Back to Journals » Drug Design, Development and Therapy » Volume 17
Isoliensinine Attenuates Renal Fibrosis and Inhibits TGF-β1/Smad2/3 Signaling Pathway in Spontaneously Hypertensive Rats
Authors Yao M , Lian D, Wu M, Zhou Y, Fang Y, Zhang S, Zhang W, Yang Y, Li R, Chen H, Chen Y, Shen A , Peng J
Received 12 April 2023
Accepted for publication 18 July 2023
Published 7 September 2023 Volume 2023:17 Pages 2749—2762
DOI https://doi.org/10.2147/DDDT.S414179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Mengying Yao,1– 3,* Dawei Lian,1– 3,* Meizhu Wu,1– 3 Yuting Zhou,1– 3 Yi Fang,1– 4 Siyu Zhang,1– 3 Wenqiang Zhang,1– 3 Yanyan Yang,1– 4 Renfeng Li,1– 3 Hong Chen,1– 3 Youqin Chen,5 Aling Shen,1– 4 Jun Peng1– 3
1Academy of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 2Fujian Key Laboratory of Integrative Medicine on Geriatrics, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 3Fujian Collaborative Innovation Center for Integrative Medicine in Prevention and Treatment of Major Chronic Cardiovascular Diseases, Fuzhou, Fujian, People’s Republic of China; 4Innovation and Transformation Center, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 5Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children’s Hospital, Cleveland, OH, USA
*These authors contributed equally to this work
Correspondence: Aling Shen; Jun Peng, Fujian University of Traditional Chinese Medicine, Fuzhou, 350122, People’s Republic of China, Tel +86 18050286255 ; +86 15806070011, Email [email protected]; [email protected]
Purpose: This study aimed to investigate the molecular mechanisms of isoliensinine, a kind of bibenzyl isoquinoline alkaloid which isolated from a TCM named Lotus Plumule (Nelumbo nucifera Gaertn), in treating renal interstitial fibrosis (RIF) by using RNA sequencing, KEGG analysis and in vivo experimental approaches.
Methods: Spontaneous hypertension rats (SHRs) were randomly assigned into five groups, consisting of SHR, SHR+Isoliensinine-L (2.5 mg/kg/day), SHR+Isoliensinine-M (5 mg/kg/day), SHR+Isoliensinine-H (10 mg/kg/day), and SHR+Valsartan (10 mg/kg/day) groups (n = 6 for each group). A control group of Wistar Kyoto rats (n = 6) was also included. Rats were treated intragastrically with isoliensinine, valsartan, or double-distilled water of equal volume for 10 weeks. To examine the therapeutic impact on hypertensive renal injury, fibrosis, and its underlying mechanisms, multiple techniques were employed, including hematoxylin and eosin staining, Masson trichrome staining, RNA sequencing, gene ontology (GO) function and pathway enrichment analysis and immunohistochemistry.
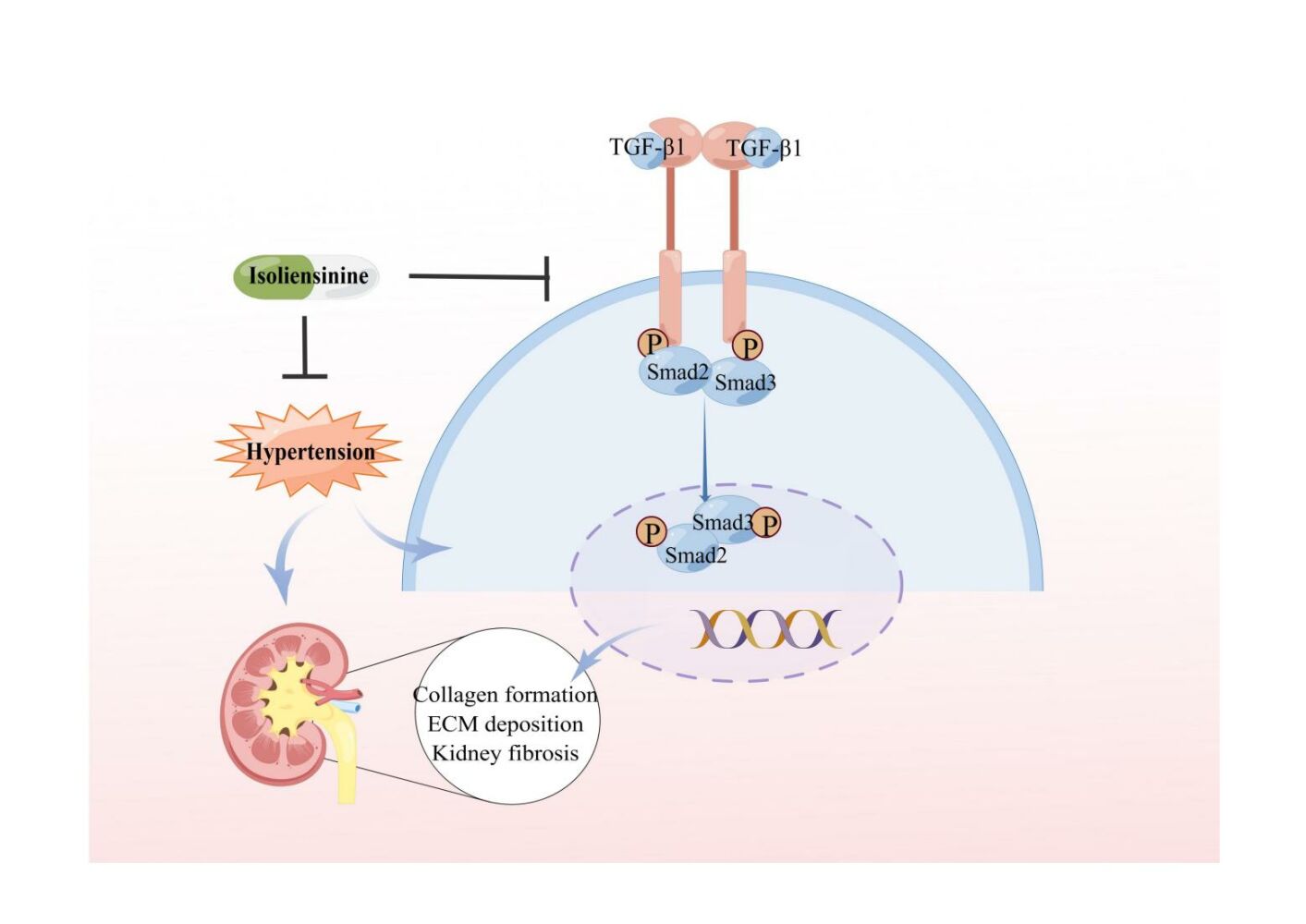
