Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Isolation and Molecular Detection of Marek’s Disease Virus from Outbreak Cases in Chicken in South Western Ethiopia
Authors Bulbula A , Borena B, Tadesse B, Aliy A, Negessu D
Received 4 August 2022
Accepted for publication 14 September 2022
Published 28 September 2022 Volume 2022:13 Pages 265—275
DOI https://doi.org/10.2147/VMRR.S376795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Abdela Bulbula,1 Bizunesh Borena,2 Biniam Tadesse,1 Abde Aliy,1 Demessa Negessu1
1Department of Bacterial Serology, Animal Health Institute (AHI), Sebeta, Oromia, Ethiopia; 2Department of Veterinary Laboratory Technology, Ambo University, Ambo, Oromia, Ethiopia
Correspondence: Abdela Bulbula, Department of Bacterial Serology, Animal Health Institute (AHI), PO Box 04, Sebeta, Oromia, Ethiopia, Tel +251 9103989, Email [email protected]
Background: Marek’s disease virus is a devastating infection, causing high morbidity and mortality in chickens in Ethiopia.
Methods: The current study was conducted from March to November, 2021 with the general objective of performing antemortem and postmortem, isolation, and molecular detection of Marek’s disease virus from outbreak cases in southwestern Ethiopia. Accordingly, based on outbreak information reported from the study sites namely, Bedelle, Yayo, and Bonga towns in southwestern Ethiopia, 50 sick chickens were sampled. The backyard and intensive farming systems of chickens were included in the sampling and priorities were given for chickens that showed clinical signs that are characteristics of Marek’s disease.
Results: By clinical examinations, paralysis of legs and wings, gray eye, loss of weight, difficulty in breathing, and depression were recorded on all chickens sampled for this study and death of diseased chickens was observed. In addition, enlargement of the spleen and gross lesions of the liver and heart were recorded during postmortem examination. The death of infected chickens was observed in both vaccinated and non-vaccinated flocks. Out of 50 pooled feather follicle samples, Marek’s disease virus was isolated from 14/50 (28%) by cell culture method and out of six tissue samples, the virus was isolated from 5/6(83.30%). By Real time polymerization chain reaction technique, which was targeted to detect the Meq gene, Marek’s disease virus was detected from 18/50 feather follicles which accounts for 36% of sampled chickens.
Conclusion: In general, current study showed that the circulating Marek’s disease virus in southwestern Ethiopia was caused by the oncogenic Gallid herpesvirus-2 (Serotype-1). Further research on molecular characterization of revolving virus in current and other regions is recommended for effective control of the disease through vaccination.
Keywords: Ethiopia, Marek’s diseases, isolation, molecular detection, outbreak
Introduction
Chicken production is an essential agricultural activity in Ethiopia. The majority of the farmers and many others, keep at least a few chickens, which can play a significant role in nutrition, poverty alleviation, and food security. The country shared about 60% of the total chickens in East Africa. This number consists of all breeds of exotic, hybrid, and local. In detail, the estimation of the chicken population was about 56.53 million and with regard to breed, 94.3%, 3.21%, and 2.49% of the total poultry population are indigenous, hybrid, and exotic, respectively.3
The majority of chicken types reared in Ethiopia are local ecotypes, which demonstrate a large variation in body position, plumage color, comb type, and productivity10 and are managed under an extensive management system.25 Village chickens have been reared as a source of family protein and income for many years and they have contributed to the country’s economy. However, the overall chicken production system in Ethiopia is commonly known as it is not for the purpose of commercial targeted, decreased input, scavenging, and outdated management system entails domestic breeds. Accordingly, the economic importance of the sector is not still proportional to the large chicken numbers.17
In addition, the Newcastle disease, Infectious Bursal disease, and Marek’s disease are making worse constraints on Ethiopia’s poultry production. Among the major constraints of chicken productions, Marek’s disease (MD) is a devastating infection, which replicates in lymphocytes, made the latent infection in CD4 (+) T lymphocytes,12 and occurred in chickens by herpesvirus, which is scientifically known as Mardivirus genus.
