Back to Journals » Infection and Drug Resistance » Volume 17
Isolation and Phenotypic Characterization of Virulent Bacteriophages Against Multidrug-Resistant Escherichia coli and Its Phage-Resistant Variant from Sewage Sources
Authors Fikadu A , Amankwah S , Alemu B, Alemu Y, Naga A , Tekle E , Kassa T
Received 12 October 2023
Accepted for publication 22 January 2024
Published 25 January 2024 Volume 2024:17 Pages 293—303
DOI https://doi.org/10.2147/IDR.S441085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ashetu Fikadu,1,2 Stephen Amankwah,3 Bikila Alemu,1,4 Yared Alemu,1 Adisu Naga,5 Esayas Tekle,6 Tesfaye Kassa1
1School of Medical Laboratory Sciences, Jimma University, Jimma, Ethiopia; 2Department of Medical Laboratory Sciences, Dambi Dollo University, Dambi Dollo, Ethiopia; 3Department of Medical Laboratory, Accra Medical Centre, Accra, Ghana; 4Medical Microbiology Laboratory Unit, Jimma Medical Center, Jimma, Ethiopia; 5Department of Public Health Emergency Management, Kelem Wollega Zone Health Office, Dambi Dollo, Ethiopia; 6Department of Medical Laboratory, Wollega University, Nekemte, Ethiopia
Correspondence: Tesfaye Kassa, School of Medical Laboratory Sciences, Jimma University, P. O. Box 788, Jimma, Ethiopia, Tel +251931057195, Email [email protected]; [email protected]
Purpose: The use of lytic bacteriophages for the control or elimination of pathogenic multidrug-resistant (MDR) bacteria is the promising alternative. However, the emergence of resistant bacterial variants after phage application may challenge its therapeutic benefit. In this study, we aimed to isolate candidate phages from sewage samples against two MDR Escherichia coli as well as their phage-resistant variant.
Methods: MDR E. coli isolates (n = 10) obtained from Jimma Medical Center that had been properly identified and stored were used to isolate bacteriophages. Two lytic coliphages were isolated from hospital sewage samples following standard protocols. Upon single phage infection, phage-resistant variant quickly evolved serving as a new host for the isolation of a third lytic phage. This virulent phage’s lytic activity against both its host and the wild host was investigated. The host infectivity of the various cocktails was assessed, and each phage’s biological properties were studied.
Results: Out of the first round of phage isolation process, two lytic phages were identified as VBO-E. coli 4307 and VBW-E. coli 4194. When exposed to VBO-E. coli 4307, the wild-type E. coli 4307 developed resistant variants. A third phage (VBA-E. coli 4307R) was isolated specific to this resistant variant (E. coli 4307R) under optimum condition. For VBO-E. coli 4307, VBW-E. coli 4194, and VBA-E. coli 4307R, the plaque assays generated under comparable conditions were 2.13 × 1010 PFU mL− 1, 9.17 × 1012 PFU mL− 1, and 3.3 × 1010 PFU mL− 1, respectively. These phages have nearly identical stability and lytic ability but differ greatly in their host ranges for VBA-E. coli 4307R.
Conclusion: While the wild-type MDR pathogen could easily evolve resistance when exposed to a single phage infection by VBO-E. coli 4307, it is still possible to isolate a novel bacteriophage from environmental samples that is effective against the phage-resistant variants. This indicates that it is possible to manage the effects of phage resistance pathogens even if they are MDR.