Results: Resultantly, the use of isoliensinine at different concentrations or valsartan showed significant improvement in renal pathological injury in SHRs. RNA sequencing and KEGG analysis uncovered 583 differentially expressed transcripts and pathways enriched in collagen formation and ECM–receptor interaction after treatment with isoliensinine. There was also a reduction in the increase of collagen and upregulation of collagen I & III, TGF-β 1, p-Smad2, and p-Smad3 in the renal tissue of SHRs. Thus, isoliensinine ameliorated renal injury and collagen deposition in hypertensive rats, and inhibiting the activation of the TGF-β 1/Smad2/3 pathway might be one of the underlying mechanisms.
Conclusion: This study showed that treatment with isoliensinine effectively reduced the renal injury and fibrosis in SHRs. In addition, isoliensinine inhibited the TGF-β 1/Smad2/3 signaling in-vivo. These findings provided strong evidence for the therapeutic benefits of isoliensinine in combating renal injury and fibrosis.
Keywords: isoliensinine, RNA sequencing, hypertension, renal injury, TGF-β 1/Smad2/3 pathway, collagen deposition
Graphical Abstract:
Introduction
Hypertension renal disease (HRD) is a kind of chronic kidney disease (CKD) in which the main etiology is primary hypertension.1 It affects a large number of people worldwide and has become a severe public healthcare problem.2 As the disease progresses, it will cause functional and structural damage to the kidney,3 then develop into end-stage renal disease (ERSD) and eventually lead to death4 The renal injury caused by hypertensive nephropathy is characterized by renal tubular injury, renal interstitial fibrosis, and glomerulosclerosis.5 Studies have shown that renal interstitial fibrosis (RIF) is the main complication of hypertensive nephropathy.6–8 Therefore, prevention and treatment of renal interstitial fibrosis is an important way to treat hypertensive nephropathy.9
The underlying mechanisms of renal fibrosis are complicated and are closely associated with the abnormal activation of multiple signaling pathways.10–13 Among them, the transforming growth factor beta (TGF-β1) pathway is crucial in virtually all types of fibrosis.14 Previous studies have found that TGF-β1 is up-regulated in the glomeruli and interstitium of fibrotic kidneys, which in turn can further promote the development of fibrosis.15,16 Meanwhile, a study targeting smad3 signaling downstream of TGF-β1 found that targeted deletion of smad3 alleviated fibrosis.17–19 Therefore, targeting these signaling pathways is vital for treating renal fibrosis. Meanwhile, it is essential to find drugs that can inhibit kidney fibrosis at present, most of the anti-fibrosis drugs used in clinical practice target the TGF-β1 pathway,20,21 such as humanized neutralizing monoclonal antibody against TGF-β1 (LY2382770), small oral molecules, and oral inhibitor of TGF-β type I receptor kinase.22 Although it has a specific therapeutic effect, it also has disadvantages such as high price, complex preparation, difficult storage, and side effects.23,24 Therefore, exploring more effective and more convenient treatment methods is advisable.