This genus is classified into three serotypes namely; Gallid herpesvirus 2 (serotype 1), Gallid herpesvirus 3 (serotype 2), and Meleagrid herpesvirus 1 or herpesvirus of turkeys (HVT) (serotype 3). Out of the MD serotypes, the first one is virulent and a small number of them were the attenuated vaccine strains. The second species/serotype is naturally recognized as avirulent and the HVT also served as a vaccine, further identified as being a recombinant viral vaccine vector recently.24
The disease, introduced in Ethiopia with the exotic breeds from outside, has now become a fear for backyard and commercial farming systems.16 Epidemiologically, MD is extensively distributed and causing the high morbidity and mortality of chickens at small-scale farming and backyard poultry farms in Ethiopia.4 In contrast,3 a study targeting the MDV isolation and molecular characterization in the central part of the country on infected chickens of diverse production systems grouped all isolated results in Gallid Herpesvirus type 2.
The decreasing weight, despair, crippling, bursal atrophy, neurological disease, and immediate beginning of T cell lymphomas that infiltrate lymphoid tissues, visceral organs, and peripheral nerves of infected chickens are identified as clinical signs of MD.11 For instance, the chronic persistence of the virus in diseased chickens supports horizontal transmission of MDV, which advances the incidence and speeds up virus-induced tumors in multiple organs. And the connection between viral quantity or very virulent (vv) MDV and that of pathology is closely correlated.16
The World Organization for Animal Health (OIE) recommended different methods to diagnose MD, which include; indirect techniques like ELISA, AGID, and IFI. Direct diagnostic methods are; histopathology, histochemistry, PCR, virus isolation, and immuno-precipitation. More recently new techniques such as quantitative polymerase chain reaction (qPCR) and loop-mediated isothermal amplification (LAMP) which are more sensitive, specific, reliable, and faster and have been applied for the detection of MDV. Hence, molecular methods, which rapidly quantify the amount of MDV genome copies in feathers and dust are used in order to detect specific serotypes present on the broiler and layer poultry farms and for monitoring vaccination status.15
Vaccination is the extreme controlling way of Marek’s disease in advancing the poultry industry. However, the major concerns are due to the latent vaccine-driven highly facilitates upsurges in pathogenicity and the achievement of controlling program by vaccination28 can be challenged when there is high growing virulence of MDV strains.23
Due to the absence of a formally organized government plan in Ethiopia, this highly contagious paralytic/oncogenic disease is causing severe economic losses resulting in serious harm to the industry of the country.4 The survey conducted on outbreaks of MD in a commercial poultry farm in central Ethiopia,14 investigated a 46% mortality rate, and another study4 on indigenous chickens in Ethiopia justified the morbidity and mortality as almost equal and as Marek’s disease is still fatal even to local breeds of chickens. To control the disease by means of vaccination supported better survival. However, these vaccines are imported with high transportation costs through foreign currency, and as a result, this made the sector more expensive.16 In contrast, effective vaccines developed from local strains created great demands for genetic analysis of the virus mingling through the country.5
A study conducted3 in central Ethiopia, which isolated and characterized the MDV1 and recommended the isolation and molecular characterization of Marek’s disease virus in other geographical areas, regarding backyard, commercial poultry farms, on different breeds and age groups. However, published information regarding isolation and molecular detection, molecular characterization, and serotype identification of MDV is scarce in southwestern Ethiopia. Thus, investigating the MD virus serotype circulating in this area is needed for good control planning and preventive measure in the country. Therefore, the objective of the current study was to perform antemortem and postmortem diagnosis, isolate, and molecular detection of Marek’s disease virus in southwestern Ethiopia.