Keywords: phage, phage-resistance, E. coli, multidrug resistance, sewage, Jimma Medical Center
Introduction
Globally, antimicrobial resistance has reached an alarming stage leading to a declaration by the World Health Organization (WHO) as one of the most critical threats confronting human health.1 Recently, the spread of resistance genes has become a public health concern particularly among members of Enterobacteriaceae, with most of the cases reported are due to Escherichia coli, as causes of both community and hospital acquired infections2 such as gastrointestinal tract infection, wound infection, sepsis, neonatal meningitis, and mainly urinary tract infections (UTI).3
Besides its causation of multiple infections, E. coli is becoming an increasingly antimicrobial resistant.4 The effect of E. coli resistance is not only limited to itself but also to other related or unrelated species by the transfer of resistance genes through conjugation, transformation, and transduction by mobile genetic elements such as plasmids, transposons, and integrons.5 The report from WHO in 2019 stated that the rate of E. coli resistance to ciprofloxacin, an antibiotic commonly used to treat UTI, varied from 8.4% to 92.9%, while resistance to fluoroquinolone was also widespread, resulting in ineffectiveness in more than half of patients in different countries of the world.6 The significant increase of its resistance to the third-generation cephalosporin and aminoglycoside is exerting a great burden on healthcare systems, which complicates the management of infections.7 A systematic review on drug-resistant pathogens of UTI among pregnant women in Africa and Asia from 2005 to 2016 shows that the pooled mean resistance of E. coli to ampicillin, nalidixic acid, amoxicillin, trimethoprim-sulfamethoxazole, gentamicin, norfloxacin, nitrofurantoin, ciprofloxacin, and ceftriaxone was 77.9, 68.7, 57.2, 52.8, 50.6, 36.9, 34.7, 34 and 14.9%, respectively.8
A study conducted in Ethiopia utilizing different clinical specimens reported increased level of resistance of E. coli to trimethoprim-sulfamethoxazole (77.6%) followed by amoxicillin-clavicle acid (70.0%), norfloxacin (64.3%), and ciprofloxacin (64.0%). In addition, its resistance level to cefotaxime, cefepime, and ceftazidime was 54.8, 53.5, and 53.1%, respectively, with the lowest level of resistance observed to meropenem (3.5%) and amikacin (11.8%).9 Treating patients infected with this strain is becoming increasingly difficult due to E. coli’s growing resistance to the gram-negative bacteria’s last-resort treatment, colistin, in over 20 countries across several continents, including Europe, Asia, South America, North America, and Africa.10 Unfortunately, antibiotic resistance in E. coli is spreading at a faster rate than new antibiotics being developed. Because of this, it is critical to take into account alternative antibacterial agents with essentially distinct modes of action. Phage therapy is one of the current approaches being employed to combat the rise of AMR besides careful utilization of available antibiotics, methods to restore indigenous microbiota, and the discovery of microbiome-sparing antimicrobial therapy.11 The use of bacteriophages to treat bacterial infections dates back to the early pre-antibiotic era, after their discovery independently by Frederick Twort and Felix d’Hérelle.12 Bacteriophages (shortly phages) are viruses that infect bacterial cells, and they are the most abundant biological entities reaching an overall abundance of 1031 viral particles on earth. Every known bacterial strain in the world is thought to have at least one type of specific phage.13 Phages are more host-specific than antibiotics and so are less likely to cause collateral damage. Phages, as opposed to antibiotics, do not need to be administered repeatedly because they can multiply in the human body for extended periods of time by exploiting the bacterial host machinery.14 However, the evolution of phage-resistant variants of bacteria is a challenge encountered in the application of phage therapy even though it does not parallel with the development of bacterial resistance to antibiotics.15 The mechanisms of bacterial resistance to bacteriophage include blocking phage adsorption, inhibiting phage DNA injection, blocking phage DNA replication, the CRISPR-Cas system, and the abortive system.16 Therefore, it is essential to attempt to search for bacteriophages against bacterial strains that are resistant to phages in the same manner as the original from environmental samples. In this way, it might be ideal to guard the emergence of potential phage resistance by combining it with the original lytic phage in a cocktail form to reduce the likelihood of emergence of phage resistant bacterial strains.16 In this study, we aimed to isolate, and biophysically characterize bacteriophages against MDR E. coli. Additionally, it was intended to isolate phages with different infection strategies from an environmental sewage sample using the rapidly evolving phage-resistant E. coli variant.
Materials and Methods
Bacterial Strains and Growth Conditions
Clinical isolates of E. coli were obtained from the microbiology laboratory at Jimma Medical Center (JMC, Ethiopia). E. coli that was identified as pathogenic isolates from extra-intestinal specimens of patients such as from urine, wound abscess, blood, cerebrospinal fluid (CSF), and eye discharge and that was stored in a deep freezer at −80°C were used. Using various conventional biochemical reactions, ten isolates were re-identified as E. coli isolates after being refreshed on MacConkey agar (Oxoid Ltd, Hampshire, UK). After their multidrug resistance characteristics were examined, pure cultures were suspended in sterile 0.85% NaCl and stored at 4°C in the refrigerator until the phage assay was conducted. Table 1 displays the source and matching MDR profile of every E. coli isolate utilized in the investigation.
