Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Isolated V-Shaped Sternal Cleft — A Rare Chest Wall Malformation
Authors Gebremariam DS , Miruts A, Desta KG
Received 13 November 2022
Accepted for publication 25 February 2023
Published 4 March 2023 Volume 2023:14 Pages 81—87
DOI https://doi.org/10.2147/PHMT.S397462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Dawit Seyoum Gebremariam,1 Asmamaw Miruts,2 Kibrom Gebreselassie Desta3
1Department of Pediatrics and Child Health, College of Health Sciences, Mekelle University, Mekelle, Tigray, Ethiopia; 2Department Surgery, Pediatric Surgery Unit, College of Health Sciences, Mekelle University, Mekelle, Tigray, Ethiopia; 3Department of Surgery, Cardiothoracic Unit, College of Health Sciences, Mekelle University, Mekelle, Tigray, Ethiopia
Correspondence: Dawit Seyoum Gebremariam, Department of Pediatrics and Child Health, College of Health Sciences, Mekelle University, P.O. Box: 1871, Mekelle, Tigray, Ethiopia, Tel +251911731454, Email [email protected]
Abstract: Sternal cleft is a rare chest wall anomaly resulting from a failure of the lateral mesodermal folds to migrate to the midline, causing a cleft in the early stage of embryological development. This can be a complete or partial defect. It can also occur as an isolated anomaly or in association with other syndromes. Fetal sonographic diagnosis of this defect is possible, but less practiced. After birth, this defect can be easily diagnosed clinically because of the presence of paradoxical chest wall movement. The flexibility of the thorax is maximal and compression of the underlying structures is minimal during the neonatal and early infancy period, and this period is the preferred time for surgical repair. We report a 39-day-old infant who presented with an isolated V-shaped inferior sternal cleft, its surgical primary closure, and postoperative course.
Keywords: sternal cleft, surgery, congenital anomaly, complication
Background
Isolated sternal cleft is one of the rare congenital midline defects of the sternum occurring commonly in association with midline supraumbilical raphe and facial hemangiomas. It results from failed ventral midline fusion of sternal bars during the ossification of the sternum, which starts in the fifth month of embryonic life. The exact etiology is unknown, but nutritional deficiency (methylcobalamin or riboflavin deficiencies) in mothers and alcohol intake may be associated with congenital skeletal deformities like sternal cleft.1,2 The incidence is 1:100,000 cases per live births, and represents less than 1% of all chest wall deformities.1 Sternal cleft can be isolated or associated with syndromes that involve various systems of the body, such as brain, cardiac, abdominal, renal, eye, and skeletally related malformations. It can also be either complete or partial. The partial form is subdivided into superior and inferior types, depending on when the developmental process is interrupted. When the space between the costal ridges reaches the xiphoid process, the defect is V-shaped, whereas with the bony bridge joining the two edges ending at the third or fourth costal cartilage, it is U-shaped.2–4
Fetal sonographic diagnosis of this defect is possible, but less practiced. It can be easily diagnosed clinically because of the presence of paradoxical chest wall movement during respiration in early infancy and due to the cosmetic deformity on the anterior chest wall.1,2 When the defect is associated with cardiac anomaly, it seems to be more easily identifiable due to the unusual movements of the heart under the skin.1–4,8
The decision for surgery depends on the age of the patient, rigidity of the chest wall, the size of the defect, and presence of other associated abnormalities in the chest wall and other systems. Maintaining and improving respiratory functions and cosmetic reasons are some of the indications for surgical closure.1–4 The closure of this defect is safe in the neonatal period because the flexibility of the thorax is maximal and compression of the underlying structures is minimal. The earlier the closure is done, the better the outcome, with no or few complications.3–5 Many authors have reported that patients diagnosed late or at older ages need complex reconstruction surgeries due to ossification of the cartilage tissue.2–5
The surgical correction can be achieved using a stepwise approach, such as primary approximation, sliding chondrotomies, prosthetic grafts, autologous grafts, or biological implants (Figure 1).4 We report a 39-day-old child who presented with V-shaped inferior partial sternal cleft, for whom primary surgical closure was done with no complications.
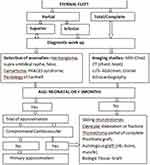 |
Figure 1 Stepwise approach for management of sternal cleft. |
Case Report
A 39-day-old female infant born to a primipara mother at term gestational age presented with anterior chest wall defect associated with sinus discharge from the defect and with hypopigmented skin lesions since birth. The mother had had regular antenatal care follow-up at a nearby health center with no sonographic evaluation and delivered at home via spontaneous vaginal delivery. The APGAR score and birth weight are not known, but she cried immediately after birth. There was no identified problem during prenatal, natal, or postnatal periods. She was vaccinated at the age of 6 weeks.