Materials and Methods
Study Areas
The present study was performed from March to November, 2021 in the Yayo from Ilubabor, Bedelle from Buno Bedelle, and Bonga town from Kaffa Zones of southwestern Ethiopia. Ilubabor and Buno Bedelle zones are found in Oromia National Regional State and While, Kaffa zone is located in Southern Nation Nationalities and People’s Regional State (SNNPR). The details of study areas are explained in Table 1 as evidence obtained from their respective animal health offices.
 |
Table 1 Details of Study Areas |
Study Design
A case-based study was employed for this research. Before beginning any outbreak investigation, proper information regarding the outbreak was gathered from district’s animal health technical personnel, officers of livestock sectors, regional laboratories, and the National Animal Health Diagnostic and Investigation Center (NAHDIC). Then, after the case-based study (outbreak investigation) was conducted with the purpose of collecting appropriate samples for isolation and molecular detection of MDV, following the outbreak information gathered.
Study Animals
The samples were collected only from the total of 50 clinically suspected chickens by MD, in an outbreak areas as 28 from Bedelle, 17 from Yayo and 5 Bonga. Three outbreaks, one from each study area were investigated. By this study, a total of 250 feathers (five from each) and six (pooled from six chickens) tissue samples of spleen, liver, and kidney; five from Bedelle and one from Yayo were collected from critically ill chickens showing clinical sign of MD. All examined chickens were exotic breeds and their age was between 12 and 13 weeks.
During the research period, the outbreak investigation was purposively employed on clinically diseased chickens in suspicion of MD. Information about the disease/outbreak was gathered by history taking from the chicken owners and collected wisely from animal health professionals working in the outbreak areas.
Clinical and Postmortem Examination of Suspected Chickens
A total of 50 sick chickens were examined for the presence of Marek’s disease clinical signs such as depression, loss of weight, loss of appetite, death without showing clinical signs, paralysis of legs and wings, flaccid neck, and rising up of the skin near the hair follicles. Also, a postmortem examination was conducted on six seriously sick chickens out of 50 to observe the gross lesions. Accordingly, enlargement of visceral organs of the spleen, liver, kidney, heart, and for the presence of tumor formation in different organs.
Sample Collection and Transportation
Prior to the observation of each case, samples were collected aseptically for the aim of virus isolation, Serotype identification and molecular detection. Accordingly, spleen, and liver, kidney were collected in universal bottles and also, feather follicles of infected chickens were put into cryovials filled with viral transporting media (VTM). The collected samples were transported cold chain to the National Animal Health Diagnostics and Investigation Center (NAHDIC) laboratory. In the laboratory, they were kept at −80°C till processing, according to the world organization for animal health20 terrestrial manual.
Laboratory Investigation
Cell Culture and Sample Preparation
The DF-1, passage-14 (immortalized chicken embryo fibroblast) which was gained from GD, Netherlands were re-cultured in 25 cm2 tissue culture flask after revitalized out of liquid nitrogen. The subculture was then employed for confluent flask and tissue culture plate with six wells and preserved in Dulbecco’s modified Eagle’s medium (DMEM) that containing 10% fetal bovine serum at the temperature of 37°C in a humidified incubator at 5% CO2.
