Back to Journals » Drug Design, Development and Therapy » Volume 16
Isoliquiritigenin Ameliorates Ischemia-Induced Myocardial Injury via Modulating the Nrf2/HO-1 Pathway in Mice
Authors Yao D , Shi B, Wang S, Bao L, Tan M, Shen H, Zhang Z, Pan X, Yang Y, Wu Y, Gong K
Received 23 February 2022
Accepted for publication 21 April 2022
Published 29 April 2022 Volume 2022:16 Pages 1273—1287
DOI https://doi.org/10.2147/DDDT.S362754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Deshan Yao,1,2 Bo Shi,3 Sichuan Wang,1,2 Liuxiang Bao,1,2 Meng Tan,1,2 Hui Shen,1,2 Zhengang Zhang,1,2 Xin Pan,1,2 Yi Yang,1,2 Yong Wu,1,2 Kaizheng Gong1,2
1Department of Cardiology, the Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, 225001, People’s Republic of China; 2Jiangsu Key Laboratory of Integrative Medicine for the Control of Geriatrics and Institute of Cardiovascular Disease, Yangzhou University, Yangzhou, 225001, People’s Republic of China; 3School of Life Science, Liaoning Normal University, Dalian, 116081, People’s Republic of China
Correspondence: Kaizheng Gong; Yong Wu, Department of Cardiology, the Affiliated Hospital of Yangzhou University, Yangzhou University, No. 368 Hanjiang Middle Road, Yangzhou, 225001, People’s Republic of China, Tel +86-514-82981199, Email [email protected]; [email protected]
Background: Oxidative stress and inflammatory reaction play critical roles in acute myocardial infarction (AMI). Isoliquiritigenin (ISL), a flavonoid monomer extracted from licorice, has been found to have antioxidant and anti-inflammatory effects in cancer studies. Here, we tested the effect and underlying mechanisms of ISL on ischemia-induced myocardial injury in a mouse AMI model.
Methods: Adult C57BL/6 mice were pre-treated by intraperitoneal injection of ISL and/or a specific nuclear factor E2-related factor 2 (Nrf2) inhibitor ML385 for 3 days, respectively. Then, the AMI model was established by ligating the anterior descending branch of the left coronary artery. Myocardial oxidative stress status, inflammatory response, cardiac function and infarction size were assessed after 7th day of surgery.
Results: Compared with sham group, the reactive oxygen species (ROS) and malondialdehyde (MDA) level in AMI group were significantly increased. However, the superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) level were dramatically decreased. ISL treatment significantly reduced the myocardial infarction area, improved cardiac function, inhibited the production of ROS and MDA and reduced the consumption of SOD and GSH-Px. Interestingly, ISL could significantly increase nuclear Nrf2 and cytosolic heme oxygenase 1 (HO-1) level in the infarcted myocardium and reduce the oxidative stress after AMI. Also, ISL treatment dramatically inhibited the activation of myocardial NF-κB pathway and reduced the expression of pro-inflammatory factors in the AMI group. However, the administration of ML385 not only suppressed the Nrf2/HO-1 activation, the anti-oxidant and anti-inflammatory effects induced by ISL, but also attenuated the beneficial role of ISL on reducing infarct size and improving cardiac function in the mouse with AMI.
Conclusion: The results suggested that activation of Nrf2/HO-1 pathway has an essential role in ISL-induced cardiac protection by alleviating myocardial oxidative stress and inflammation response in mice with AMI.
