Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Is Routine Intraoperative Frozen Section Analysis of Sentinel Lymph Nodes Necessary in Every Early-Stage Breast Cancer?
Authors Lerttiendamrong B, Treeratanapun N, Vacharathit V, Tantiphlachiva K, Vongwattanakit P, Manasnayakorn S, Vongsaisuwon M
Received 30 June 2022
Accepted for publication 8 September 2022
Published 19 September 2022 Volume 2022:14 Pages 281—290
DOI https://doi.org/10.2147/BCTT.S380579
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Bhoowit Lerttiendamrong, Nattanan Treeratanapun, Voranaddha Vacharathit, Kasaya Tantiphlachiva, Phuphat Vongwattanakit, Sopark Manasnayakorn, Mawin Vongsaisuwon
Department of Surgery, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Correspondence: Mawin Vongsaisuwon, Department of Surgery, Faculty of Medicine, Chulalongkorn University, 1873 King Chulalongkorn Memorial Hospital, Rama IV Road, Pathum Wan, Bangkok, 10330, Thailand, Tel +66 897158888, Email [email protected]
Purpose: Clinical application of the ACOSOG Z0011 trial results allows clinically node-negative breast cancer patients who meet criteria to avoid axillary dissection even when 1– 2 sentinel lymph nodes (SLNs) are positive for metastatic disease. Intraoperative frozen section (iFS) analyses of SLNs were thought to reduce re-operation rates despite variable reported sensitivity and possibility of a false negative result. This study evaluated the rate of re-operations prevented by SLN iFS in a tertiary care hospital in Bangkok, Thailand, over a 6-year time-frame.
Patients and Methods: From April 2016 to April 2022, 1284 sentinel lymph node biopsy (SLNB) procedures were performed. Of these, 214 cases were breast-conserving surgery in accordance with the ACOSOG criteria with concomitant usage of iFS. Clinicopathological features of these cases were collected and analyzed. Re-operation rates prevented by the additional intervention were reported.
Results: Only five additional operations were prevented with the usage of 214 iFS. The discordance rate between frozen and permanent sections in terms of presence of metastatic disease and number of total lymph nodes was around 15%. Tumor staging, node staging, Nottingham histologic grading and lymphovascular invasion are significant predictors of SLN metastasis.
Conclusion: iFS results in a very low prevention rate for follow-up ALND in patients with preoperative clinically negative axillary nodes and is associated with a non-negligible discordance rate with permanent sections. Our study suggests iFS may be avoided in most cases of early-stage clinically and radiographically node-negative breast cancer patients. Doing so may reduce surgical costs and total operative time without a significant impact on the overall quality of treatment and standard of care.
Keywords: breast cancer, sentinel lymph node biopsy, intraoperative frozen section analysis, ACOSOG Z0011
Introduction
Breast cancer is one of the leading causes of death in both developed and developing countries.1 Surgery is the main treatment method in early-stage invasive breast cancer.2 Treatment goals in early-stage breast cancer are tumor eradication and prevention of recurrence. Surgical therapy includes breast-conserving surgery with or without axillary lymph node dissection (ALND) and/or postoperative radiation as indicated.3 ALNDs have historically been a critical part in surgical breast cancer treatment.4 However, ALNDs were more likely to result in lymphedema compared to sentinel lymph node biopsy (SLNB), with no significant differences between the overall survival of both procedures.5 A historical switch from ALND to SLNB was implemented in the 1990s4. The ACOSOG Z0011 randomized controlled trial provided concrete evidence for the trend and demonstrated that the 10-year overall survival for breast cancer patients who had undergone SLNB was not inferior to those treated with ALND. Inclusion criteria for the Z0011 trial were breast cancer patients treated with breast-conserving therapy and adjuvant systemic therapy with clinical T1 or T2 node-negative disease (by palpation) and 1 or 2 sentinel lymph node (SLN) metastases.6,7 The International Breast Cancer Study Group 23–01 (IBCSG 23–01) and After Mapping of the Axilla: Radiotherapy Or Surgery? (AMAROS) clinical trials had also been performed to address the necessity of ALND.8,9 In response to the Z0011 trial, surgeons at MD Anderson, the United States of America, had significantly reduced ALND surgeries since 2012.10 As of 2017, SLNB has replaced ALND as the standard of care for those with a clinically negative axilla or N0 disease to reduce morbidity.11 It had also been proven that SLNB was more cost-effective than ALND.12
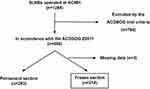 |
Figure 1 Patient selection flowchart. Abbreviations: SLNB, sentinel lymph node biopsy; KCMH, King Chulalongkorn Memorial Hospital. |