There have been many reports on the promising use of Traditional Chinese Medicine (TCM) and its active ingredients in the cure of hypertensive nephropathy.25–27 Lotus Plumule (Nelumbo nucifera Gaertn) is a TCM.28 Isoliensinine is a kind of bibenzyl isoquinoline alkaloid which isolated from it.29–31 It has antioxidant,32 cardioprotective33 and anti-vascular proliferation.34 A previous study indicated that isoliensinine is a naturally occurring molecule with “Drug-Like” potential.35 Our unpublished data indicated that the treatment of isoliensinine significantly lowers the elevation of blood pressure and improves the functional and pathological changes of the abdominal aorta in SHRs, suggesting the antihypertension of isoliesinine. However, the pharmacological efficacy and molecular mechanisms of isoliensinine in the therapy of hypertensive renal fibrosis have still remained unclear. Therefore, this study endeavors to examine the pharmacodynamic effects of isoliensinine on renal injury and determine its molecular mechanisms.
Materials and Methods
Reagents
Isoliesinine with a purity > 95% was purchased from Key Pharmtech Co., Ltd (Shanghai, China). Hematoxylin (Cat. no. G1140), eosin (Cat. no. G1100), and Masson staining kit (Cat no. G1340) were purchased from Solarbio Technology Co., Ltd (Beijing, China). Ultra Sensitive™ SP (Mouse/Rabbit) immunohistochemistry kit (Cat no. KIT-9720) and DAB kit (Cat no. DAB-0031), antibodies against p-Smad2 (Cat no.13429), Smad2 (Cat no. 41442), p-Smad3 (Cat no.12838), Smad3 (Cat no.41445) were purchased from SAB (College Park, Maryland, USA). Antibody against TGF-β1 (Cat no. ABP52598) was obtained from Abbkine (Wuhan, Hubei, China). Antibodies against collagen I (Cat. no. 14695-1-AP), and collagen III (Cat. no. 22734-1-AP) were obtained from Proteintech (Rosemont, IL, USA).
Animals and Experimental Protocols
A total of 30 spontaneously hypertensive rats (SHRs) and 6 Wistar Kyoto rats (WKY) were obtained from Beijing Vital River Laboratory Animal Technology Co Ltd (Beijing, China). These rats (male, 4 weeks old, weighing 96 g ± 9 g) were housed under specific pathogen-free and controlled conditions with appropriate humidity (55% ± 5%), temperature (24 °C ± 2 °C), Given a 12 hour alternating light/dark cycle and were given standard chow and water. The SHRs were divided into five groups: SHR, SHR+Isoliensinine-2.5 (2.5mg/kg/day), SHR+Isoliensinine-5 (5mg/kg/day), SHR+Isoliensinine-10 (10mg/kg/day), and SHR+Valsartan-10 (10mg/kg/day) group with 6 rats in each group. The WKY rats were used as the control group (n = 6). The rats were acclimatized for a week before the start of the experiment and the study was approved by the Ethics Committee of Fujian University of Traditional Chinese Medicine.
Hematoxylin-Eosin (H&E) Staining
The rats were sacrificed using 0.2% isoflurane, and kidneys were fixed in the pre-prepared 4% paraformaldehyde solution after 48 hours of fixation, placed in 70% alcohol, and routinely embedded with paraffin. For subsequent experiments, kidney tissues were sliced into 4-μm thick slices. Paraffin sections underwent H&E staining, and the resulting images were viewed through a 400x magnification light microscope (Leica DM6000B, Leica Microsystems, Wetzlar, Germany).
RNA Sequencing
The dichotomized renal tissues were stored in RNA later and total RNA was extracted using Trizol (Tiangen, Beijing, China). RNA integrity (RIN) was assessed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and RNA concentration was determined using the Qubit RNA Assay Kit and Qubit Fluorometer (Invitrogen, CA, USA). Samples with an RIN score of 7 or greater were selected for further experiments. RNA-seq was conducted by Capital Bio Technology (Beijing, China) as described previously,36 with data processing methods outlined in reference.37
Masson’s Trichrome Staining
The content of collagen fibers in the kidney was determined by Masson trichrome staining. The paprffinized kidney sections were dewaxized and stained with lichun red stain, phosphomolybdic acid differential stain, and aniline blue staining were performed according to the instructions. The sections were dyed and rinsed with running water and sealed with gum. To quantify kidney interstitial fibrosis, the blue pixeled content of the images was captured. Image J software was utilized to determine collagen content as the percent of positive area relative to the total area.