Keywords: isoliquiritigenin, acute myocardial infarction, oxidative stress, Nrf2, NF-κB
Introduction
The pathogenesis of AMI is complex and involves overproduction of reactive oxygen species (ROS), DNA damage, apoptosis, calcium overload, necrosis, mitochondrial dysfunction, and inflammatory responses.1,2 It is well known that the excessive accumulation of ROS disrupts the balance between oxidation and antioxidant system and induced oxidative stress.2–4 The increased production of free radicals formed in the process of oxidative stress can trigger lipid peroxidation and protein oxidation to an inactive state, thereby resulting in protein dysfunction, apoptosis, autophagy, mitochondrial dysfunction, endoplasmic reticulum stress, extracellular matrix deposition, and inflammatory reaction.1,5–7
Recently, accumulating evidences has shown that oxidative stress is the leading cause of the poor outcome of ischemic myocardial injury in AMI;8,9 it is an important promoter of myocardial cell death and a decline in systolic and diastolic function.10 Besides, ROS can act as second messengers to activate stress-related nuclear transcription factors in cardiomyocytes through mitogen-activated protein kinase (MAPK), Src family of tyrosine protein kinases, and cytokines, thereby leading to cell hypertrophy and apoptosis.11 Nuclear factor E2-related factor 2 (Nrf2) is an important transcription factor for cellular activation of defense against oxidative stress. When oxidative stress damage occurs, Nrf2 enters the nucleus and activates the expression of downstream target molecules such as heme oxygenase 1(HO-1) by binding to antioxidant response elements (AREs), thereby exerting antioxidant and anti-inflammatory effects.12–14 Therefore, Nrf2 has been shown a cardiac protective effect in atherosclerosis, myocardial hypertrophy, diabetic cardiomyopathy, and ischemia–reperfusion models by resisting tissue damage and myocardial remodeling.15
In recent decades, compounds derived from plants have received considerable attention in the treatment of various life-threatening diseases. Isoliquiritigenin (ISL) is a flavonoid monomer with antioxidant and anti-inflammatory activity. A previous study showed that ISL inhibited oxidative stress injury in acute pancreatitis by regulating the Nrf2/HO-1 pathway; however, the protective role of ISL in acute pancreatitis was abolished by a selective Nrf2 inhibitor, ML385.16 In a traumatic brain injury (TBI) model, ISL promoted the translocation of Nrf2 protein to the nucleus and inhibited oxidative stress induced by TBI; in Nrf2-knockout mice, TBI-induced oxidative stress was further aggravated, and ISL treatment could not alleviate oxidative stress damage.17 Additionally, ISL exerted anti-inflammatory and antioxidant protective effects in a streptozotocin-induced diabetic cardiomyopathy model and a diabetic nephropathy model by inhibiting the MAPK signaling pathway.18,19
However, the effect of ISL on ischemia-induced myocardial injury after AMI and the underlying mechanism remains unclear so far. Here, we hypothesized that ISL alleviates oxidative stress and inflammatory response after AMI by activation of Nrf2/HO-1 pathway. To test this hypothesis, we constructed an AMI model to test the effect of ISL on ischemia-induced myocardial injury and further explore its underlying mechanism.
Materials and Methods
Animals and Drugs
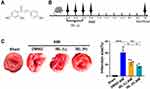
Male C57BL6J mice aged 8–10 weeks, weighing 23–25 g, were purchased from the Institute of Comparative Medicine, Yangzhou University, license number SCXK (Su) 2017-0007. All laboratory procedures and animal care were approved by the Yangzhou University Ethics Committee (No. 202111013) and were conducted in accordance with the guidelines of the European Parliament directive 2010/63/EU on the protection of animals used for scientific purposes. During the experiment, the mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and then killed by dislocation of the cervical spine, and the heart was quickly obtained. All of the mice were exposed to a specific pathogen-free (SPF) environment (12 h of light/dark cycle, constant temperature of about 25°C, and relative humidity of about 55%). During the experiment, the mice had free access to standard food and water. ISL was purchased from Aladdin (Shanghai, China). The molecular formula is C15H12O4, and the structural formula is shown in Figure 1A. In this study, ISL was used by dissolving in 5% dimethyl sulfoxide (DMSO). ML385 was purchased from Selleck (Shanghai, China).
AMI Model
The mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and were ventilated with endotracheal intubation. The thoracic cavity was opened from the fourth intercostal space; the heart was exposed; and the left anterior descending coronary artery was ligated with an 8-wire 2 mm below the left atrial appendage (the sham group only opened the chest to expose the heart without ligation). Electrocardiographic changes were observed, and ST-segment elevation was observed to determine the success of the operation.
Experimental Design and Groups
The mice were randomly assigned to either sham or surgery groups. A total of 50 mice were randomly divided into the following five groups: sham surgery group (Sham), DMSO+AMI group (DMSO MI), ISL+AMI group (ISL MI), Nrf2 inhibitor ML385+AMI group (ML385 MI), and ISL+ML385+AMI group (ISL+ML385 MI). Intraperitoneal injection of ISL (100 mg/kg/day) and (or) ML385 (30 mg/kg/day) was started 3 days before surgery. The DMSO MI group was administered an equal volume of normal saline containing equal amount of DMSO as control.
Echocardiography to Measure Cardiac Function
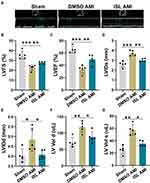
On the seventh day after myocardial infarction, B-mode and M-mode echocardiography was performed with a Vevo 770 machine (Visual Sonics Toronto, Canada). The end-diastolic left ventricular diameter (LVIDd), end-systolic left ventricular diameter (LVIDs), end-diastolic left ventricular volume (LV Vold), end-systolic left ventricular volume (LV Vols), left ventricular fractional shortening (LVFS), left ventricular ejection fraction (LVEF) were measured.