Sample Preparation
Samples collected were pooled together. The samples of tissue and feather follicle/tips broken down by sterile scalpel blade and scissor to small pieces. To facilitate maceration, the small pieces of both samples were ground by the help of mortar and pestle by sterile sand. A 10% suspension of tissue or feather tips were made in pure suspension known as phosphate buffer saline (PBS) with 2% antibiotic-antimycotic solution. The suspensions were then centrifuged at 3000 rpm for 10 min and the supernatant is harvested, filtered by 0.45 μL Millipore filter paper and kept at −20°C prior to inoculation.27
Virus Isolation
The sample suspensions prepared and stored at −20°C were thawed and 0.1 mL of the samples inoculated into confluent DF-1 cultures in six-well plates along with cell controls. After 60 min adsorption at 37°C, maintenance medium is added to each well including a negative control and incubated at 37°C in a humidified incubator at 5% CO2. Cells were monitored every 24 h postinfection and inspected for cytopathic effects (CPEs), plaque and foci formed using an inverted microscope. On sixth day, the cultures were freeze-thawed and the resulting lysates were again inoculated into fresh cultures using 25 cm2 tissue culture flasks until the third passage as this method supported the growth of MDV.13,18
Virus Isolation from Feather Follicles
The feather follicle samples has been used for diagnosis of Marek’s diseases virus and feather tips were used to extract the cell free MDV. Accordingly, feather tips about 5 mm were suspended in sucrose, phosphate, glutamate and albumin/ethylenediaminetetraacetic acid (SPGA/EDTA) buffer and the buffer was added with different volumes in biosafety class-II based on laboratory procedures for the purpose of extraction and titration of cell-free virus. The suspension was filtered through a 0.45 μm membrane filter and cultured on DF-1. Then the medium was added and cultures were incubated for 15 days after 40 min absorption. This method was used to isolate serotype 1 and serotype 2 with serotype 3 (HVT) of Marek’s disease virus. The CPEs that are formed as a result of MDV virus serotypes were identified properly on the basis of their foci of plaque development.21
Virus Isolation from Tissues
The MDV Was isolated from suspected tissue as DF-1 obtained from GD, Netherlands protocol. So, 1000 μg/mL of streptomycin and 100 IU/mL of penicillin were added to chopped pooled tissue suspension to avoid crossing contaminants. Supernatants were harvested and inoculated on primary DF-1 cells in maintenance Dulbecco’s modified Eagle’s medium (DMEM) that have 2% bovine fetal calf serum (incubated at 37°C). The culture medium was observed every day until 14 days to see the visible cytopathic effects (CPE).21
DNA Extraction
In the NAHDIC Biotechnology laboratory, from a 10% (w/v) sample of tissue/feather, the DNA was extracted and homogenates have been carried out using the Qiagen® DNeasy Mini kit (cat. no. 51306, Germany) according to the manufacturing company instruction. A small piece of tissue was placed (<25 mg) in a 2 mL reaction vessel and re-suspended in the tissue pellet in180 µL in a viral lysing buffer (ATL). Then, 20 µL of proteinase K was added and incubated at 56°C until it is lysed (1–3 h), vortices during incubation. Then, after it was centrifuged briefly below and 200 µL buffer AL was added, vortexes for 15 seconds and incubated for 10 min at 70°C. Two hundred microliters of ethanol (96–100%) was added to the samples after brief centrifugation and then mixed for 15 seconds.
The spin column was centrifuged for one minute at 6000 (8000 rpm) and QIAamp spin was placed into clean collection tube (then collection tube with filtrate was discarded). The washing buffer (AW1) was added to opened spin column tube with volume of 500 µL and centrifuged for one minute at 6000 (8000 rpm). Five hundred microliters of washing buffer (AW2) was added to spin column tube (located in new collection tube) following the careful opening and centrifuged for three minutes by 20,000 g (14,000 rpm). Then 200 µL of buffer AE (elusion buffer) was added to QIAmp spin column tube and incubated for one minute at room temperature and centrifuged for one minute at 6000 (8000 rpm) and the filtrate was retained at 4°C for 48 h at the latest (prolonged storage at −20°C). At the end, the extracted DNA from isolates and fresh samples were amplified by PCR.
Real-time Polymerization Chain Reaction (RT-PCR)
The quantitative or real time PCR has been used to quantify the genome copy of MDV virus serotypes that are responsible for Marek's disease.1 Thus, the PCR was performed targeting the MDV meq gene using forward and reverse primers of MDV1 (5’-GGAGCCGGA GAG GCTTTA TG-3’) and (5ʹATCTGG CCC GAATACAAG GAA-3’) respectively and MDV1 Pobe 5’-(FAM) CGT CTT ACC GAG GAT CCC GAA CAG G-3ʹaccording to (Qiagen, Germany) QIAamp kit now available in NAHDIC molecular lab. All procedures were employed as recommended by kit manufacturers and the test protocol was performed with steps of initial denaturation at 95°C for 15 min followed by 45 cycles at 95°C for 15 min, annealing at 60°C for 15 min, extension at 72°C for 10 min and was cooled at 40°C.
