performed a CCK8 assay in the shRNA-interfering group. The cell proliferation in the isoorientin-treated group was not significantly different from that in the untreated group in both the PANC-1 and PATU-8988 cell lines (Figure 1B).
AMPK is constitutively activated by isoorientin
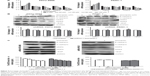
After the cells were cultured with isoorientin (0, 20, 40, 80, and 160 μM for PANC-1; 0, 20, 40, 80, 160, and 320 μM for PATU-8988) for 24 hours, we assessed the expression of p-AMPK and AMPK by Western blotting. After the isoorientin treatment, the p-AMPK expression was increased (Figure 2A). Then, in the shRNA group, we choose the concentration of 80 μM to detect the effects of isoorientin. As shown in Figure 2B, the expression levels of AMPK and p-AMPK were much lower in the shRNA group than in the wild-type PC cells (WT) and the group that was transfected with a negative control lentivirus (NC). In the shRNA group, with or without isoorientin treatment, the expression of AMPK and p-AMPK was not different (Figure 2B).
PC cells are susceptible to isoorientin-induced apoptosis
Because AMPK is interrelated with the regulation of cell apoptosis in a large variety of cancer cells, the effect of isoorientin treatment was analyzed for up to 24 hours on the cell apoptosis rate of PANC-1 and PATU-8988 cells. Except for the cells that were cultured with or without isoorientin, all cells were treated the same way. The isoorientin-induced apoptotic response was increased in the normal PC group (Figure 3A–C). In contrast, apoptosis was not significantly different in the shRNA groups with or without isoorientin treatment (Figure 3D–F). Thus, we can conclude that the proapoptotic response of isoorientin was increased via the activation of the AMPK signaling pathway.
Isoorientin downregulates the expression of VEGF, MMPs, and the proteins involved in the EMT
Since VEGF, MMPs, and EMT are associated with changes in the survival, motility, migration, and invasion of tumors and, thus their malignancy, we evaluated whether isoorientin is able to regulate the expression of VEGF, MMPs, and EMT. The cells were treated with isoorientin (0, 20, 40, 80, and 160 μM) for 24 hours, and the expression of VEGF, EMT hallmarks (N-cadherin and E-cadherin), and MMP hallmarks (MMP2 and MMP9) was evaluated by real-time PCR assay and Western blotting. We also test the releasing VEGF level in medium by ELISA. We found that PC cells treated with isoorientin (20, 40, 80, and 160 μM) had significantly reduced expression levels of VEGF, N-cadherin, MMP2, and MMP9 and increased expression levels of E-cadherin both at the mRNA and the protein levels (Figure 4). Meanwhile, in the shRNA group, these changes were not observed (Figure 4C–E).
Isoorientin inhibits the migration and invasion of PC cells
To further evaluate the effects of isoorientin on PC cell migration and invasion, we utilized transwell migration/invasion assays. The cells were plated onto the upper chamber of the transwell system in the presence or absence of isoorientin (20, 40, 80, and 160 μM) for 24 hours. After the cells were stained with crystal violet nuclear dye, micrographs of the cells migrating onto the lower surface of the transwell membrane were obtained. Compared with the respective untreated control, the density of the migrated cells, which were stained purple, was clearly decreased in the groups treated with isoorientin (20, 40, 80, and 160 μM; Figure 5A). In the shRNA group, no significant difference was found between the isoorientin-treated and untreated groups (Figure 5B).