Assessment of Infarct Size
Five mice were selected from each of the groups. After anesthesia, the chest cavity was opened quickly, and the heart was irrigated with normal saline. A 2-mm-thick tissue was cut from the ligation point along the long axis of the heart to the apex of the heart, and the tissue was soaked in 1% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma, USA) and incubated at 37°C for 15 min without light. Normal myocardial tissues stained red, and myocardial tissues in the infarct area stained white. The images were taken with a digital camera, and the infarct area was calculated by ImageJ software.
ROS Staining
The apical part of the heart was collected for frozen section. The tissue was circled with a histochemical pen, and a self-fluorescence quenching agent was added into the circle for 5 min, followed by rinsing with water for 10 min. We added ROS dye drops and placed the section in a 37°C incubator for 30 min away from light. Then, the section was washed with phosphate buffer solution (PBS) three times, 5 min each time. DAPI solution was added dropwise and incubated at room temperature in the dark for 10 min. After washing three times with PBS for 5 min each time, an anti-fluorescence quenching agent was added to seal the tissue slice. A fluorescence microscope was used to acquire images.
Determination of the Levels of Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GSH-Px)
About 20 mg of cardiac tissue was added to 0.5 mL PBS, and tissue suspension was obtained using a homogenizer. After centrifugation, the pellet was discarded, and the protein content in the suspension was determined with a BCA protein quantification kit. MDA, SOD, and GSH-Px contents in each sample were detected in accordance with the kit instructions.
Hematoxylin-Eosin (HE) Staining
The myocardial tissue was immersed in 4% paraformaldehyde solution for 24 h and then embedded in paraffin. Slices with a thickness of 5 μm were dewaxed, dehydrated, and stained in accordance with the staining procedure of the modified HE staining kit (Solarbio, Wuhan, China).
Real-Time RT-PCR
Ventricular RNA was extracted using TRIzol reagent (Tiangen, Beijing, China), and RNA was reverse-transcribed into cDNA using a reverse transcription kit (Thermo Scientific, USA). The list of primers is shown in Supplementary Table 1. All cytokine mRNA levels were normalized to GAPDH mRNA.
Western Blot Analysis
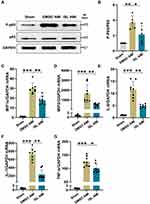
Left ventricular tissue proteins were extracted with RIPA lysis buffer (Beyotime, Shanghai, China). Cardiac tissue nuclear proteins were extracted using nuclear and cytoplasmic extraction reagents (Thermo Scientific, USA). Proteins were separated by SDS-PAGE gel, transferred to PVDF membranes (Millipore, Billerica, MA), and sealed with 5% nonfat milk. The membranes were then blotted with primary antibodies diluted in 5% nonfat milk-TBST buffer. Antibodies used in this study were Nrf2, HO-1 (Abcam, USA), p-IKKα/β, IKKα, IKKβ, p-P65, P65, p-IκBα, IκBα (Cell Signaling, USA), TBP (Absin, China), and GAPDH (Santa Cruz, USA). This was followed by incubation with an HRP-conjugated secondary antibody (Cell Signaling, USA). The bands were visualized using ECL Chemiluminescence solution (Thermo Scientific, USA). Grayscale measurements were performed using ImageJ software.
Statistical Analysis
SPSS20.0 software was used for data analysis, and the data were expressed as mean ± standard deviation (mean ± SD). The Student–Newman–Keuls test was used for comparison between the two groups, and one-way ANOVA was used for comparison between multiple groups. P < 0.05 was considered statistically significant.
Result
ISL Reduces Infarct Size and Improves Cardiac Function After AMI
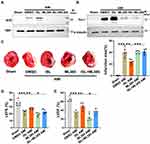
To test the effect of ISL on cardiac function after AMI in mice, different concentrations of ISL (30 mg/kg/day, 100 mg/kg/day) were intraperitoneally injected 3 days before surgery (Figure 1B). The AMI model was established by ligating the left anterior descending coronary artery, and the ECG monitoring of ST-segment elevation confirmed the success of the operation (Supplementary Figure 1A). Seven days after the surgery, significant myocardial infarction occurred in the AMI group, as revealed by TTC staining. High-dose ISL treatment significantly diminished the size of myocardial infarction (Figure 1C). AMI caused severe tissue damage and inflammatory cell infiltration, as revealed by HE staining; however, after ISL treatment, tissue damage was alleviated and inflammatory cell infiltration decreased, especially in high ISL doses (Supplementary Figure 1B).