Results
Clinical Examinations and Postmortem Findings
A total of 50 chickens (28 from Bedelle, 17 from Yayo and 5 chickens from Bonga towns) were examined (Table 2). Clinical signs of MD like; paralysis of legs and wings, gray eye, loss of weight, difficulty in breathing and depression were recorded for all the chickens sampled for this study and death of diseased chickens were reported by animal owners.
 |
Table 2 Number of Clinically and Postmortem Examined Chickens in the Study Areas |
Postmortem examination was applied on six chickens with serious clinical signs of MD. Five postmortem-examined chickens were from Bedelle, one from Yayo and there were no examined chickens by postmortem from Bonga town. Upon examination, the enlargement of spleens were observed. Also, enlargement, congestion, and nodular lesion of the liver were found (Figure 1).
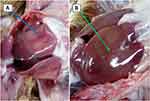 |
Figure 1 Gross lesions found on liver. (A) enlargement and congestion and (B) nodular/lymphomatous lesion. |
In general, gross Lesions observed on 3/5 from Bedelle (60%), 1/1 from Yayo (100) (Table 3) and no lesions appeared on two from Bedelle. Conversely, enlargement of spleen appeared on two chickens from Bedelle and was not observed on remaining 4 (three from Bedelle and one from Yayo).
 |
Table 3 Gross Lesions on Organs of Chickens Suspected of MD from the Study Sites |
Isolation of Marek’s Disease Virus
The total of 50 pooled feather follicle samples as collected five feathers from each chicken were cultured on DF-1 cells. Out of them, MDV virus was isolated in 14/50 (28%) samples which were 9/28 (32.14%) from Bedelle town, 4/17 (23.53%) from Yayo, and 1/5 (20%) from Bonga town as shown in Table 4.
 |
Table 4 Marek’s Disease Isolation from Feather Follicle Samples |
In addition, the MDV was isolated from pooled tissue from spleen, liver, and kidney of seriously sick chickens, 5/6 chickens tissue samples from Bedelle and 1/6 tissue samples collected from Yayo. Out of six pooled tissue samples of spleen, liver, and kidney the MDV was isolated in 4/5 (80%) from Bedelle and 1/1 (100%) tissues from Yayo. In total, the virus was isolated in 5/6 (83.30%) pooled tissues of chickens (Table 5).
 |
Table 5 Marek’s Disease Isolation Rate from Tissue Samples of Chicken |
All of the isolated tissue samples and feather follicles showed small plaques, which are suggestive of the cytopathic effect (CPE) of MDV observed on DF-1 cells.
Initially, the CPE appeared as small and rounded cells, and at the end, it was observed as small plaque, after the formation of foci separated from the body of the culture flask. The bright and enlarged cell was also demonstrated with the help of an inverted microscope beginning from three days of the third passage as observed after 11 days of inoculation (Figure 2).
Real-time Polymerase Chain Reaction (RT-PCR)
A total of 50 feather follicles pooled (as collected from every 50 chickens) and six pooled tissue samples were examined by RT-PCR targeting to detect the meq gene of MDV1 using forward (5’-GGAGCCGGA GAG GCTTTA TG-3’) and reverse primers of MDV1 (5ʹATCTGG CCC GAATACAAG GAA-3’). DNA of 18 feather follicles was detected as MDV1 (serotype1) positive which accounts for 36%. Out of six pooled tissue samples, 5 (83.3%) of them were positive for MDV1. However, the DNA of one, 1/6 (17.3%) chicken tissue samples was not detected as MDV and resulted as negative. Thus, a summary of the PCR results was discussed in Tables 6 and 7.
 |
Table 6 The Summary of RT-PCR Results for Feather Follicle Samples |
 |
Table 7 The Summary of RT-PCR for Tissue Samples |
All positive results of present study were indicated Gallid herpesvirus type 2 (GaHV-2), also known as Marek’s disease virus 1 (Serotype-1, MDV-1). The RT-PCR results of examined samples were indicated (Figure 3).