Discussion
PC is considered one of the most invasive tumor diseases, and it has a very poor prognosis. The mortality rate will not decrease significantly unless new chemotherapy, radiotherapy, and biotherapy treatments emerge. It has been reported that AMPK is a potential therapeutic target for tumor treatment. AMPK is a ubiquitous and highly conserved sensor of the cellular energy status, and it regulates cellular energy homeostasis by phosphorylating various enzymes that are involved in glucose, protein, and lipid metabolism.32 It has been reported that increasing the activation of AMPK in papillary thyroid cancer cell lines leads to a conspicuous antitumor response, as measured by the inhibition of cell proliferation and migration, the induction of cell death, and the reversal of the EMT.33 Furthermore, the exposure of cancer cells to OSU-53, a novel AMPK activator, was sufficient to reverse the EMT in those cells.34 Several other AMPK activators, such as metformin, phenformin, and AICAR, have been shown to regulate cell survival and metastasis in a variety of cancer types.25–31,35 Herbal medicine has become a hot topic in the search of antitumor agents. Many studies have noted that various herbal medicines that have antitumor effects can be separated into several compounds, including isoorientin.5–7 Flavonoids, such as baicalin, quercetin, luteolin, and epigallocatechin, have been shown to activate the AMPK signaling pathway.36,37 Here, we hypothesized that, as a flavone, isoorientin could induce cancer cell death and lower tumor malignancy via the AMPK signaling pathway. In this study, we choose the PANC-1 and PATU-8988 cell lines to test the antitumor capacity of isoorientin. We found that isoorientin can lower the malignancy of PC cells, at least partly, by activating the AMPK signaling pathway, further demonstrating isoorientin’s potential as a new therapeutic agent for PC.
Apoptosis plays a vital role in the progress of cell proliferation, differentiation, senescence, and death. Previous studies have concluded that BCL-2 and BAX control programmed cell death (apoptosis).38–41 Therefore, we determined the levels of BCL-2 and BAX after isoorientin treatment and found that isoorientin effectively inhibited the expression of BCL-2, whereas a contrary trend was observed in the expression of BAX. To further examine the apoptotic effect of isoorientin, we examined the apoptosis rates by flow cytometry.
To some extent, the biological behaviors of cancer cell malignancy are invasiveness and high vascularization. EMT is a biological process in which epithelial cells transform into special cells with mesenchymal phenotypes, and these cells can be isolated during cancer metastasis. Previous studies have found that changes in the EMT are often followed with increased expression of MMPs.42,43 Thus, we determined the levels of E-cadherin, N-cadherin, MMP2, and MMP9 after isoorientin treatment and found that isoorientin efficaciously inhibited the expression of N-cadherin, MMP2, and MMP9, while the level of E-cadherin was increased. The EMT and the degradation of the extracellular matrix make cancer cell migration possible.44,45 We performed a transwell invasion and migration assay to further validate the inhibitory effect of isoorientin on cancer cell invasiveness in vitro. VEGF is one of the most effective proangiogenic growth factors. The inhibition of VEGF can suppress tumor growth in vivo.46 Moreover, VEGF can promote the migration and invasion capacity of tumor cells, resulting in an increase in the degree of the tumor cell malignancy.47,48 In our experiments, VEGF was significantly reduced by isoorientin treatment in PC cell lines, both at the mRNA and protein levels. All the data demonstrated that isoorientin can decrease the malignancy of PC. More importantly, in this study, we observed that the phosphorylation of AMPK was accompanied by the upregulation of E-cadherin and the downregulation of VEGF, N-cadherin, and MMPs, similar to apoptosis. This suggested that isoorientin lowers malignancy and induces the apoptosis of PC cell through the activation of the AMPK signaling pathway.
Although the antitumor effects of AMPK were tested in our experiments, the specific mechanism by which AMPK regulates cell apoptosis, migration, and invasion was not fully demonstrated in this article. In many reports, it has been confirmed that mTOR, which is negatively regulated by AMPK, is involved in cancer cell death.49,50 Similarly, the tumor suppressors TSC251–54 and p5354–57 are downstream targets of AMPK activity. Intriguingly, a survey of the literature available shows that the activation of AMPK is a necessary but not sufficient condition for the induction of the EMT.30 Altogether, it is certain that the relationship of isoorientin with the antitumor effect of AMPK and their relationship with other signaling pathways are very complex and need to be further investigated.