SLNB is based on the concept that if the SLN is negative for cancer, the other lymph nodes in that group will also be negative. Intraoperative SLNB was used to spare a second procedure, while permanent histopathology sections were used as a confirmatory assessment.13 However, frozen section (FS) detection rates are not ideal, and a meta-analysis has demonstrated that pooled sensitivity for FS was 0.86.14 Chao et al have shown that even when intraoperative SLNs were negative, results can be positive on permanent sections in over 8% of cases performed. Even with a non-100% detection rate and false-negative results, over 60% of the patients were spared a re-operation.15 Despite the evidence, a recent study questioned the need for SLN intraoperative frozen sections (iFS) for patients in whom additional ALND is unlikely to be performed according to the ACOSOG Z0011.16 A Korean study suggested that permanent section analyses alone without iFS can be performed in clinically node-negative patients.17 On the other hand, Cipolla et al concluded that the intraoperative procedure remained useful in preventing second axillary surgeries in patients with early breast cancer, however only 7.7% of the patients avoided a delayed ALND when the Z0011 criteria was applied.18
Surgeons at the King Chulalongkorn Memorial Hospital, a tertiary care university hospital in Bangkok, Thailand, implement ACOSOG Z0011 in their clinical practice. However, most surgeons still send iFS in breast-conserving surgeries. There is currently no research validating the benefit of the additional iFS analyses in Thailand. We suspect that very few patients satisfying ACOSOG criteria will turn out to have more than two positive nodes on final pathology and as such, aim to evaluate whether iFS confer additional diagnostic value or provide benefit in terms of change to treatment plan. Our secondary outcome was to analyze clinical and pathological factors that correlate with findings of positive SLNs.
Materials and Methods
A 6-year retrospective review of all SLNB operations conducted at King Chulalongkorn Memorial Hospital (KCMH) was performed. A total of 1284 SLNB operations were found from electronic-based and paper-based medical records from April 2016 to April 2022. Information on laterality of breast cancer, type of operation, tumor staging, Nottingham score grading, tumor histopathology and immunohistochemistry status were extracted from the medical records. Signed patient informed consent forms were not required in this study. This study was approved by the Institutional Review Board of Chulalongkorn University (IRB Number 555/64).
Inclusion and exclusion criteria were in accordance with the Z0011 trial. We included all breast cancer patients with clinical T1 or T2 disease and negative axillae by palpation on physical examination and radiological findings who then went on to have breast-conserving surgery. Patients who had received neoadjuvant chemotherapy and patients who had non-invasive breast cancer were excluded. A single patient who had bilateral SLNB satisfied our inclusion criteria and was classified as two separate cases. As demonstrated in Figure 1, there were in total 500 cases that were in accordance with our exclusion and inclusion criteria; 217 of these cases had an SLN iFS analysis, while the remainder relied on permanent section alone. Out of 217 cases that had iFS, 3 cases with missing data were excluded from our study. A final sum of 214 cases were statistically analyzed.
We evaluated cases that benefit from iFS in terms of re-operations prevented. We define treatment plan changes or reduction of re-operations as detection of more than two positive SLNs on final pathology, since according to ACOSOG, no additional ALND is indicated for patients with 0, 1 or 2 positive lymph nodes. We also performed data analysis to find predictive factors correlating with finding positive SLNs on final pathology. Tumor staging, Nottingham score grading and tumor histopathology results were based on the final report confirmed by pathologists at KCMH. Data were compiled using Microsoft Excel 2021 version 16.48 and analyzed using IBM SPSS Statistics Version 28.0. Categorical data were described as numbers and percentages. Age was classified into three groups and analyzed as a categorical variable. Chi-square and Fisher's exact test were used to compare categorical variables’ significance in influencing the final pathological SLN status. Statistical significance was defined as p-value <0.05. Sensitivity and specificity of finding positive nodes on iFS compared to those of permanent section were also reported.
Radiological findings were based on mammography and ultrasonography, which was performed on all patients. Our study utilized both single-agent mapping tracer (isosulfan blue dye) and dual tracer technique (isosulfan blue dye and radioisotope) for SLN detection; however, the majority of SLNs in this study were detected using a single tracer technique, which is the current standard of care in our center and across Thailand.19,20 The tracers were injected into breast skin or parenchymal tissue prior to the operation, although the selection of tracer technique was dependent on the operating surgeon. Hematoxylin and eosin were used as the main pathological staining method in this study.