Immunohistochemistry of Renal Tissue
Detection of expression of related proteins by using immunohistochemicalSP kit, paraffin sections of kidney tissues of rats in each group were prepared, dewaxed gradient rehydration, microwave antigen repair was placed in citric acid buffer (pH 6.0), naturally cooled to room temperature, endogenous peroxidase was incubated for 10 min, and 1x PBS was washed for three times. Block solution was added for 60 min and then dried by rotation. Primary antibody was added and incubated overnight in a wet box at 4 °C. On the second day, after room temperature was restored, PBS was washed 3 times, and the corresponding secondary antibody labeled with horseradish peroxidase was dropped. The reaction was incubated at room temperature for 60 min, followed by PBS washing 3 times, streptomycin was incubated for 60 min, and DAB was performed for color rendering. The color developing time was controlled under microscope, and the reaction was terminated by washing with distilled water. After the nucleus was restained with hematoxylin, the tablets were washed and sealed with neutral gum, and the expression of positive marker area was calculated using Image J.
Statistical Analysis
The results were expressed as mean ± standard deviation, and the statistical analysis was conducted using SPSS Social Sciences (version 25.0, IBM, Armonk, NY, USA). To determine differences among three groups, one-way analysis of variance was employed when the data conformed to a normal distribution, and the Kruskal–Wallis test was used for non-normal distribution. Results were considered statistically significant with P-value < 0.05.
Results
Isoliensinie Alleviates Kidney Injury in Spontaneously Hypertensive Rats
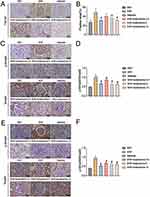
Compared with the WKY group, obvious renal damage was found in SHR group, such as abnormal glomerular structure. On the contrary, pathological condition was relieved after isoliensinine treatment (Figure 1).
Genome-Wide Gene Expression Profiling Analysis
To uncover the molecular mechanism behind isoliensinine treatment, we conducted RNA sequencing to analyze the kidney gene expression profiles in each group. The results were depicted in cluster maps (Figure 2A) and volcano maps (Figure 2B). We found that after 5 mg/kg/day doses of isoliensinine treatment, 1328 transcripts were upregulated and 1495 were downregulated, compared to the SHR group which showed 1945 transcripts upregulated and 2060 downregulated (Figures 2C). The intersection analysis between the SHR vs WKY group and the SHR + Isoliensinine vs SHR group showed that 389 genes upregulated by SHR were downregulated after isoliensinine treatment (top ranked genes: Tmlhe, Tgfbrap1, Usp45, Usp45, Pacsin3) (Table 1), while 194 genes downregulated by SHR were upregulated after isoliensinine treatment (top ranked genes: Rps27a, Erc1, Slc9a6, Tgfbrap1, Oxr1) (Table 2). Meanwhile, we have identified a number of genes associated with fibrosis, such as P4ha1,38,39 Stra6,40,41 Fubp1,42 Stat6,43,44 Myh945 (Table 3).
 |
Table 1 Details of the Top Ranked Genes Up-Regulated in the SHR Group and Down-Regulated in the Isoliensinine Groups |
 |
Table 2 Details of the Top Ranked Genes Down-Regulated in the SHR Group and Up-Regulated in the SHR + Isoliensinine Groups |
 |
Table 3 Details of Some Genes Related to Fibrosis |
Annotationally Analysis of Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Pathways
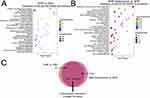
To identify potential pathways, we conducted Gene Ontology (GO) and KEGG pathway analysis on related processes and gene expression. The top 30 enriched GO terms were presented in Figures 3A and B. The results showed that the biological processes were mainly enriched for kidney development and the mRNA was mainly distributed in the cytoplasm, organelle, and nucleus as shown in the cell component analysis. The molecular function was enriched for enzyme binding, transferase activity, and molecular function regulation. Additionally, we performed KEGG pathway enrichment analysis on the DEGs identified between the SHR vs WKY group and the SHR + Isoliensinine vs SHR group to further study the relevant pathways in the mechanism of isoliensinine in hypertension treatment. The top 30 signaling pathways are depicted in Figures 4A and B. Out of the enriched pathways between the two groups, there were 222 overlapping pathways (Figure 4C). Among these, we focused on the ECM pathway and collagen formation.
Isoliensinine Ameliorates ECM Deposition
The Masson staining results indicated that the collagen fiber accumulation in the kidney of the SHR group was significantly greater compared to the WKY control group (Figures 5A and B). To assess the impact of isoliensinine on ECM and fibrosis markers, immunohistochemistry was performed on kidney tissue. The accumulation of ECM components, such as collagen I and collagen III, is a key characteristic of renal interstitial fibrosis. The immunohistochemical analysis showed that the expression of collagen I and collagen III proteins was higher in the SHR group compared to the WKY group, and every dose of isoliensinine significantly reversed this trend (Figure 5C–F). These results suggest that isoliensinine can prevent the expression of ECM components and reduce pathological collagen expression.