In conclusion, our study shows that isoorientin can inhibit the expression of VEGF, VEGF, N-cadherin, MMP2, MMP9, and BAX and promote the expression of E-cadherin and BCL-2 via the AMPK signaling pathway. It provides a new role for isoorientin, a flavone, as an anticancer drug. We expect that isoorientin will be a new therapeutic strategy that targets apoptosis, migration, invasion, and angiogenesis.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (Nos 81370563 and 81570583), the Outstanding Youth Fund of Zhejiang Province (No LR14H30001), and the Health and Family Planning Commission of Zhejiang Province (2013ZDA014).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Antoniou G, Kountourakis P, Papadimitriou K, Vassiliou V, Papamichael D. Adjuvant therapy for resectable pancreatic adenocarcinoma: Review of the current treatment approaches and future directions. Cancer Treat Rev. 2014;40(1):78–85. | ||
Pliarchopoulou K, Pectasides D. Pancreatic cancer: current and future treatment strategies. Cancer Treat Rev. 2009;35(5):431–436. | ||
Conforti F, Menichini F, Rigano D, Senatore F. Antiproliferative activity on human cancer cell lines after treatment with polyphenolic compounds isolated from Iris pseudopumila flowers and rhizomes. Z Naturforsch C. 2009;64(7–8):490–494. | ||
Li L, Henry GE, Seeram NP. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem. 2009;57(16):7282–7287. | ||
Rashed KN, AĆ, Glamočlija J, Calhelha RC, Ferreira IC, Soković M. Antimicrobial activity, growth inhibition of human tumour cell lines, and phytochemical characterization of the hydromethanolic extract obtained from Sapindus saponaria L. aerial parts. Biomed Res Int. 2013;2013(4):659183. | ||
Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76(11):1223–1238. | ||
Huang X, Dai S, Dai J, et al. Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. Onco Ther. 2015;8:2989–3001. | ||
Lee W, Ku SK, Bae JS. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vascul Pharmacol. 2014;62(1):3–14. | ||
Li Y, Wang J, Xiao H, Wu W, Wang Y, Liu X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem Toxicol. 2013;53(3):62–68. | ||
Ju HL, Park HS, Choi JK, Lee IS, Choi HJ. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Arch Pharm Res. 2007;30(12):1590–1598. | ||
Alonsocastro AJ, Zapatabustos R, Gómezespinoza G, Salazarolivo LA. Isoorientin reverts TNF-α-induced insulin resistance in adipocytes activating the insulin signaling pathway. Endocrinology. 2012;153(11):5222–5230. | ||
Yuan L, Wei S, Wang J, Liu X. Isoorientin induces apoptosis and autophagy simultaneously by reactive oxygen species (ROS)-related p53, PI3K/Akt, JNK, and p38 signaling pathways in HepG2 cancer cells. J Agric Food Chem. 2014;62(23):5390–5400. | ||
Adam RD. The Giardia lamblia genome. Int J Parasitol. 2000;30(4):475–484. | ||
Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546(1):113–120. | ||
Faubert B, Boily G, Izreig S, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–124. | ||
Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–665. | ||
Shackelford D, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2011;23(2):143–158. | ||
Shackelford DB, Verma IM, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106(27):11137–11142. | ||
Buzzai M, Bauer DE, Jones RG, et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24(26):4165–4173. | ||
Liu L, Ulbrich J, Müller J, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483(7391):608–612. | ||
Martin MJ, Hayward R, Viros A, Marais R. Metformin accelerates the growth of BRAFV600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012;2(4):344–355. | ||
Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33(2):237–247. | ||
Chen MB, Shen WX, Yang Y, Wu XY, Gu JH, Lu PH. Activation of AMP-activated protein kinase is involved in vincristine-induced cell apoptosis in B16 melanoma cell. J Cell Physiol. 2011;226(7):1915–1925. | ||
Chen MB, Zhang Y, Wei MX, et al. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013;25(10):1993–2002. | ||