 |
Table 1 The Antimicrobial Resistance Profile, and Sample Source of MDR E. coli from Clinical Isolates Obtained from Jimma Medical Center |
Isolation of Bacteriophages from Sewage
Isolation of bacteriophages specific to E. coli from sewage sources (JMC) was carried out according to the standard protocol described earlier by Twest and Kropinski17 with some modifications. Briefly, sewage samples were collected in sterile 500 mL bottles and quickly transported to the medical microbiology laboratory of Jimma University in a cold box. To remove particulate matter, fifty milliliters of each hospital sewage sample was centrifuged at 10,000 rpm for 10 minutes at 4°C using a refrigerated centrifuge (Laby, Ambala, India). The supernatant was filter sterilized by passing through a 0.45 µm membrane filter (Merck Euro Lab 0.45μm PTTE, Germany) and mixed with equal volume (50 mL) of sterile double strength Nutrient Broth (Oxoid Ltd, Hampshire, UK) containing 2mM MgCl2, alongside 5 mL log phase grown host bacteria (E. coli 4307 and E. coli 4194 strains were chosen randomly and used as the host strains for phage isolation). After an overnight aerobic incubation at 37°C with frequent shaking, the lysate was centrifuged at 10,000 rpm and 4°C for 10 minutes, the supernatant was filter sterilized through 0.45 μm membrane filters and enriched for the second round with the same host strain to amplify the filtrate.
Spot Assay
The amplified filtrates obtained above were tested for phage activity using spot assay method previously described by Ullah and his team.5 After thoroughly mixing 5 mL of molten 0.8% soft agar with 100 μL of exponentially grown host strains, the mixture was poured over a fresh Nutrient Agar base plate (Oxoid Ltd, Hampshire, UK). Following solidification, 10 μL of the amplified filtrates were spotted, and the plates were then allowed to dry (absorb) at room temperature (RT) for approximately 15 minutes and incubated overnight at 37°C. The presence of phages was presumptively identified by the formation of clearance at the sites of lysate application compared with a normal saline used as control. For positive spotted phage activities, the central part was picked aseptically with the use of sterile toothpick and transferred into a tube containing 5 mL broth, followed by the addition of 100 μL of log phase-grown indicator host. Another tube containing the indicator strain was left as control without adding the plaque material. Both tubes were incubated at 37֩C under shaking conditions until complete lysis was seen in the test preparation. Afterwards, the tube was centrifuged at 10,000 rpm for 15 minutes at 4°C. The supernatant was filter sterilized and serially diluted for plaque assay following the standard protocol described by Twest and Kropinski.17 The procedure was repeated at least twice to ascertain the isolation of a single plaque, the purity of the phage, and the titration and activity of isolated phages.
Isolation of Candidate Bacteriophage Against Phage-Resistant Variant
The bacteriophage-resistant E. coli was identified by using the double agar overlay method of plaque assay described previously.16 One hundred μL of E. coli isolate adjusted to the turbidity of a 0.5 McFarland standard and equal volume of the specific coliphage was pipetted into the tube and incubated for 15 minutes for the phages to attach to host cells. The mixture was added to 5 mL of top agar and poured over an already solidified Nutrient Agar base plate and incubated for 48 hr at 37°C. The overgrowth of bacteria after the susceptible variants were lysed by the lytic bacteriophage indicated the emergence of resistant variants to the phage infection. The resistant variant was further confirmed by spot assay where there was no clearance and plaque formation. Re-identification of the grown colonies was performed on MacConkey agar and different biochemical media. Using the standard protocol previously mentioned, this resistant variant pure colonies were used as a host to isolate virulent phages from dirty water samples taken from Awetu River intersecting Jimma town, located in southwest Ethiopia.17 Then after, the lytic activity of this phage on the wild host as well as the resistant variant bacterial host were checked first by the spot assay followed by the plaque assay for spot positive result using the double agar overlay method described earlier.
Host Range Determination of Phages
The host range of propagated phages were tested for phage activity following the standard spot test procedure described earlier18 against E. coli strains and other non-E. coli bacteria. One hundred μL of each of the host cells at log phase were added to 5 mL of sterile molten soft agar (0.8%) and poured over a previously prepared Nutrient Agar plate surface. Ten μL of the purified phage lysates were spotted on each plate peripherally on the labelled area with their respective code. Sterile normal saline drop was employed as a control in each plate. The plates were allowed to dry at room temperature and incubated at 37°C for 24 hours. Bacterial sensitivity to phages was presumptively identified by the presence of a zone of clearance at the sites of phage application. Spot positive test was further assayed for plaque assay to verify the lysis was due to the phage particles. According to the degree of clarity, the observed results were differentiated into clear plaques (++), turbid plaques (+), or no plaques (–).