Isoliensinine Attenuates Renal Fibrosis in SHR by Inhibiting the TGF-β1/Smad2/3 Pathway
Due to TGF-β1/smad2/3 pathway activation and ECM overaccumulation has close relationship.46–48 Based on the RNA-seq results, we detected the TGF-β1, p-Smad2, Smad2, p-Smad3, and Smad3 expression in kidney tissues. TGF-β1, p-Smad2, and p-Smad3 were highly expressed in the SHR group and were relieved by isoliensinine intervention (Figure 6). To sum up, it appears that the TGF-β1/Smad2/3 pathway was involved in the development of renal interstitial fibrosis (RIF). Additionally, isoliensinine may have a therapeutic effect by suppressing renal fibrosis through the inhibition of the TGF-β1/Smad2/3 pathway.
Discussion
RIF is a major complication of hypertensive nephropathy49 and a common outcome of various chronic kidney diseases, contributing significantly to ESRD in many countries.50 Despite the growing number of drugs for treating renal fibrosis, their effectiveness remains inadequate, making it imperative to seek more potent pharmacological treatments in this field. Isoliensinine is a bisbenzylisoquinoline alkaloid isolated from Lotus Plumule (Nelumbo nucifera Gaertn). It has a cardiovascular protective effect. However, the protective effect of isoliensinine on the kidney has yet to be studied. Therefore, it is necessary to investigate the renoprotective effects of isoliensinine and to explore the mechanisms by which it regulates renal fibrosis.
Prior to exploring its mechanism of action, we evaluated the effect of isoliensinine on renal injury related to hypertension, the significant reduction of renal pathological changes in SHR due to the use of isoliensinine suggesting its efficacy in the alleviation of hypertension-induced renal injury. To determine the extent of renal fibrosis, we performed an analysis by using Masson staining, which suggested that renal interstitial fibrosis was occurring and was improved by isoliensinine intervention.
It is noteworthy that in spontaneously hypertensive rats, the renin–angiotensin aldosterone system (RAAS) is present,51 a system that has been shown to be closely linked to fibrosis,52,53 and the secretion of angiotensin II via the RAAS system, a multifunctional effector molecule that acts systemically via G protein-coupled receptors,54 and the binding of AngII to its type 1 receptor (AT1R) activates Smads, leading to TGF-β1 synthesis and release, and generates a positive feedback loop to amplify fibrotic TGF-β1 signalling.55,56 Thus, inhibition of TGF-β1 signaling may be one way to treat renal fibrosis caused by hypertensive nephropathy. In the progression of renal interstitial fibrosis, another hallmark of the disease is the over-accumulation of ECM proteins in both the glomerular and interstitial regions. This results in the characteristic pathological changes seen in this condition. Thus, inhibiting ECM deposition is an important way to treat renal fibrosis.57 Our in vivo results showed that isoliensinine treatment can reduce the expression of two main components of ECM, collagen I and III in renal tissue of SHRs. This indicates that isoliensinine may treat renal fibrosis by reducing the accumulation of ECM. It is well known that ECM proteins can be induced to be expressed by TGF-β1, and also directly affects the synthesis of ECM, leading to its excessive accumulation.58,59 TGF-β1, which contributes to fibrosis, plays a role in every stage of kidney fibrosis.60 As the classical pathway of TGF-β1 mediated fibrosis,61 The TGF-β1/smad2/3 pathway plays an important role. In the disease state, TGF-β1 can bind to its type II receptor TβRII and recruit TβRI, which phosphorylates smad2 and smad3,62,63 thereby promoting the development of fibrosis.64 In our study, we found that the expression levels of p-Smad2, p-Smad3 were significantly increased in the kidney tissues of SHR rats, and the expression levels of TGF-β1, p-Smad2 and p-Smad3 were greatly reduced after isoliensinine treatment. Renal fibrosis caused by hypertension involves multiple signaling pathways, including Nrf2 pathway,65 FOXO3 pathway,66 and Notch pathway,67,68 etc. However, the most classic pathway is TGF- β 1/Smad2/3 pathway.61,69 In this study, we focused on this pathway, which is not only the most widely studied pathway in fibrosis, but also based on previous studies that reported that isoliensinine can pass through TGF- β 1/Smad2/3 pathway treats Pulmonary fibrosis.70
Concurrently, we utilized RNA sequencing to examine gene transcript variances. The intersection analysis of DEGs between SHR vs WKY and SHR + Isoliensinine vs SHR demonstrated that 389 genes, which were elevated by SHR, were decreased after isoliensinine treatment. Among them, many genes are closely related to fibrosis, such as P4ha1,38,39 Stra6,40,41 Fubp1,42 Stat6,43,44 Myh9,45 These genes have been reported in liver fibrosis and renal fibrosis, respectively. Meanwhile, we found through sequencing results that isoliensinine could significantly alter the expression of these genes, but the specific regulatory mechanism of isoliensinine on these genes has not been reported. We will continue to conduct in-depth research in the future.
Limitation
Our study confirmed the TGF-β1/Smad2/3 pathway in-vivo and revealed that isoliensinine decreased its expression. Furthermore, isoliensinine either blocked the release of TGF-β1 or the expression of its downstream proteins (phosphorylated proteins). Further investigation into the mechanism of isoliensinine in regulating renal interstitial fibrosis is planned in in-vitro studies in the future.