Wu WD, Hu ZM, Shang MJ, et al. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int J Mol Sci. 2014;15(7):12778–12790. | ||
Sun Y, Tao C, Huang X, et al. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. Onco Ther. 2016;9:2845–2853. | ||
Lennon JC, Butini S, Campiani G, O’Meara A, Williams DC, Zisterer DM. Involvement of AMP-activated protein kinase in mediating pyrrolo-1,5-benzoxazepine–induced apoptosis in neuroblastoma cells. Invest New Drugs. 2016;34(5):1–14. | ||
Wang X, Pan X, Song J. AMP-activated protein kinase is required for induction of apoptosis and epithelial-to-mesenchymal transition. Cell Signal. 2010;22(11):1790–1797. | ||
Qu C, Zhang W, Zheng G, Zhang Z, Yin J, He Z. Metformin reverses multidrug resistance and epithelial–mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol Cell Biochem. 2014;386(2):63–71. | ||
Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Ann Rev Biochem. 1998;67(1):821–855. | ||
Cazarin JM, Coelho RG, Hecht F, Andrade BM, Carvalho DP. 5′-AMP-activated protein kinase (AMPK) regulates papillary (TPC-1 and BCPAP) thyroid cancer cell survival, migration, invasion and epithelial-to-mesenchymal transition. Thyroid. 2016;26(7):933–942. | ||
Chou CC, Lee KH, Lai IL, et al. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74(17):4783–4795. | ||
Steinberg GR, Kemp BE. AMPK in health and disease. J Clin Invest. 2002;110(110):583–590. | ||
Xi Y, Wu M, Li H, et al. Baicalin attenuates high fat diet-induced obesity and liver dysfunction: dose-response and potential role of CaMKKβ/AMPK/ACC pathway. Cell Physiol Biochem. 2015;35(6):2349–2359. | ||
Wu J, Xu X, Yi L, et al. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol. 2014;745:59–68. | ||
Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74(4):609–619. | ||
Yin XM1, Oltvai ZN, Veis-Novack DJ, Linette GP, Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cold Spring Harb Symp Quant Biol. 1994;59(3):387–393. | ||
Larsen CJ. [The BCL2 gene, prototype of a gene family that controls programmed cell death (apoptosis)]. Ann Genet. 1994;37(3):121–134. French. | ||
Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993;4(6):327–332. | ||
Asuthkar S, Nalla AK, Gondi CS, et al. Gadd45a sensitizes medulloblastoma cells to irradiation and suppresses MMP-9-mediated EMT. Neuro Oncol. 2011;13(10):1059–1073. | ||
Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–127. | ||
Lu W, Xia YH, Qu JJ, et al. p21-activated kinase 4 regulation of endometrial cancer cell migration and invasion involves the ERK1/2 pathway mediated MMP-2 secretion. Neoplasma. 2013;60(5):493–503. | ||
Xu C, Hu DM, Zhu Q. eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin Exp Metastasis. 2013;30(7):933–944. | ||
Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. | ||
García-Román J, Zentella-Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013;335(2):259–269. | ||
Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. | ||
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. | ||
He K, Zheng X, Li M, Zhang L, Yu J. mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation. Oncogene. 2016;35(2):148–157. | ||
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. | ||
Kim M, Lee JH. Identification of an AMPK phosphorylation site in drosophila TSC2 (gigas) that regulate cell growth. Int J Mol Sci. 2015;16(4):7015–7026. | ||
Park IJ, Yang WK, Nam SH, et al. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis. 2014;19(4):615–628. | ||
Agarwal S, Bell CM, Rothbart SB, Moran RG. AMP-activated protein kinase (AMPK) control of mTORC1 Is p53- and TSC2-independent in pemetrexed-treated carcinoma cells. J Biol Chem. 2015;290(46):27473–27486. | ||
Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-Dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–293. | ||
Xie SB, He XX, Yao SK. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int J Oncol. 2015;47(2):517–526. | ||
Ji HK, Lee JO, Lee SK, et al. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53–polo like kinase 2 (PLK-2) pathway. Cell Signal. 2013;25(4):805–813. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.