According to TTC staining and HE staining results, we selected high-dose ISL (100 mg/kg/day) for the subsequent experiments. Echocardiographic results (Figure 2A) showed that the left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were lower in the AMI group than that in the Sham group (Figure 2B and C). AMI caused cardiac pathological changes, including increased LVID and LV Vol (Figure 2D–G). However, ISL treatment significantly improved left ventricular dysfunction in AMI mice, as reflected by increased LVFS and LVEF and decreased LVID and LV Volume (Figure 2B–G). These results suggest that ISL can reduce myocardial infarction size and restore cardiac dysfunction in AMI.
ISL Treatment Alleviates Oxidative Stress After AMI
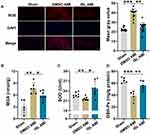
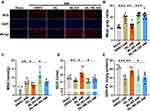
Next, we tested the effect of ISL treatment on AMI-induced oxidative stress. The content of ROS in myocardial tissue was detected using DHE fluorescent probe;20 we found that the positive staining of ROS was very weak in normal myocardial tissue, while it was significantly increased after AMI (Figure 3A). With ISL treatment, the level of ROS in mouse myocardial tissue was decreased significantly.
The damaged tissue released a large amount of ROS. This process was accompanied by an increase in MDA production and a decrease in SOD and total GSH-Px levels, indicators of the cellular reduction–oxidation context. Therefore, we detected the content of MDA, SOD, and GSH-Px in mouse hearts (Figure 3B–D). In the AMI model, the production of MDA increased, while the contents of SOD and GSH-Px distinctly decreased. However, after ISL treatment, the production of MDA was obviously reduced, and the contents of GSH-Px and SOD were visibly increased. These results indicate that ISL has an inhibitory effect on AMI-induced oxidative stress.
ISL Treatment Alleviates Myocardial Inflammation Induced by AMI
There is strong evidence that oxidative stress can induce inflammation during AMI.21,22 After induction of AMI in mice, the expression of p-p65 was significantly increased, as revealed by Western blot analysis (Figure 4A and B). Based on semiquantitative real-time RT-PCR analysis, the mRNA expression levels of IL-1β, IL-6, TNFα, MIP1α, and MIP2 were significantly elevated in the AMI group (Figure 4C–G). Compared with the AMI group, ISL treatment significantly suppressed the activation of p65 and reduced the mRNA expression levels of IL-6, IL-1β, TNF-α, MIP1α, and MIP2. These results indicate that ISL treatment has an anti-inflammatory effect in mouse with AMI.
Nrf2 Pathway Inhibitor Restrains the Protective Effect of ISL on Mice with AMI
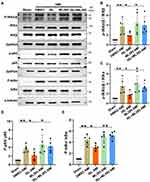
Previous studies have confirmed that Nrf2 is an important target against oxidative stress.16,23,24 Given that Nrf2 mainly enters the nucleus to exert its biological functions,16 we detected Nrf2 level in the nucleus after AMI. Western blot analysis showed that the level of Nrf2 in the nucleus and its downstream target HO-1 were increased due to oxidative stress (Figure 5A and B). After ISL treatment, both the levels of Nrf2 and HO-1 were significantly higher compared with those in the AMI group. To further explore the potential role of Nrf2 on the cardiac protection of ISL in AMI, we used a selective inhibitor of Nrf2, ML385, which has been shown to block Nrf2 binding to the promoter of its target gene in the nucleus.16 We found that ML385 significantly reduced the presence of Nrf2 in the nucleus and the expression of downstream antioxidant HO-1.
Based on the previous study,18 we used ML385 at 30 mg/kg/day to inhibit the Nrf2 activity for the function test in mice. TTC staining showed that with the inhibition of Nrf2 by ML385, the effect of ISL on reducing the size of AMI was attenuated (Figure 5C). The echocardiography results also showed that the effect of ISL on restoring cardiac function was abolished by ML385 (Figure 5D and E). Therefore, these results suggested that ISL alleviates ischemia-induced cardiac injury and restores cardiac function in AMI through modulating the Nrf2 activity.
ISL Alleviates Oxidative Stress After AMI by Activating the Nrf2 /HO-1 Pathway
Next, we tested whether the antioxidant activity of ISL could be inhibited by the specific Nrf2 inhibitor, ML385. In ROS staining (Figure 6A and B), the inhibition of Nrf2 activity by ML385 fully abolished the function of ISL and reversed the ROS production level back to that of the AMI group. In addition, as shown in Figure 6C–E, ML385 also effectively attenuated the effect of ISL on the production of MDA, the consumption of SOD and GSH PX after AMI. All these data indicate that the antioxidant activity of ISL involves the activation of the Nrf2/HO-1 signaling pathway.