On physical examination, the vital signs were in normal ranges (RR 36 breath/min, PR 112 beat/min, temperature 36.6°C, SpO2 96%, weight 3500 g) with normal anthropometric interpretation. The pertinent finding was on the anterior chest wall, where there was a vertical hypopigmented supraumbilical midline raphe 15 cm in length extending from the sternal defect to the umbilicus and a 0.2×0.2 cm sinus oozing serous discharge over the midpoint of the defect (3 cm below the sternal notch). The sternum was not palpable, but there was palpable xiphisternum. The chest was clear and resonant with good air entry, and there was no abnormal cardiac or abdominal finding. Complete blood count and creatinine were normal, as were echocardiography and abdominal and cranial sonography. Chest X-ray and CT scan showed only sternal cleft, with no other thoracic or cardiac malformations.
The procedure was done at the age of 39 days (10 days after admission).
- Under general anesthesia, the infant was put in a supine position and the defect marked as V. An endotracheal tube was inserted, and the neck and sternal defect area were cleaned and draped (Figure 2A and B).
- A midline vertical incision was made from the suprasternal notch towards the xiphisternum. Skin flaps were raised on both sides, sternal bars exposed, and substernal, pleural, and mediastinal spaces freed by blunt dissection (Figure 3A and B).
- The edge of the sternal bars was refreshed to create a raw surface and a wedge excision made of the lower end. Approximation of the sternal bars with PDS number 2 was done and observed, and no cardiovascular compromise was seen (Figure 4A and B).
- The chest wall was closed and observed, and her vital signs were stable. Since the right pleura had been inadvertently opened upon dissection, a right-sided chest tube was inserted and connected to underwater seal bottle drainage. The incision was closed layer by layer (Figure 5A–C).
 |
Figure 2 Preoperative: (A) defect on the anterior chest wall with scar-like hypopigmented skin and supraumbilical raphe; (B) marked V-shaped sternal defect. |
 |
Figure 4 Immediately postprocedure: (A) approximation of sternal bars and muscle flaps done; (B) complication of inward chest retraction is not seen. |
 |
Figure 5 Postoperative: (A) the incision closed layer by layer, cleaned; (B) observed for infection at 2–7 days; (C) at discharge. |
Postoperatively, the infant remained intubated for 4 hours in order to achieve a good tolerance of intrathoracic pressure. The chest tube was functioning, and her stay in the recovery room was uneventful. She was transferred to the general ward and stayed for 1 week. There was no complication. The patient was discharged on her 47th day of age and followed up at 3, 4, 7, and 12 months of age. Currently, the age of the child is 4 years and 4 months. No complications have been reported. The figures show preoperative, intraoperative, and immediate postoperative conditions.
Discussion
Sternal cleft is a rare chest wall anomaly formed by a failure of the lateral mesodermal folds to migrate to the midline. Sternal cleft can expose mediastinal viscera and vessels to injuries and introduces multiple risks like chest wall infections, hypothermia, and insensible fluid losses. Other important factors that can be related to this defect are respiratory and hemodynamic sequelae.1–4
In Embryonic Life
The sternum is formed from two mesenchymal bars, which fuse between the seventh and tenth weeks of gestation. It starts cranially at the manubrium and ends distally at the xiphoid process.1,2 The ossification of the sternum starts in the fifth month of embryonic life. Very rarely, the sternal bars fail to join in the midline, which results in a complete or partial (superior/upper or inferior/lower) sternal cleft, depending when the development process has paused. This defect can be isolated or associated with syndromes. Anda et al mentioned that superior cleft is usually associated with other malformations like abdominal raphe and facial hemangioma, whereas inferior cleft can be associated with ectopia cordis alone or syndromic as the pentalogy of Cantrell (ectopia cordis, intracardiac defects, sternal cleft, omphalocele, pericardial defect).1,2,5 Zamfir et al reported that other malformations, such as PHACES syndrome, VACTERL association, gastroschisis, pectus excavatum, and chest wall hamartomas, are rare associations with either complete or incomplete forms of defects. Most of these defects are asymptomatic and have a female preponderance.1–3 de Campos et al reviewed eight patients (six girls and two boys), five of which were asymptomatic. Pectus excavatum and Cantrell’s pentalogy were associated syndromes for two patients. Our case was symptomatic. The sternal cleft was associated with serous sinus discharge and midline supraumbilical raphe, but no associated syndromes were seen. Manuel et al described that a nutritional deficiency in mothers, such as methylcobalamin deficiency and alcohol abuse during pregnancy, are some of the risk factors of skeletal defects.1–3,5 Our infant’s mother had no history of alcohol abuse.
The diagnosis can easily be done clinically at birth by physical examination. Bulging of overlying skin, sunken sternal defect, visible pulse, scarring, and supraumbilical midline raphe are the most common findings. The imaging identifies any associated anomaly besides the visible congenital abnormalities, such as abdominal raphe and facial hemangioma, and gives a detailed description of the position of clavicles, sternal bars, and ribs and other common cardiac anomalies. Our patient had mildly sunken sternal defect, band-like scarring extending from the defect to a supraumbilical midline raphe, and small sinus.
Prenatal diagnosis of isolated or syndromic sternal cleft is possible. This diagnosis by fetal ultrasound is done between the 18th and 26th weeks of gestation when the number and size of ossification centers are small or absent and prenatal identification of sternal cleft is easier when cardiac anomalies exist.2,4,6,8 Kemal et al and Twomey et al reported that two patients were diagnosed with sternal cleft by ultrasound at 21 and 22 weeks’ gestation. Similarly, Manuel et al reviewed five cases of sternal anomalies, one of which was identified prenatally but the rest diagnosed at birth.