External Factors Stability Tests on Phages
The stability of phages to different physical and chemical factors, including temperature, pH, and organic solvents was tested according to the protocols described earlier with some modifications.5 All assays were performed in triplicates and plating was done by double agar overlay procedure.
Temperature Stability Assay
Thermal stability test of isolated phage was done by incubating 100 μL of phage lysate in duplicate tubes at the specified temperature including 4°C (control), 25°C, 37°C, 50°C, 70°C or 90°C for 1 hour. Then, the phage suspensions were serially diluted and plated by double agar overlay technique. The percentage of viable phages able to survive the exposed temperature was determined. The initial plaque count of phages kept at 4°C was taken as control for comparison.
pH Stability Assay
Phages were exposed to a different pH value of 2, 4, 7, 10 and 12 by adjusting SM buffer with 1M of hydrochloric acid or 1M of sodium hydroxide and incubated at 37°C for 1 hour. Then, the phage suspension was serially diluted and plated by the double agar overlay techniques indicated previously. The percentage of viable phage particles after exposure to the respective pH was calculated using plaque assay. The plaque count incubated in an SM buffer at pH 7.0 was taken as control.
Organic Solvents Tolerance
The stability of phage particles was tested against three different organic solvents: ethanol, acetone, and chloroform. A stock solution of phage lysates was added to SM buffer, 99% chloroform, pure acetone, 96% and 48% ethanol. The mixture was incubated for 1hr at room temperature, and the phage tolerance was checked by the double agar overlay techniques indicated previously. The percentage of viable phages after chemical exposure was estimated compared with the SM control tube which was handled in the same way as the experiment.
Results
Phage Isolation from Sewage Sources
The lytic bacteriophages named as VBO-E. coli 4307 and VBW-E. coli 4194 were isolated from JMC sewage sources using the corresponding host, E. coli 4307 and E. coli 4194, respectively. Both species of E. coli were MDR with the first was uropathogenic E. coli isolated from a urine sample, and the latter one was isolated from a wound abscess. The phages with the first VBO-E. coli 4307 were isolated from emergency outpatient department drained sewage source, and the second VBW-E. coli 4194 was isolated from medical ward drained sewage point. JMC bacteriology laboratory assigned code number to the host bacteria were used throughout this study (E. coli 4307 and E. coli 4194).
Screening of Phage-Resistant Host Variant and Its Phage Isolation
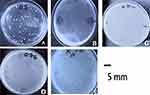
Plaque assay of VBO-E. coli 4307 resulted in the emergence of resistant variant colonies grown for 48 hr surrounding the plaques of killed E. coli 4307 (Figure 1A). The spot assay of phage VBO-E. coli 4307 infections of its host showed lysed bacteria on lawn of E. coli 4307 at sites it was applied (Figure 1B). The resistant variant colonies were isolated to make pure culture, and it was confirmed as E. coli by inoculating on Eosin methylene blue (EMB) agar plate as well as on conventional biochemical tests. The biochemical tests showed the characteristic reaction with Gram-negative bacilli, Kligler Iron agar (Acid slant/Acid butt, Gas +), urease (-), indole and motility test (+), Citrate utilization (-), and Methyl red and VP (±), respectively. Its colonies on EMB were lactose fermenter with a typical metallic shine. This single phage resistant variant E. coli was named E. coli 4307R.
A spot assay result of the previously isolated bacteriophage against this variant did not show lytic activity (Figure 1C), thus indicating that phage VBO-E. coli 4307 was not adsorbed onto E. coli 4307R. Using the phage-resistant E. coli 4307R as indicator host, lytic phage was isolated and named as VBA E. coli 4307R (Figure 1D).
Based on plaque assay, the phages were classified as lytic (virulent) bacteriophage. The plaques of the purified phages were uniform in shape and size and transparent without halos (Figure 1E). The physicochemical properties of the third phage were not different from the previously purified phages which is summarized in Figure 2.