ISL Alleviates Myocardial Inflammatory Response After AMI by Inhibiting the NF-κB Signaling Pathway
To further verify the detailed mechanisms by which ISL reduces myocardial inflammation response after AMI, we next tested whether Nrf2 could affect the NF-κB signaling pathway. The expression levels of p-IKKα/β, p-p65, and p-IκBα in the NF-κB signaling pathway in the myocardium were measured by Western blot analysis. As shown in Figure 7A–E, after AMI, the expression levels of p-IKKα/β, p-P65, and p-IκBα in the AMI group were significantly increased; after ISL treatment, p-IKKα/β, p-P65, and p-IκBα expression levels were significantly reduced compared with DMSO treatment. After inhibiting Nrf2 by ML385, the effect of ISL was inhibited, and the expression levels of p-IKKα/β, p-P65, and p-IκBα again increased. Also, the real-time RT-PCR analysis showed that the expression levels of IL-6, IL-1β, TNFα, MIP1α, and MIP2 significantly increased after AMI, but these inflammatory factors and chemokines significantly decreased after ISL treatment. However, pretreatment of ML385 resulted into the increase of the expression levels of these inflammatory factors and chemokines compared with ISL treatment alone (Supplementary Figure 2 A–E). These results suggest that ISL attenuates the inflammatory response after AMI by inhibiting the NF-κB signaling pathway, which may be related to the activity of Nrf2.
Discussion
ISL has been extensively studied in multiple pathological processes, such as diabetic cardiomyopathy23 and intracerebral hemorrhage.25 The main function of ISL is to counteract oxidative stress and inflammation.24,26 Since oxidative stress and inflammation play essential roles in the pathophysiology of the acute phase of AMI, inhibiting them might be beneficial;5,27,28 thus, we tested the effect of ISL in the treatment of mouse with AMI. We found that the dose of 100 mg/kg/day of ISL could reduce the myocardial infarction size and restore cardiac function. Further experiments showed that ISL activated the Nrf2/HO-1 signaling pathway to inhibit oxidative stress caused by AMI. Besides, ischemia-induced myocardial inflammatory response after AMI was alleviated by ISL treatment, which is characterized by the inhibition of the activation of NF-κB pathway and the reduced expression of the related pro-inflammatory factors (IL-1, IL-6, TNF-α) and chemokines (MIP1α, MIP2). When Nrf2 was specifically inhibited by ML385, the cardiac protective effect of ISL on oxidative stress and inflammatory response were abolished in AMI mice. Collectively, the results suggested that ISL has potential therapeutic effects on ischemia-induced oxidative stress and inflammatory responses, mainly by regulating the Nrf2/HO-1 and NF-κB pathways.
Nrf2 is a vital transcription factor that regulates the levels of antioxidant enzymes in cells.16,24,26 In normal conditions, cytoplasmic Nrf2 content is strictly regulated by Keap1 (redox-sensitive E3 ubiquitin ligase substrate adapter).29 Two Keap1 molecules bind to one Nrf2 to regulate the continuous ubiquitination and degradation. Under Keap1 control, cytoplasmic Nrf2 has a short half-life of 10 to 30 min, and nuclear Nrf2 is undetectable. Therefore, Keap1-mediated Nrf2 level in the nucleus is at an extremely low basal level in normal conditions.30 During oxidative stress, Keap1 is oxidized and inactivated, which leads to Nrf2 stabilization and translocation into the nucleus. In the nucleus, Nrf2 can bind to sMaf and form a complex, which binds to AREs in the promoter region of Nrf2 target gene through a sequence-specific manner to promote the transcription of downstream antioxidants such as HO-1.31 Our experiments showed that ISL could effectively increase the level of Nrf2 in the nucleus and lead to the up-regulation of transcription of the downstream antioxidant HO-1 in the AMI mouse model. ML385, a specific inhibitor of Nrf2, can selectively block the binding of Nrf2–sMaf complex to the ARE sequence in the promoter and reduce the transcriptional activity of Nrf2 (summarized in Figure 8).32 The main mechanism by which ISL can treat AMI involves the increased entry of Nrf2 into the nucleus, which promotes the transcription of the downstream antioxidant HO-1. This is the core function of ISL in AMI treatment. With the reduction of oxidative stress injury, a series of pathological impairments, such as inflammatory response, calcium imbalance, apoptosis, and necrosis, might be improved.33–35
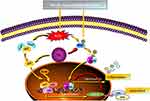 |
Figure 8 Schematic representation of the role of ISL in response to AMI-induced oxidative stress and inflammation. |