Results
From April 2016 to April 2022, 500 cases out of 1284 SLNB operations satisfied both the inclusion and exclusion criteria. Additional categorizations were made based on the usage of the adjunctive iFS; 217 patients (43.40%) had both FS and permanent sections performed, while just over 55% had permanent sections alone. Data were incomplete in three cases, which were excluded from our study. A final 214 cases were analyzed in our research, which was performed by 13 surgeons at a single institution.
Analysis of those 214 cases revealed a patient age range from 26 to 90 years with a mean age of 56.7 years. Participants were then stratified by age group, with an interval of 10 years, from 21–30 to 81–90 years old. The 51–60-year-old age bracket had the greatest number of patients at 58 patients (27.10%), which was followed by 61–70-year-old category with 55 patients. The 21–30-year-old bracket held the least number of patients with two patients, corresponding to just less than 1% of the total participants.
Histopathologic results from SLN iFS and permanent sections were analyzed. The majority of FS results found no lymph node metastasis (174 out of 214 cases, corresponding to 81.31%). Discordance between the total number of lymph nodes detected by frozen and permanent sections was identified. The permanent sections had higher total detected lymph nodes than iFS in 32 cases (14.95%), with differences of up to 4 nodes as demonstrated in Table 1. Of the 38 cases with nodal disease on iFS, 19 (50%) of the total cases had a single positive node. Fourteen cases (36.84%) had two metastatic nodes on iFS, while only five patients with more than two positive nodes (13.16%) had indications for further ALND in accordance with the ACOSOG classification as shown in Table 2. Out of the five ALNDs performed, four ALNDs found additional metastatic nodes. There was a discrepancy between metastatic nodes detected by frozen and permanent sections in seven cases. Out of those seven cases, six were false-negative results with one false-positive iFS. Four out of the six false-negative cases revealed one under-detected metastatic node on final pathology compared to the intraoperative analysis, while the latter two cases showed a discordance of two and three metastatic nodes respectively. Significantly, one case had two positive nodes on iFS and three positive nodes on permanent section. This patient required a follow-up ALND even though iFS was performed. As for the single false-positive iFS, the number of metastatic nodes found on the frozen and permanent sections were 2 and 1, respectively. In 5 patients, each with one or two positive SLNs, ALNDs were performed even without ACOSOG indication due to surgeon judgement. No additional metastatic nodes were found in three cases, while one and two metastatic nodes were found in each of the remaining two cases, respectively. Sensitivity and specificity of iFS for positive SLNs compared to the permanent section gold-standard were 95% and 100%, respectively.
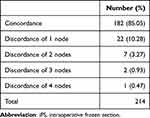 |
Table 1 Concordance/Discordance Rates of Total Number of Lymph Nodes Detected Between iFS and Permanent Sections |
 |
Table 2 Number of Positive SLNs by Number of Nodes Detected on iFS Vs Permanent Section |
 |
Table 3 Evaluation of Clinical and Pathological Factors Predicting Positive Sentinel Lymph Node Status in Early-Stage Breast Cancer Patients |
As shown in Table 3, 113 operations (52.80%) were performed on the left breast; the remaining 101 procedures were performed on the right side. One hundred and ninety-nine SLNBs cases (92.99%) were performed concurrently with a wide excision, while SLNB was performed after wide excision in 15 cases. Tumor status and nodal status were a statistically significant factor in predicting positive SLN status. Over 64% of the patients were reported as T1, while 75 patients (35.05%) were classified as T2. Twenty-three cases out of 75 patients with T2 disease were found to have positive axillary nodes, while just over 10% of the patients with T1 disease were node positive. Most of our cases (84.11%) were found to be N0. Of 34 patients with either N1 and N2 disease, 33 patients had metastatic nodes on final pathological analysis while a mere 7 out of 180 N0 cases were ultimately confirmed as nodal positive. No patients were found with distant metastasis. Nottingham histologic grading was a significant factor in predicting positive lymph node status. More than half of our cases were Nottingham histologic grade 2. Twenty-nine out of 111 cases (26.13%) with histologic grade 2 disease also had a positive lymph node status, while the figures were 5.66% and 16% for grade 1 and grade 3 disease, respectively.