Plaques Morphology and Quantitative Assay of Bacteriophages
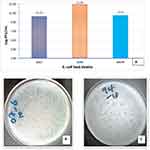
The phages were propagated against their respective host strain (MDR E. coli 4307 and 4194) by the double agar overlay method forming a visible plaque. Subjecting the phage lysates to further analysis revealed that both phages formed clear plaques on the lawn of the host and produced mean countable number of plaques: 2.13 × 1010 PFU/mL for VBO-E. coli 44307, 3.3 × 1010 PFU/mL for VBA-E. coli 4307R and 9.17 × 1012 PFU/mL for VBW-E. coli 4194 (Figure 2A). The plaques of VBO-E. coli 4307 (Figure 2B) and VBW-E. coli 4194 (Figure 2C) had a diameter of about 1 to 2 mm size.
Host Range Determination
The host ranges of phages VBO-E. coli 4307, VBW-E. coli 4194 and VBA-E. coli 4307R were determined using spot assay. The phages were tested against clinical isolates of MDR E. coli and non-E. coli strains, and the lysis spectra were indicated in Table 2. Strikingly, VBO-E. coli 4307 and VBW-E. coli 4194 lysed 50% of the E. coli isolates tested but did not lyse non-E. coli strains (Pseudomonas aeruginosa ATCC-27853, Klebsiella pneumoniae ATCC-700603, Staphylococcus aureus ATCC-25923, and Enterococcus faecium ATCC-29212) (Table 2). Furthermore, the isolates which could be killed by VBO-E. coli 4307 and VBW-E. coli 4194 were not at all lysed by phage of the later one (VBA-E. coli 4307R).
 |
Table 2 Lytic Activity of Phages VBO-E. coli 4307, VBW-E. coli 4194 and VBA-E. coli 4307R Against Their Corresponding Hosts and Other Different Bacterial Species |
Stability Tests for the Phages Against Different Factors
The stability of isolated phages to various physical and chemical factors, including different temperatures, pH ranges, and organic solvents, was tested. They appeared stable over various temperatures and pH ranges. Phages incubated at 50°C resulted in a lower titer. At 70°C, a considerable number of plaques were produced. A higher phage titer was obtained when incubated at 37°C with its specific host. Remarkably, no plaques were seen at 90°C as shown in Figure 3A.
The bacteriophages were treated at various pH ranges of 2, 4, 7, 9, and 12. No plaques were observed at pH = 2 and 12, while few PFU/mL plaques were observed at pH = 4 which shows that virions cannot tolerate extreme conditions. At pH = 7, highest titer was comparable with the considerable titer, and plaques were developed at pH = 10 (Figure 3B).
The virions exhibited a moderate effect in 48% ethanol, but they were unable to survive in absolute acetone or 96% ethanol. Phages treated with chloroform exhibit less effect than as compared to the SM buffer control sample (Figure 3C).
The Bactericidal Effect of the Phage Mixture in vitro Against Each Host
The four different phage combinations were designed using these three phages, and their lytic ability was investigated against two of bacterial hosts: E. coli 4307 and E. coli 4307R (named cocktail I = VBO-E. coli 4307 and VBW-E. coli 4194; cocktail II = VBW-E. coli 4194 and VBA-E. coli 4307R; cocktail III = VBO-E. coli 4307 and VBA-E. coli 4307R; and cocktail IV = VBO-E. coli 4307, VBW-E. coli 4194 and VBA-E. coli 4307R) have shown different effects on the E. coli 4307 and E. coli 4307R hosts. The combinations were used to compare infection strategy and plaque formation. The combination produced lytic effect showing synergistic effect on E. coli 4307 and E. coli 4194, but it was shown the antagonistic effect on a resistant variant of E. coli 4307 (Figure 4).
Discussion
In our study, the first two E. coli bacteriophages (VBO4307 and VBW4194) were isolated from hospital sewage sources. It is estimated that there are approximately 1031 phage particles on earth, ten-fold more than the bacterial population, making them the most abundant biological entities in the biosphere including in ocean, soil, freshwater, and in another complex living environments. Hospital sewage is a favorite niche for the bacteriophages as it contains a wide variety of microbes due to contamination with different clinical sources and organic wastes.19,20 On repeated assay, the clear plaques formed on the lawn of host strain indicated that the isolated phages were virulent bacteriophages and they can be candidate in phage therapy. The therapeutic application of bacteriophages in human emerged at the beginning of the 20th century but was progressively replaced by the use of antibiotics in most parts of the world after the Second World War. Nowadays it is getting attention due to the antibiotic crises associated with the spread of MDR bacteria. Among those known MDR bacteria, E. coli is one, and it is the most prevalent causes of community and hospital-acquired infections across the globe.21
The greater phage titers (1010–1012 PFU/mL) obtained in this investigation compared to earlier studies5,22,23 can be explained by the quantitative assay of these phages through three rounds of bacteriophage enrichment. Their unique architecture and viral family membership may account for their high degree of similarity in how they react to environmental stimuli. When stability or shelf life and purification processes are taken into account, this production level corresponds with the production required for therapeutic or biocontrol reasons (>1 × 1010 PFU mL-1).