Discussion
The clinical examination of the diseased chickens showed paralysis of legs and wings, grayness of eyes color, depression and difficulty in breathing, weight loss, and mortality. The postmortem findings in the current study also demonstrated the enlargement of the spleen, liver blood cell congestion, and lymphomatous lesion of visceral organs such as liver and lesions appeared on 4/6 of the slaughtered chickens. Both the clinical signs and postmortem examination findings indicated MDV and it was in agreement with results of previous studies.6,8,9,20
In this study, MDV was isolated from pooled feather follicles of chickens which were 14/50 of sampled chickens and accounted for 28%. This finding was in agreement with previous studies,13,18 which isolated MDV from feather tips on the culture of DF-1. MDV was isolated from 5/6 (83.30%) of seriously ill chicken’s pooled tissues of liver, spleen, and kidney. This result was related to a study for Demeke et al3 as their isolated 11/12 pooled spleen and dissimilar by types of tissues and cells. All isolates developed, small plaques, the specific and clear (CPE), which were the indicative characteristics of MDV, and this was confirmed by RT-PCR. The bright and enlarged cell was demonstrated with the help of an inverted microscope, beginning from three days of the third passage and a clear CPE was observed after 11 days of inoculation as stated by previous studies.19,20,26
The real-time PCR was used due to its high sensitivity, high specificity, efficiency, and mostly its capacity of detecting the quantity of virus accurately as it was appreciated and performed by Baigent et al.1 By this method Marek’s disease virus was detected from feather follicles of 18/50 (36%) examined chickens. This is related to the study that detected Serotype-1 (MDV1) from feather follicles22 where less than half of the positive results were 22/173 samples tested and 13/173 tissue samples.
In addition, the Marek’s disease virus was detected in pooled tissues of the chicken’s spleen, liver, and kidneys as collected from critically ill chickens, during this study by RT-PCR. Accordingly, the virus was detected in 5/6 (83.30%) examined tissues of clinically suspected chickens for MD, which this result agrees with a prior study7 as it detected the oncogenic MDV1 virus from similar tissue samples by RT-PCR, but differs from their results which found the virus in 16/83 examined tissues.
The present study findings also confirmed the existence of Gallid herpesvirus type 2 (GaHV-2), also known as Marek’s disease virus 1 (MDV-1) in southwestern Ethiopia which was devoid of previously published findings regarding MDV, based on clinical signs, isolation, and molecular detection. This result has an agreement reports4,14 which underlined the presence of MD in the country.
Also, the present finding was supported by that of Demeke et al,3 who conducted research on isolation and molecular characterization of MDV from clinically diseased chickens reared under different a production system in central Ethiopia and concluded that the Marek’s disease virus isolates were clustered under Gallid herpesvirus type 2;16 a study based on isolation and molecular detection of MDV from chickens in the area of central Ethiopia and a study performed in central Ethiopia3 on the isolation and molecular characterization of Marek’s disease which identified the presence of Meq genes of MDV1 that is circulating in the country. But, they used different diagnostic methods, specifically conventional PCR which is a qualitative method. This makes the present study findings differ as it was performed by real-time PCR.
The isolation and molecular detection of Marek’s disease virus is a new one for southwestern Ethiopia as it had been justified by this study. Hence, another study focusing on viral characterization and sequencing of the strains is the key point to successful control, and prevention and better knowledge of the behavior of the virus; such as pathotypes or genotypes for eradication program.