Conclusions
This study revealed that treatment with Isoliensinine effectively reduced the renal injury and fibrosis in SHRs. In addition, we have revealed by immunohistochemistry that Isoliensinine inhibits the TGF-β1/Smad2/3 signaling in-vivo. These findings provided strong evidence for the therapeutic benefits of isoliensinine in combating renal injury and fibrosis.
Availability Statement
The following RNA sequencing supporting information can be downloaded at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE225146.
Ethics Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Fujian Traditional Chinese Medicine University (The ethics number: 2021189). The experiments were conducted in strict compliance with the People’s Republic of China Guidelines for Ethical Review of Laboratory Animal Welfare.
Author Contributions
Mengying Yao and Dawei Lian share the first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by the National Natural Science Foundation of China (82074363), the National Natural Science Foundation of China (82204662), the National Natural Science Foundation of China (U22A20372) the Science and Technology Major Project of Fujian Province (2019YZ014004), the Young Elite Scientists Sponsorship Program of the China Association of Chinese Medicine (2021-QNRC2-B19), Scientific and economic integration service platform for translational medicine of cardiovascular diseases in Fujian Province, Fuzhou (2021XRH004); the Development Fund of Chen Keji Integrative Medicine (CKJ2020003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stompór T, Perkowska-Ptasińska A. Hypertensive kidney disease: a true epidemic or rare disease? Pol Arch Intern Med. 2020;130(2):130–139. doi:10.20452/pamw.15150
2. Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(Suppl 1):A6–A7. doi:10.1053/j.ajkd.2019.09.003
3. Gusev E, Solomatina L, Zhuravleva Y, Sarapultsev A. The pathogenesis of end-stage renal disease from the standpoint of the theory of general pathological processes of inflammation. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222111453
4. Liu F, Zhuang S. New therapies for the treatment of renal fibrosis. Adv Exp Med Biol. 2019;1165:625–659. doi:10.1007/978-981-13-8871-2_31
5. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28(2):74–79. doi:10.1038/jhh.2013.55
6. Son M, Oh S, Choi J, Jang JT, Son KH, Byun K. Attenuating effects of dieckol on hypertensive nephropathy in spontaneously hypertensive rats. Int J Mol Sci. 2021;22(8). doi:10.3390/ijms22084230
7. Gu D, Fang D, Zhang M, et al. Gastrin, via activation of PPARα, protects the kidney against hypertensive injury. Clin Sci. 2021;135(2):409–427. doi:10.1042/CS20201340
8. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–340.
9. Chen X, Wei S-Y, J-S L, et al. Overexpression of heme oxygenase-1 prevents renal interstitial inflammation and fibrosis induced by unilateral ureter obstruction. PLoS One. 2016;11(1):e0147084. doi:10.1371/journal.pone.0147084
10. Zhu H, Wang X, Wang X, Liu B, Yuan Y, Zuo X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle. 2020;19(15):1941–1951. doi:10.1080/15384101.2020.1784599
11. Sun Y, Jin D, Zhang Z, et al. The critical role of the Hippo signaling pathway in kidney diseases. Front Pharmacol. 2022;13:988175. doi:10.3389/fphar.2022.988175
12. Zhang M, Chen Y, Yang M-J, et al. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-κB pathway. Phytother Res. 2019;33(4):1191–1198. doi:10.1002/ptr.6314
13. Patel S, Tang J, Overstreet JM, et al. Rac-GTPase promotes fibrotic TGF-β1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways. FASEB J. 2019;33(9):9797–9810. doi:10.1096/fj.201802489RR
14. Y-Y G, Liu X-S, Huang X-R, X-Q Y, Lan H-Y. TGF-β in renal fibrosis: triumphs and challenges. Future Med Chem. 2020;12(9):853–866. doi:10.4155/fmc-2020-0005
15. Yoshioka K, Takemura T, Murakami K, et al. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68(2):154–163.
16. Isaka Y. Targeting TGF-β signaling in kidney fibrosis. Int J Mol Sci. 2018;19(9). doi:10.3390/ijms19092532
17. Inazaki K, Kanamaru Y, Kojima Y, et al. Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int. 2004;66(2):597–604.
18. Qin W, Chung ACK, Huang XR, et al. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22(8):1462–1474. doi:10.1681/ASN.2010121308
19. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112(10):1486–1494.
20. Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21(1):104. doi:10.1186/s12943-022-01569-x
21. Li X, Ding Z, Wu Z, Xu Y, Yao H, Lin K. Targeting the TGF-β signaling pathway for fibrosis therapy: a patent review (2015–2020). Expert Opin Ther Pat. 2021;31(8):723–743. doi:10.1080/13543776.2021.1896705
22. Lee S-Y, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res. 2015;165(4):512–530. doi:10.1016/j.trsl.2014.07.010
23. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi:10.1016/S0140-6736(11)60405-4
24. Luangmonkong T, Suriguga S, Bigaeva E, et al. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol. 2017;174(18):3107–3117. doi:10.1111/bph.13945
25. Zhu Y, Chai Y, Xiao G, et al. Astragalus and its formulas as a therapeutic option for fibrotic diseases: pharmacology and mechanisms. Front Pharmacol. 2022;13:1040350. doi:10.3389/fphar.2022.1040350
26. Qin T, Wu L, Hua Q, Song Z, Pan Y, Liu T. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: a network pharmacology study and experimental validation. J Ethnopharmacol. 2020;246:112128. doi:10.1016/j.jep.2019.112128
27. Wang M, Chen D-Q, Chen L, et al. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175(13):2689–2708. doi:10.1111/bph.14333
28. Arooj M, Imran S, Inam-Ur-Raheem M, et al. Lotus seeds (Nelumbinis semen) as an emerging therapeutic seed: a comprehensive review. Food Sci Nutr. 2021;9(7):3971–3987. doi:10.1002/fsn3.2313
29. Itoh A, Saitoh T, Tani K, et al. Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Chem Pharm Bull. 2011;59(8):947–951.
30. Sharma BR, Gautam LNS, Adhikari D, Karki R. A comprehensive review on chemical profiling of Nelumbo nucifera: potential for drug development. Phytother Res. 2017;31(1). doi:10.1002/ptr.5732
31. Xie Y, Zhang Y, Zhang L-T, Zeng S-X, Guo Z-B, Zheng B-D. Protective effects of alkaloid compounds from Nelumbinis Plumula on tert-butyl hydroperoxide-induced oxidative stress. Molecules. 2013;18(9):10285–10300. doi:10.3390/molecules180910285
32. Liu Z, Hu L, Zhang Z, et al. Isoliensinine eliminates afterdepolarizations through inhibiting late sodium current and L-type calcium current. Cardiovasc Toxicol. 2021;21(1):67–78. doi:10.1007/s12012-020-09597-z
33. Qian J-Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin. 2002;23(12):1086–1092.
34. Xiao JH, Zhang YL, Feng XL, Wang JL, Qian JQ. Effects of isoliensinine on angiotensin II-induced proliferation of porcine coronary arterial smooth muscle cells. J Asian Nat Prod Res. 2006;8(3):209–216.
35. Cheng Y, H-L L, Zhou Z-W, et al. Isoliensinine: a natural compound with “Drug-Like”. Potential Front Pharmacol. 2021;12:630385. doi:10.3389/fphar.2021.630385
36. Long L, Zhang X, Wen Y, et al. Qingda granule attenuates angiotensin ii-induced renal apoptosis and activation of the p53 pathway. Front Pharmacol. 2021;12:770863. doi:10.3389/fphar.2021.770863
37. Wu M, Wu X, Cheng Y, et al. Qingda granule attenuates angiotensin II-induced blood pressure and inhibits Ca2+/ERK signaling pathway. Front Pharmacol. 2021;12:688877. doi:10.3389/fphar.2021.688877
38. Li J, Ghazwani M, Zhang Y, et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J Hepatol. 2013;58(3):522–528. doi:10.1016/j.jhep.2012.11.011
39. Zhao T, Chen H, Cheng C, et al. Liraglutide protects high-glucose-stimulated fibroblasts by activating the CD36-JNK-AP1 pathway to downregulate P4HA1. Biomed Pharmacother. 2019;118:109224. doi:10.1016/j.biopha.2019.109224
40. Hwang I, Lee EJ, Park H, Moon D, Kim H-S. Retinol from hepatic stellate cells via STRA6 induces lipogenesis on hepatocytes during fibrosis. Cell Biosci. 2021;11(1):3. doi:10.1186/s13578-020-00509-w
41. Chen C-H, L-Y K, Chan H-C, et al. Electronegative low density lipoprotein induces renal apoptosis and fibrosis: STRA6 signaling involved. J Lipid Res. 2016;57(8):1435–1446. doi:10.1194/jlr.M067215
42. Nie W, Li M, Liu B, et al. A circular RNA, circPTPN14, increases MYC transcription by interacting with FUBP1 and exacerbates renal fibrosis. Cell Mol Life Sci. 2022;79(12):595. doi:10.1007/s00018-022-04603-9
43. Li J, Yang Y, Li Q, et al. STAT6 contributes to renal fibrosis by modulating PPARα-mediated tubular fatty acid oxidation. Cell Death Dis. 2022;13(1):66. doi:10.1038/s41419-022-04515-3
44. Yang Y, Li Q, Ling Y, et al. m6A eraser FTO modulates autophagy by targeting SQSTM1/P62 in the prevention of canagliflozin against renal fibrosis. Front Immunol. 2022;13:1094556. doi:10.3389/fimmu.2022.1094556