The VBO-E. coli 4307 and VBW-E. coli 4194 phages showed a narrow host range with activities on clinical MDR E. coli isolates from different patient sources, while VBA-E. coli 4307R was only lytic to its own host. Differentiation between bacteriophage binding in Gram-negative bacteria may be caused by their difference in the somatic O, flagellar or other surface antigens that serve as a receptor. Strain-specificity of the isolated phage may suggest that it has the potential to further develop as a candidate for phage typing, phage therapy or as a bio-control agent on abiotic materials.24 When used in phage therapy, the preservation of beneficial microflora may be an added advantage to antibiotics used to combat pathogens.23
Several studies have reported that bacteriophage effectiveness may vary in different environmental conditions (pH, temperature ranges, and organic solvents) that play important roles in bacteria-phage interactions. Our investigation revealed a tolerance to a wide range of temperatures, from 4°C to 50°C, but the stability was rapidly dropped at 70°C, but all phages were inactivated at 90°C. These findings were in agreement with other previous studies,25,26 suggesting that the phages could be stored at room temperature, particularly in resource-limited settings. It could be applied, with minimal denaturation on surfaces of inanimate objects or animate as physiologic systems do not change abruptly.
The phages also showed stability over a wide pH ranges from pH 4 to 10, with maximum stability at pH 7 which is in agreement with earlier studies.25,26 The native virion stability and activity were maintained throughout this pH value. The isolated phages were inactivated rapidly at very low pH values of 2 and very high pH values of 12. Bacteriophages are usually less stable in acidic environments due to denaturation of their proteins.27 The human gastrointestinal tract acidity in particular may be endured by the addition of neutralizing agents for oral administration of phages. Oral phage dosing in mice along with the addition of 0.025% CaCO3 was found effective in keeping phage activity from acidic body sites such as the urinary tract and gastrointestinal tract.21 On the other hand, the phages exhibited tolerance to chloroform treatment justifying that all the phages did not possess lipid covering envelope residue. Chloroform may be used to preserve the phages from contamination for prolonged use.25
The main challenges regarding phage therapy are related to the possible emergence of phage-resistant bacterial variants, which should not be underestimated. In this regard, efforts were being made to develop methodologies for monitoring this challenge.28 One study isolated bacteriophages against the phage-resistant variants and subsequently mixed them with the original lytic phage to prevent resistance.16 In our finding, a candidate lytic phage was isolated against the original phage-resistant variants of E. coli 4307 (E. coli 4307R). Importantly, this bacteriophage was successively tested and successful in destroying it in vitro. This third phage was isolated from riverside water sample, and its physicochemical characteristics were almost similar to the first two phages isolated. The E. coli 4307R variant was the only host that was lysed by VBA-E. coli 4307R thus justifying the high specificity toward the receptors of its host. The reason for the remarkable specificity of VBA-E. coli 4307R phage targeting host receptors is because it only lysed the E. coli 4307R variant, even leaving E. coli 4307 unaffected.