Conclusion
The clinical and postmortem examination of the current study indicated clearly Marek’s disease virus and the disease is causing morbidity and mortality of infected chickens in both vaccinated and non-vaccinated flocks in newly emerging intensive farms and backyard production systems of chickens in southwestern Ethiopia districts/towns of Bedelle, Yayo, and Bonga. Generally, the current study confirmed that the MD, which is the reason for the occurrence of an outbreak, mortality, and morbidity in chickens in the study area, was caused by Gallid herpesvirus 2 or serotype 1 (MDV-1) as it was clinically and postmortem examined, isolated and detected molecularly. Thus, this research results can contribute greatly to disease control and prevention. Hence, more extended study based on molecular characterization in the current study area, and isolation and molecular characterization of MDV in other regions of the country is recommended to identify pathotypes of the MDV that are becoming a threat to chicken production for effective control of the disease through vaccination.
Data Management and Analysis
Data obtained from laboratory tests like; virus isolation by cell culture and MDV Molecular detection by real-time polymerase chain reaction (RT-PCR) and characteristics of sampled chickens collected during sample collections were stored in Microsoft Excel prior to being analyzed using descriptive statistics and to summarize the results, the frequency table was used.
Ethical Approval Clearance
Ethical clearance was received from the National Animal Health Diagnostics and Investigation Center (NAHDIC), with reference number (ARSERC/EC/013/22/12/2020).
Acknowledgments
The authors have extended thanks to the former National Animal Health Diagnostic and Investigation Center (NAHDIC)/present Animal Health Institute (AHI) for unreserved support from the beginning to the end of this work by technical, all performed laboratory works, and financial support. We are grateful to AHI Director General, Dr Tesfaye Rufael for his great support in facilitating every material needed for this research. Also, we would like to thank poultry owners for their unreserved willingness during sample collection and for sharing necessary information.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baigent S, Petherbridge L, Howes K, Smith L, Currie R, Nair V. Absolute quantitation of Marek’s disease virus genome copy number in chicken feather and lymphocyte samples using real-time PCR. J Virol Methods. 2005;123:53–64. doi:10.1016/j.jviromet.2004.08.019
2. CSA. Central statistic authority: federal democratic Republic of Ethiopia, agricultural sample enumeration statistical abstract; 2017.
3. Duguma R, Yami A, Dana N, Hassen F, Esatu W. Marek’s disease in local chicken strains of Ethiopia reared under confined management regime in central Ethiopia. Rev Med Vet. 2005;156:541–546.
4. Duguma R, Dana N, Yami A. Marek’s disease vaccination opened the door to rear indigenous chickens of Ethiopia under confined management. IJARVM 2006;4:121–127.
5. El-Kenawy AA, Emad A, El-Tholoth M. Isolation and identification of Marek ’ s disease virus (MDV) from feather follicle epithelium and internal organs of diseased chickens in Dakahlia. Mansoura Vet Med J. 2019;2:6–11. doi:10.21608/mvmj.2019.22.102
6. Gall S, Kőrösi L, Cortes AL, Delvecchio A, Mitsch P, Gimeno IM. Use of real-time PCR to rule out Marek ’ s disease in the diagnosis of peripheral neuropathy. Avian Pathol. 2018;47. doi:10.1080/03079457.2018.1473555
7. Gimeno IM. Stepwise diagnostic approach to inves- tigate a Marek’s disease outbreak. In: Pandiri AR, editor. American Association of Avian Pathologist Symposium: An Update on Marek’s Disease Vaccination, Diagnosis, and Immunosuppression. Boston, MA: American Association of Avian Pathologists; 2015.
8. Gimeno IM, Wakenell PS. Marek’s disease. In: Williams SM, Dufour-Zavala L, Jackwood MW, Lee MD, Lupiani B, Reed WM, editors. A Laboratory Manual for the Isolation, Identification and Characterization of Avian Pathogens. Jacksonville, FL: American Association of Avian Pathologists; 2016:249–258.
9. Halima H. Phenotypic and genetic characterization of indigenous chicken populations in North-West Ethiopia PhD Thesis Submitted to the faculty of natural and agricultural sciences department of animal, wildlife and grassland Sciences. Bloemfontein, South Africa: University of the Free State; 2007.