In an early acute stage of AMI, oxidative stress and inflammation are closely coupled to each other, and they can promote each other in the pathological process.36 Excessive inflammatory mediators aggravate myocardial cellular damage and increase the production of mitochondrial ROS in myocardial cells.37 Previous studies have demonstrated that mitochondria are not only cellular energy factories, but the key triggers of cardiac ischemic injury.38 Compared with the cells in other organs, cardiomyocytes have more mitochondria, which provide energy for cellular contraction and relaxation.39 Mitochondria are the main organelles of cardiomyocytes that respond to various stresses during ischemic pathological process. Under ischemic stress conditions, the mitochondrial quality control (MQC) system is activated to restore mitochondrial homeostasis by altering the coordination of processes such as mitochondrial redox reactions, mitochondrial fission, mitochondrial fusion, mitophagy, and bioenergetics, ensuring that cardiomyocyte survival and cardiac function after ischemic myocardial injury.40,41 Mitochondrial dysfunction can release a large number of ROS and trigger or enhance multiple pathological processes such as calcium overload, oxidative stress, endoplasmic reticulum stress, and immune responses.38 The release of ROS from mitochondria into the cytoplasm further increases ROS production through multiple mechanisms.37 Large amounts of ROS disrupt the structure and function of macromolecules (nucleic acids, proteins, lipids), and these oxidative modifications can generate new “oxidation-specific epitopes” (OSEs) that induce proinflammatory responses in vitro and in vivo, thereby exacerbating inflammatory response.42,43 In summary, high oxidative stress can induce a high inflammatory response, and high inflammatory response can amplify oxidative stress.
In this study, ISL markedly inhibited oxidative stress through Nrf2 regulation; at the same time, inflammation was also well inhibited. One of the main mechanisms is that the up-regulation of Nrf2 pathway can inhibit NF-κB-mediated inflammation response in AMI. Previous studies have demonstrated a strong link between Nrf2 and NF-κB signal transduction,44 in which HO-1 is the target gene of Nrf2 and is the core of Nrf2-mediated inhibition of NF-κB activation. For example, in endothelial cells, increased HO-1 expression inhibited NF-κB-mediated transcription of adhesion molecule.45 Therefore, our findings in AMI are consistent with previous studies in other fields. In the present study, we demonstrated that ISL inhibited NF-κB-related inflammation by regulating the Nrf2/HO-1 pathway in mouse with AMI. Notably, several studies have reported that ISL could also inhibit inflammatory responses by inhibiting the activation of NLRP3 inflammasome in different models.26,46,47 Therefore, Nrf2 and NF-κB pathway may be one of the main inflammatory regulators in ISL treatment for AMI in the early acute stage. We could not exclude cross-talking with other pathways, which could be effective to control inflammation response in different conditions.
In the early stage of myocardial infarction, oxidative stress and inflammatory reaction are the essential elements.48,49 With the development of AMI, various features such as myocardial fibrosis, apoptosis, necrosis, angiogenesis, and scar formation can also affect cardiac prognosis and further aggravate cardiac dysfunction.48,50,51 Therefore, our study confirmed that ISL had potential cardiac protective effects in the early acute stage of AMI and alleviated ischemia-induced oxidative stress and inflammation, mainly by up-regulating Nrf2/HO-1 pathway and inhibiting NF-κB pathway activation. However, with respect to the long-term benefit of ISL treatment for myocardial infarction, other pathogenic processes such as fibrosis, apoptosis, necrosis, and arrhythmia also need to be considered in the future.
In conclusion, the results suggest that activation of Nrf2/HO-1 pathway has an essential role in ISL-induced cardiac protection by alleviating myocardial oxidative stress and inflammation response in mice with AMI.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was partly supported by the National Natural Science Foundation of China (81770262, 82000257 and 81970225), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (20KJB320006), the Social Development Project of Yangzhou City (YZ2020087), the Project of “333” Jiangsu Province.
Disclosure
The research was conducted in the absence of any business or financial relationships that could be construed as potential conflicts of interest.