Cocktails of these phages (named category I = VBO-E. coli 4307 and VBA-E. coli 4194; II = VBO-E. coli 4194 and VBW-E. coli 4307R; III = VBA-E. coli 4307 and VBW-E. coli 4307R; and IV = VBO-E. coli 4307, VBW-E. coli 4194 and VBA-E. coli 4307R) have shown different effects on the E. coli 4307 and E. coli 4307R hosts. For the phage-resistant variant (E. coli 4307R), there was no lytic effect observed in all combination categories of bacteriophages used. This may indicate that receptors of the phage-resistant bacterium were competitively attached to the receptor-binding proteins of the first phages and blocked the binding of its candidate bacteriophage.29 The combinations of VBO-E. coli 4307 and VBW-E. coli 4194, VBW-E. coli 4194 and VBA-E. coli 4307R, VBW-E. coli 4307 and VBA-E. coli 4307R were worsened in its lytic potential than the single phage (VBA-E. coli 4307R) to infect the resistant variants of E. coli (Figure 4C). This may indicate that those different phage mixtures were recognized by the same host receptors. In all those combinations, VBA-E. coli 4307R faces resistance for its lost adsorption capability on its host. The application of multiple phages in combinations could also affect the lytic activity of the susceptible bacterial hosts. Sequential exposure assessment may be important for cocktail application of phages that may share same receptors for adsorption.30,31
Conclusion
In this study, two bacteriophages were isolated from a medical center sewage water and their capabilities of lysing clinical MDR E. coli strains were investigated. The obtained results showed that the isolated bacteriophages were highly specific and lytic coliphages and are promising candidates for the treatment of MDR E. coli infections. However, phage-resistant strains still occur despite the use of phage to infect MDR E. coli. It was possible to get candidate novel bacteriophages specific to a resistant strain from environmental sources. This made it possible to solve resistance problems of bacteria which could hinder phage therapy. However, still attention should be given to assess their combined (cocktail) activity as there exist antagonistic or interference action to destruct their bacterial hosts.
Abbreviations
CDC, Center of Disease Control and Prevention; ESBL, Extended-Spectrum β –lactamases; JMC, Jimma Medical Center; MDREC, Multi-Drug Resistant Escherichia coli; MDR, Multi-Drug Resistance; NA, Nutrient Agar; NB, Nutrient Broth; UPEC, Uropathogenic Escherichia coli; UTI, Urinary Tract Infection; PFU, Plaque Forming Unit; RPM, Revolution per minute; WHO, World Health Organization.
Ethics Approval and Consent to Participate
Ethical clearance and approval were obtained from the Institutional Review Board (IRB) of Jimma University Institute of Health with reference number IHRPED/850/98. The clinical bacterial isolates and their antimicrobial susceptibility test results were obtained without patient names to maintain patient confidentiality.
Acknowledgments
We would like to express our sincere thanks to Jimma University Medical Microbiology laboratory for giving us the space to carry out this study.
Disclosure
The authors report no conflicts of interest in relation to this work and declare that the study was carried out in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rajagopal K, Chandy SJ, Graham JP. A One Health Review of Community-Acquired Antimicrobial-Resistant Escherichia coli in India. Int J Environ Res Public Health. 2021;18(22):12089. doi:10.3390/ijerph182212089
2. Bitew A, Tsige E. High Prevalence of Multidrug-Resistant and Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: a Cross-Sectional Study at Arsho Advanced Medical Laboratory, Addis Ababa, Ethiopia. J Trop Med. 2020;2020:1–7. doi:10.1155/2020/6167234
3. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi:10.1038/nrmicro818
4. Ordaz-López VI, García-Herrera HM Cerda-Rivera. Urinary Tract Infection in Pregnancy: a Study of Pathogen and Bacterial Resistance in Mexico. J Fam Med. 2016;3(11):1–4.
5. Ullah A, Qamash T, Khan FA, et al. Characterization of a coliphage as1 isolated from sewage effluent in Pakistan. Brazilian J Biol. 2022;82:1–7. doi:10.1590/1519-6984.240943
6. Iredell J. Antimicrobial resistance. Microbiol Aust. 2019;40(2):55–56. doi:10.1071/MA19016
7. Farkas A, Tarco E, Butiuc-Keul A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. GERMS. 2019;9(1):17–27. doi:10.18683/germs.2019.1153
8. Belete MA, Saravanan M. A systematic review on drug resistant urinary tract infection among pregnant women in developing countries in Africa and Asia; 2005-2016. Infect Drug Resist. 2020;13:1465–1477. doi:10.2147/IDR.S250654
9. Teklu DS, Negeri AA, Legese MH, Bedada TL, Woldemariam HK, Tullu KD. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob Resist Infect Control. 2019;8(1):39. doi:10.1186/s13756-019-0488-4
10. Zafer MM, El-Mahallawy HA, Abdulhak A, Amin MA, Al-Agamy MH, Radwan HH. Emergence of colistin resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli strains isolated from cancer patients. Ann Clin Microbiol Antimicrob. 2019;18(1):2021. doi:10.1186/s12941-019-0339-4
11. Knoll BM, Mylonakis E. Antibacterial bioagents based on principles of bacteriophage biology: an overview. Clin Infect Dis. 2014;58(4):528–534. doi:10.1093/cid/cit771
12. Vandamme EJ, Mortelmans K. A century of bacteriophage research and applications: impacts on biotechnology, health, ecology and the economy! J Chem Technol Biotechnol. 2019;94(2):323–342. doi:10.1002/jctb.5810
13. Zalewska-Piątek B, Piątek R. Phage therapy as a novel strategy in the treatment of urinary tract infections caused by E. coli. 2020;9:1–20.
14. Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ. Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J Appl Microbiol. 2007;104(1):070802123828004. doi:10.1111/j.1365-2672.2007.03498.x
15. Moghadam MT, Amirmozafari N, Shariati A, et al. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect Drug Resist. 2020;13:45–61. doi:10.2147/IDR.S234353
16. Yu L, Wang S, Guo Z, et al. A guard-killer phage cocktail effectively lyses the host and inhibits the development of phage-resistant strains of Escherichia coli. Appl Microbiol Biotechnol. 2018;102(2):971–983. doi:10.1007/s00253-017-8591-z
17. Young ARJ, Narita M, Narita M. Cell senescence as both a dynamic and a static phenotype. Methods Mol Biol. 2013;965(1):1–13.
18. Amankwah S, Adisu M, Gorems K, Abdella K, Kassa T. Assessment of Phage-Mediated Inhibition and Removal of Multidrug-Resistant Pseudomonas aeruginosa Biofilm on Medical Implants. Infect Drug Resist. 2022. doi:10.2147/IDR.S367460
19. Kassa T. Bacteriophages Against Pathogenic Bacteria and Possibilities for Future Application in Africa. Infect Drug Resist. 2021;14:17–31. doi:10.2147/IDR.S284331
20. Batinovic W, Knowler R, Stanton R, et al. Bacteriophages in Natural and Artificial Environments. Pathogens. 2019;8(3):100. doi:10.3390/pathogens8030100
21. Romero-Calle D, Guimarães Benevides R, Góes-Neto A, Billington C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. MDPI. 2019;8(3):138.
22. Rahimzadeh G, Resch G, Rezai MS, Hevelaee EN. Characterization and lytic activity of isolated Escherichia coli bacteriophages against Escherichia coli in vitro. Iran J Med Sci. 2020;45(4):298–303. doi:10.30476/ijms.2019.45420
23. Alexyuk P, Bogoyavlenskiy A, Alexyuk M, Akanova K, Moldakhanov Y, Berezin V. Isolation and Characterization of Lytic Bacteriophages Active against Clinical Strains of E. coli and Development of a Phage Antimicrobial Cocktail. Viruses. 2022;14(11):2381. doi:10.3390/v14112381
24. Lukman C, Yonathan C, Magdalena S, Waturangi DE. Isolation and characterization of pathogenic Escherichia coli bacteriophages from chicken and beef offal. BMC Res Notes. 2020;13(1):8. doi:10.1186/s13104-019-4859-y
25. Hyman P. Phages for Phage Therapy: isolation, Characterization, and Host Range Breadth. MDPI. 2019;12(35):56.
26. Chaudhary N, Narayan C, Mohan B, Taneja N. Characterization and in vitro activity of a lytic phage RDN37 isolated from community sewage water active against MDR Uropathogenic E. coli. Indian J Med Microbiol. 2021;39(3 343–348). doi:10.1016/j.ijmmb.2021.04.011
27. Jamal M, Hussain, T, Rajanna Das, C, Andleeb, S. Isolation and Characterization of a Myoviridae MJ1 Bacteriophage Against Multi-Drug Resistant Escherichia coli 3. Jundishapur J Microbiol. 2015;8(11):. doi:10.5812/jjm.25917
28. Oechslin F . Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses. 2018;10(7):351. doi:10.3390/v10070351
29. Kim J, Park H, Ryu S, Jeon, B. Inhibition of Antimicrobial-Resistant Escherichia coli Using a Broad Host Range Phage Cocktail Targeting Various Bacterial Phylogenetic Groups. Front Microbiol. 2021;12(August):1–10. doi:10.3389/fmicb.2021.699630
30. Friman VP . Resistance Evolution against Phage Combinations Depends on the Timing and Order of Exposure. MBio. 2019;10(5):1–15. doi:
31. Abedon ST, Danis-Wlodarczyk, KM, Wozniak, DJ. Phage Cocktail Development for Bacteriophage Therapy: toward Improving Spectrum of Activity Breadth and Depth. Pharmaceutical. 2021;14(10):1019 doi:10.3390/ph14101019.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.