Surgical repair of sternal cleft is critical and important. Timing of the surgical treatment of the defect depends on the size of the defect. The preferred time of sternal cleft repair is in the neonatal period, because the flexibility of the thorax is at its maximum at this age, leading to minimal compression on the underlying structures like heart, lung, and airways.2–4,6 Hbibi et al described that even though surgical closure is done in the neonatal period if it is associated with serious cardiac or vascular abnormality, the outcome may be unfavorable. The life‑threatening nature of this defect may be related to the severity of vascular malformations.3,7,9,10
Manuel et al and Singh et al both mentioned that as the age of the patient increases, chest wall compliance will decrease and repair of the defect become progressively difficult as venous return and lung compliance are increasingly compromised.2,6 Beyond the neonatal period, particularly after the age of 3 months, the chest wall becomes rigid and noncompliant, which makes the procedure more complex than primary approximation. For those age-groups different techniques, including sliding chondrotomies, fracturing the clavicle, autologous bone/cartilage-graft interposition, muscle-flap interposition, and prosthetic or biological mesh grafts can be used.3,4,7,9
de Campos et al reported that among sternal clefts managed by different techniques of surgery based on the type and range of age, all patients who underwent surgical correction had good aesthetic and structural results.5 Many authors suggest that the choice of an appropriate surgical technique depends mainly on the age of patient, rigidity of the chest wall, and presence of other associated abnormalities in the chest wall (Table 1).
 |
Table 1 Summary of seven case reports presented with different types of sternal defect, associated syndromes or anomalies and various techniques of management2,3,6,8–10 |
Conclusion
Careful clinical examination and radiological imaging play a key role in diagnosis, classification, and searching for other associated malformations. When the sternal cleft is associated with a congenital anomaly, particularly cardiac and vascular, the clinical outcome may be unfavorable. Early surgical repair of sternal cleft is a preferred intervention, and the chest wall elasticity allows for primary approximation in the neonatal period.
Abbreviations
PR, pulse rate; RR, respiratory rate; APGAR, appearance, pulse, grimace, activity, respiration; SC, sternal cleft; SVD, spontaneous vaginal delivery; PHACES, posterior fossa brain malformations, facial hemangioma, arterial anomalies, cardiac defects, eye abnormalities, sternal cleft; VACTERL, vertebral abnormalities, anorectal malformations, cardiac anomalies, tracheoesophageal fistula, renal and limb abnormalities; PDS, polydiaxanone suture.
Consent and Ethics
Written informed consent for publication of her clinical details and images was obtained from the patient’s parents (guardians). No identifiers were used. A copy of the written consent is available for review (written in local language) by the editors of this journal. Ethics approval from the IRB of Mekelle University College of Health Sciences was also obtained (MU-IRB 2028/2023).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tour ADL, Varlet F, Patural H, et al. Isolated and syndromic con-genital sternal cleft. J Rare Dis Diagn Ther. 2015;1:3.
2. Zamfir C, Zamfirescu A, Tanase C, BascaI. Sternal cleft –A rare congenital malformation. Pediatr Sur Case Rep. 2014;2(3):97–100. doi:10.1016/j.epsc.2014.01.012
3. Alshomer F, Aldaghri F, Alohaideb N, et al. Reconstruction of congenital sternal cleft: surgicalexperience and literature review. Plastic Reconstr Surg Glob Open. 2017;5:e1567. doi:10.1097/GOX.0000000000001567
4. Kamal YA, et al. Sternal cleft: appropriate approach to diagnosis and treatment. SCSOAJ. 2018;1(3):1–5.
5. de Campos JR, Filomeno LT, Fernandez A, et al. Repair of congenital sternal cleft in infants and adolescents. Ann Thorac Surg. 1998;66:1151–1154. doi:10.1016/S0003-4975(98)00596-7
6. Dumitrescu A, Ryan CA, Green A. Sternal cleft malformation in a newborn. BMJ Case Rep. 2017;2017. doi:10.1136/bcr-2017-2202
7. Engum SA. Embryology, sternal clefts, ectopia cordis, and Cantrell’s pentalogy. Seminar Pediatr Surg. 2008;17:154–160. doi:10.1053/j.sempedsurg.2008.03.004
8. Twomey EL, Moore AM, Ein S, et al. Prenatal ultrasonography and neonatal imaging of complete cleft sternum: a case report. Ultrasound Obstet Gynecol. 2005;25(6):599–601. doi:10.1002/uog.1835
9. Singh S, Lahoti BK, Garge S, Negi A, Jain V. Sternal cleft repair: a report of two cases and review of literature. Afr J Paediatr Surg. 2010;7(3):211. doi:10.4103/0189-6725.70432
10. Fouzia H, Mohamed H, Mohammed J, Siham T, Samir A, Abdelhak B. Sternal cleft associated with congenital aortic aneurysm in a neonate. J Clin Neonatol. 2015;4:49–50. doi:10.4103/2249-4847.151170
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