References
1. Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 2019;125:496–502. doi:10.1016/j.ijbiomac.2018.11.190
2. Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovacs Res. 2009;81(3):457–464. doi:10.1093/cvr/cvn335
3. Hawkins CL, Davies MJ. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem. 2019;294(51):19683–19708. doi:10.1074/jbc.REV119.006217
4. Zhang H, Gomez AM, Wang X, Yan Y, Zheng M, Cheng H. ROS regulation of microdomain Ca (2+) signalling at the dyads. Cardiovacs Res. 2013;98(2):248–258. doi:10.1093/cvr/cvt050
5. Neri M, Fineschi V, Di Paolo M, et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13(1):26–36. doi:10.2174/15701611113119990003
6. Maldonado E, Rojas DA, Urbina F, Solari A. The oxidative stress and chronic inflammatory process in Chagas disease: role of exosomes and contributing genetic factors. Oxid Med Cell Longev. 2021;2021:4993452. doi:10.1155/2021/4993452
7. Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10(2):509–547.
8. Lei Q, Yi T, Chen C. NF-kappaB-Gasdermin D (GSDMD) axis couples oxidative stress and nacht, lrr and pyd domains-containing protein 3 (nlrp3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–6052. doi:10.12659/MSM.908529
9. Bezerra OC, Franca CM, Rocha JA, et al. Cholinergic stimulation improves oxidative stress and inflammation in experimental myocardial infarction. Sci Rep. 2017;7(1):13687. doi:10.1038/s41598-017-14021-8
10. Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56(25):2115–2125. doi:10.1016/j.jacc.2010.07.033
11. Fuentes E, Moore-Carrasco R, de Andrade PA, Trostchansky A. Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J Cardiovasc Pharmacol Ther. 2019;24(6):509–520. doi:10.1177/1074248419861437
12. Tanaka M, Kishimoto Y, Sasaki M, et al. Terminalia bellirica (Gaertn.) Roxb. extract and gallic acid attenuate lps-induced inflammation and oxidative stress via MAPK/NF- κ B and Akt/AMPK/Nrf2 pathways. Oxid Med Cell Longev. 2018;2018:1–15. doi:10.1155/2018/9364364
13. Han L, Chen A, Liu L, Wang F. Leonurine preconditioning attenuates ischemic acute kidney injury in rats by promoting Nrf2 nuclear translocation and suppressing TLR4/NF-kappaB pathway. Chem Pharm Bull. 2022;70(1):66–73. doi:10.1248/cpb.c21-00740
14. Huang CY, Deng JS, Huang WC, Jiang WP, Huang GJ. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients. 2020;12(6):6. doi:10.3390/nu12061742
15. Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50(2):77–97. doi:10.1152/physiolgenomics.00041.2017
16. Liu X, Zhu Q, Zhang M, et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2018;2018:1–12. doi:10.1155/2018/7161592
17. Zhang M, Huang L, Teng C, et al. Isoliquiritigenin provides protection and attenuates oxidative stress-induced injuries via the Nrf2-ARE signaling pathway after traumatic brain injury (vol 43, pg 2435, 2018). Neurochem Res. 2019;44(2):510–511. doi:10.1007/s11064-019-02718-3
18. Huang X, Shi Y, Chen H, et al. Isoliquiritigenin prevents hyperglycemia-induced renal injuries by inhibiting inflammation and oxidative stress via SIRT1-dependent mechanism. Cell Death Dis. 2020;11(12):1040. doi:10.1038/s41419-020-03260-9
19. Alshehri AS, El-Kott AF, Eleawa SM, et al. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J Physiol Pharmacol. 2021;72:3.
20. Wang J, Huang J, Wang L, et al. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J Thorac Dis. 2017;9(11):4398–4412. doi:10.21037/jtd.2017.09.135
21. Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007;334(3):197–205. doi:10.1097/MAJ.0b013e318157388f
22. Huang J, Shen H, Jiang M, Huang L, Yuan Y, Wang Q. Calycosin reduces infarct size, oxidative stress and preserve heart function in isoproterenol-induced myocardial infarction model. Pak J Pharm Sci. 2020;33:1341–1347.
23. Alzahrani S, Said E, Ajwah SM, et al. Isoliquiritigenin attenuates inflammation and modulates Nrf2/caspase-3 signalling in STZ-induced aortic injury. J Physiol Pharmacol. 2021;73(2):193–205.