10. Heidari M, Fitzgerald SD, Zhang H. Immune responses in cecal tonsils of Marek’s disease virus-infected chickens. Avian Dis. 2015;59:213–226. doi:10.1637/10950-093014-Reg.1
11. Heidari M, Wang D, Fitzgerald SD, Sun S. Severe necrotic dermatitis in the combs of line 63 chickens infected with Marek’s disease virus. Avian Pathol. 2016;45:582–592. doi:10.1080/03079457.2016.1189511
12. Himly M, Foster DN, Bottoli I, Iacovoni JS, Vogt PK. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virol 1998;248:295–304. doi:10.1006/viro.1998.9290
13. Lobago F, Woldemeskel M. An outbreak ofMarek’sdisease in chickens in central Ethiopia, tropical animal health and protection. Trop Anim Health Prod. 2004;36:397–406. doi:10.1023/B:TROP.0000026665.78878.f4
14. López-Osorio S, David Villar A, Jenny Chaparro G. Challenges in the diagnosis and control of Marek’s disease virus in Colombia. Revista MVZ Córdoba. 2019;24:7157–7165.
15. Mirtneh A. Isolation and molecular characterization of Marek’s Disease virus in central Ethiopia and evaluation of its vaccine trial MSc thesis. Addis Ababa University College of Veterinary Medicine and Agriculture. Bishoftu, Ethiopia; 2015.
16. Neme Y, Ayele J, Aserat M. Village chicken production performances and producers trait preference in buno bedele and Ilu Aba bor Zone South Western Ethiopia; 2019:34–43.
17. Niikura M, Kim T, Silva RF, Dodgson J, Cheng HH. Virulent Marek’s disease virus generated from infectious bacterial artificial chromosome clones with complete DNA sequence and the implication of viral genetic homogeneity in pathogenesis. J Gen Virol. 2011;92:598–607. doi:10.1099/vir.0.026864-0
18. OIE. OIE Manual of diagnostic tests and vaccines of terrestrial animals. In: Marek’s Disease. Paris, France: Office International des Epizootics (OIE); 2008:565–571.
19. OIE. OIE Manual of diagnostic tests and vaccines of terrestrial animals. In: Marek’s Disease. Paris, France: Office International des Epizootics (OIE); 2010:1–11.
20. OIE. Marek’s disease in OIE terrestrial manual. Paris, France: Office International des Epizootics (OIE); 2018. Available from: http://www.oie.int/fileadmin/Home/eng/Healthstandards/tahm/2.03.13MAREK.
21. Raja A, Raj GD, Bhuvaneswari P, Balachandran C, Kumanan K. Detection of virulent Marek ’ s disease virus in poultry in India. Acta Virol. 2009;53(4):255. doi:10.4149/av_2009_04_255
22. Schat KA. History of the first-generation Marek’s disease vaccines: the science and little-known facts. Avian Dis. 2016;60:715. doi:10.1637/11429-050216-Hist
23. Schippers T, Jarosinski K, Osterrieder N. The ORF012 gene of Marek’s disease virus type 1 produces a spliced transcript and en- codes a novel nuclear phosphoprotein essential for virus growth. J Virol Methods. 2015;89:1348–1363. doi:10.1128/JVI.02687-14
24. Tadelle D, Million T, Yami A, Peters KJ. Village chicken production systems in Ethiopia: use patterns and performance valuation and chicken products and socio-economic functions of chicken. Livest Res Rural Dev. 2003;15:1.
25. Demeke B, Jenberie S, Tesfaye B, et al. Investigation of Marek’s disease virus from chickens in central Ethiopia. Trop Anim Health Prod. 2017;49:403–408. doi:10.1007/s11250-016-1208-1
26. Tan J, Cooke J, Clarke N, Tannock GA. Optimization of methods for the isolation of Marek’s disease viruses in primary chicken cell cultures. J Virol Methods. 2008;147:312–318. doi:10.1016/j.jviromet.2007.09.011
27. Tip T. Culture of Animal Cells - Basic Techniques. Wiley; 2012:1–16.
28. Witter RL. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 1997;41:149–163. doi:10.2307/1592455
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