45. Sun X, Zhu M, Chen X, Jiang X. MYH9 inhibition suppresses TGF-β1-stimulated lung fibroblast-to-myofibroblast differentiation. Front Pharmacol. 2020;11:573524. doi:10.3389/fphar.2020.573524
46. Meng X-M, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi:10.1038/nrneph.2016.48
47. Geng X-Q, Ma A, J-Z H, et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacol Sin. 2020;41(5):670–677. doi:10.1038/s41401-019-0324-7
48. Yao Y, Hu C, Song Q, et al. ADAMTS16 activates latent TGF-β, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc Res. 2020;116(5):956–969. doi:10.1093/cvr/cvz187
49. Xiao H, Liao Y, Tang C, et al. RNA-Seq analysis of potential lncRNAs and genes for the anti-renal fibrotic effect of norcantharidin. J Cell Biochem. 2019;120(10):17354–17367. doi:10.1002/jcb.28999
50. Shen Y, Miao N, Xu J, et al. Metformin prevents renal fibrosis in mice with unilateral ureteral obstruction and inhibits Ang II-induced ECM production in renal fibroblasts. Int J Mol Sci. 2016;17(2). doi:10.3390/ijms17020146
51. Gupta G, Dahiya R, Singh Y, et al. Monotherapy of RAAS blockers and mobilization of aldosterone: a mechanistic perspective study in kidney disease. Chem Biol Interact. 2020;317:108975. doi:10.1016/j.cbi.2020.108975
52. Ikeda Y, Aihara K-I, Sato T, et al. Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem. 2005;280(33):29661–29666.
53. Wong CKS, Falkenham A, Myers T, Légaré J-F. Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-β signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis. J Renin Angiotensin Aldosterone Syst. 2018;19(1):1470320318759358. doi:10.1177/1470320318759358
54. Wang N-P, Erskine J, Zhang -W-W, et al. Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II. J Renin Angiotensin Aldosterone Syst. 2017;18(2):1470320317706653. doi:10.1177/1470320317706653
55. Watson S, Burnside T, Carver W. Angiotensin II-stimulated collagen gel contraction by heart fibroblasts: role of the AT1 receptor and tyrosine kinase activity. J Cell Physiol. 1998;177(2):224–231.
56. AlQudah M, Hale TM, Czubryt MP. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020;91–92. doi:10.1016/j.matbio.2020.04.005
57. Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. doi:10.1056/NEJMra1300575
58. Zheng R, Zhu R, Li X, et al. N6-(2-Hydroxyethyl) adenosine from cordyceps cicadae ameliorates renal interstitial fibrosis and prevents inflammation via TGF-β1/Smad and NF-κB signaling pathway. Front Physiol. 2018;9:1229. doi:10.3389/fphys.2018.01229
59. Sutariya B, Jhonsa D, Saraf MN. TGF-β: the connecting link between nephropathy and fibrosis. Immunopharmacol Immunotoxicol. 2016;38(1):39–49. doi:10.3109/08923973.2015.1127382
60. Gifford CC, Tang J, Costello A, et al. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin Sci. 2021;135(2):275–303. doi:10.1042/CS20201213
61. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584.
62. Xu P, Liu J, Derynck R. Post-translational regulation of TGF-β receptor and Smad signaling. FEBS Lett. 2012;586(14):1871–1884. doi:10.1016/j.febslet.2012.05.010
63. Hata A, Chen Y-G. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8(9). doi:10.1101/cshperspect.a022061
64. Wang H, Jiang Q, Zhang L. Baicalin protects against renal interstitial fibrosis in mice by inhibiting the TGF-β/Smad signalling pathway. Pharm Biol. 2022;60(1):1407–1416. doi:10.1080/13880209.2022.2097700
65. X-T L, Song J-W, Zhang -Z-Z, et al. Sirtuin 7 mitigates renal ferroptosis, fibrosis and injury in hypertensive mice by facilitating the KLF15/Nrf2 signaling. Free Radic Biol Med. 2022;193(Pt 1):459–473. doi:10.1016/j.freeradbiomed.2022.10.320
66. Liu Y, Dong Z-J, Song J-W, et al. MicroRNA-122-5p promotes renal fibrosis and injury in spontaneously hypertensive rats by targeting FOXO3. Exp Cell Res. 2022;411(2):113017. doi:10.1016/j.yexcr.2022.113017
67. Lavoz C, Droguett A, Burgos ME, et al. Translational study of the Notch pathway in hypertensive nephropathy. Nefrologia. 2014;34(3):369–376. doi:10.3265/Nefrologia.pre2014.Jan.12436
68. Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12(7):426–439. doi:10.1038/nrneph.2016.54
69. H-H H, Chen D-Q, Wang Y-N, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi:10.1016/j.cbi.2018.07.008
70. Xiao J-H, Zhang J-H, Chen H-L, Feng X-L, Wang J-L. Inhibitory effects of isoliensinine on bleomycin-induced pulmonary fibrosis in mice. Planta Med. 2005;71(3):225–230.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.