24. Yu D, Liu X, Zhang G, Ming Z, Wang T. Isoliquiritigenin inhibits cigarette smoke-induced COPD by attenuating inflammation and oxidative stress via the regulation of the Nrf2 and NF-kappaB signaling pathways. Front Pharmacol. 2018;9:1001. doi:10.3389/fphar.2018.01001
25. Zeng J, Chen Y, Ding R, et al. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-kappaB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation. 2017;14(1):119. doi:10.1186/s12974-017-0895-5
26. Wang L, Wang X, Kong L, et al. Isoliquiritigenin alleviates LPS/ D-GalN-induced acute liver failure by activating the PGC-1alpha/ Nrf2 pathway to reduce oxidative stress and inflammatory response. Int Immunopharmacol. 2021;100:108159. doi:10.1016/j.intimp.2021.108159
27. Xie S, Deng W, Chen J, et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. 2020;16(1):12–26. doi:10.7150/ijbs.37269
28. Donnarumma E, Trivedi RK, Lefer DJ. Protective actions of H2S in acute myocardial infarction and heart failure. Compr Phtsiol. 2017;7(2):583–602.
29. Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi:10.1101/gad.13.1.76
30. Yates MS, Tran QT, Dolan PM, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30(6):1024–1031. doi:10.1093/carcin/bgp100
31. Baird L, Swift S, Lleres D, Dinkova-Kostova AT. Monitoring Keap1-Nrf2 interactions in single live cells. Biotechnol Adv. 2014;32(6):1133–1144. doi:10.1016/j.biotechadv.2014.03.004
32. Singh A, Venkannagari S, Oh KH, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. Acs Chem Biol. 2016;11(11):3214–3225. doi:10.1021/acschembio.6b00651
33. Periyasamy P, Shinohara T. Age-related cataracts: role of unfolded protein response, Ca (2+) mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog Retin Eye Res. 2017;60:1–19. doi:10.1016/j.preteyeres.2017.08.003
34. Ogura S, Shimosawa T. Oxidative stress and organ damages. Curr Hypertens Rep. 2014;16(8):452. doi:10.1007/s11906-014-0452-x
35. Yu J, Wang WN, Matei N, et al. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid Med Cell Longev. 2020;2020:4717258. doi:10.1155/2020/4717258
36. Garcia N, Zazueta C, Aguilera-Aguirre L. Oxidative stress and inflammation in cardiovascular disease. Oxid Med Cell Longev. 2017;2017:5853238. doi:10.1155/2017/5853238
37. Aimo A, Castiglione V, Borrelli C, et al. Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510. doi:10.1177/2047487319870344
38. Wang J, Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm Sin B. 2020;10(10):1866–1879. doi:10.1016/j.apsb.2020.03.004
39. Zhou H, Ren J, Toan S, Mui D. Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev. 2021;66:101250. doi:10.1016/j.arr.2020.101250
40. Tan Y, Mui D, Toan S, Zhu P, Li R, Zhou H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol Ther Nucleic Acids. 2020;22:696–707. doi:10.1016/j.omtn.2020.09.013
41. Zhu H, Tan Y, Du W, et al. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021;38:101777. doi:10.1016/j.redox.2020.101777
42. Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc Med. 2001;11(3–4):142–147. doi:10.1016/S1050-1738(01)00098-6
43. Aimo A, Borrelli C, Vergaro G, et al. Targeting mitochondrial dysfunction in chronic heart failure: current evidence and potential approaches. Curr Pharm Des. 2016;22(31):4807–4822. doi:10.2174/1381612822666160701075027
44. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc Trans. 2015;43(4):621–626. doi:10.1042/BST20150014
45. Seldon MP, Silva G, Pejanovic N, et al. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J Immunol. 2007;179(11):7840–7851. doi:10.4049/jimmunol.179.11.7840
46. Gnanaguru G, Choi AR, Amarnani D, D’Amore PA. Oxidized lipoprotein uptake through the CD36 receptor activates the NLRP3 Inflammasome in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2016;57(11):4704–4712. doi:10.1167/iovs.15-18663
47. Honda H, Nagai Y, Matsunaga T, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 2014;96(6):1087–1100. doi:10.1189/jlb.3A0114-005RR
48. Ong SB, Hernandez-Resendiz S, Crespo-Avilan GE, et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. doi:10.1016/j.pharmthera.2018.01.001
49. Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116(6):1101–1112. doi:10.1093/cvr/cvz336
50. Curley D, Lavin PB, Shah AM, Botnar RM. Molecular imaging of cardiac remodelling after myocardial infarction. Basic Res Cardiol. 2018;113(2):10. doi:10.1007/s00395-018-0668-z
51. Lucas A, Mialet-Perez J, Daviaud D, Parini A, Marber MS, Sicard P. Gadd45gamma regulates cardiomyocyte death and post-myocardial infarction left ventricular remodelling. Cardiovasc Res. 2015;108(2):254–267. doi:10.1093/cvr/cvv219
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.