Histopathologically, invasive ductal carcinoma and invasive lobular carcinoma account for over 90% of the total cases. One hundred eighty-three tumor pathological results (85.51%) were found to be of invasive ductal carcinoma in origin, while 16 cases (7.48%) were invasive lobular carcinoma. Twenty-five out of 51 patients with positive lymphovascular invasion also had metastatic nodal disease. As for patients without lymphovascular invasion, 15 patients out of 162 cases revealed positive nodes on the final pathological analysis. Lymphovascular invasion was a statistically significant factor in predicting metastatic lymph nodes status (p-value <0.001). None of the immunohistochemistry status: estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), correlated with a positive nodal status. Most of the patients’ immunohistochemistry results in this study were ER positive, PR positive and HER2 negative.
Discussion
The ACOSOG Z0011 trial revolutionized surgical treatment of breast cancer allowing for a less radical intervention. The trial concluded that for patients that fulfilled its criteria, the 10-year overall survival for patients treated with SLNB was not inferior to those who were treated with ALND.6,7 In the post-Z0011 era, ALND operations have decreased as demonstrated by various studies in the United States, Ireland and Italy.10,21,22 With reduction of ALNDs in early-stage breast cancer patients, lymphedema as a complication from ALND can also be mostly avoided.23,24 The IBCSG 23–01 and AMAROS trials recommended alternatives to conventional ALND. The IBCSG trial demonstrated no difference between disease-free survival in the ALND and non-ALND groups for patients with micrometastases and tumors ≤5 centimeters.8 Moreover, the AMAROS trial found that for early breast cancer patients, axillary radiotherapy may be a comparable alternative to ALND, without the complications associated with ALND.9 As evident from all three studies, ALND was replaced as a standard of care by newer treatment options. SLNB has been incorporated into the current standard of care for early breast cancer patients since 2015.25
As the global trend is shifting towards SLNB, SLNB technique must be revised in order to maintain the quality of care and maximize cost-effectiveness. Additional iFS analyses were utilized with a main goal of eliminating a second surgical procedure. In the early 2000s, without implementation of the ACOSOG trial, studies have shown that frozen sections were highly accurate and served as a useful predictor in preventing ALND.26,27 With ACOSOG implemented as a current standard of care, the benefits and the indications for using this additional procedure must be reviewed.
A study summarized that a mere six re-operations were avoided from the utilization of 132 additional procedures and concluded that permanent section alone may be sufficient in clinically node-negative patients.17 Treeratanapan et al also confirmed that out of 239 patients with permanent section alone, no patients were indicated for re-operation. Additional iFS would not alter any standard of care in patients who fulfill the ACOSOG classification.28 An Iranian study reinforced that low tumor burdens were detected from FS in early-stage breast cancer patients.29
From a total of 214 cases analyzed in this 6-year retrospective review, 5 cases were found with more than two metastatic nodes which was an indication for further ALND in accordance with the Z0011 trial. Five patients (2.34%) directly benefited from avoidance of a re-operation out of 214 SLNBs and iFS performed. Four out of five patients indicated for additional ALND had additional metastatic nodes. Otherwise stated, 82% of the patients with early-stage breast cancer and clinically negative axillae did not benefit from iFS as evidenced by zero metastatic nodes detected.
Additionally, FS have variable reported sensitivity. According to a meta-analysis, FS sensitivity ranged from 66.7% to 95.8%. Pooled sensitivity was 0.86 and pooled specificity was 0.96.14 Additionally, iFS is known to have a wide range of false-negative rates from 5.5% to 43% across numerous studies, due to a protocol variation.15,30–32 False-negative rates are critical as each false-negative case causes an additional patient to undergo a second operation even with though iFS was used, defeating this extra intervention’s sole purpose. Our study found a discordance of total lymph node numbers detected by iFS and the permanent section of around 15%. If the nodes detected exclusively on the permanent section turned out to be positive, the following error may contribute to a false-negative result. We also found seven instances of unequal numbers of metastatic nodes found on FS compared to permanent sections. Notably, a patient had two metastatic nodes on iFS analysis, while three nodes were positive on permanent section due to additional total nodes detected on the final analysis. This patient required a follow-up ALND even with the usage of iFS.
Cost-effectiveness of the additional intervention must also be brought into consideration. Current FS at King Chulalongkorn Memorial Hospital stands at approximately 37–191 USD. Our research found that a baseline cost of over 42,000 USD was needed to prevent 5 follow-up ALND operations, without considering additional surgery time and additional medical personnel cost. We encourage future research to explore the cost-effectiveness of FS analyses in clinically and radiographically lymph node-negative patients.
Intraoperative results may also create unnecessary hesitancy for general surgeons. Three different surgeons who performed five separate cases received a iFS result each with one or two metastatic nodes. All five cases did not have indications for additional surgery by the Z0011 trial; however, ALNDs were performed to ensure total tumor removal. No further metastatic nodes were detected from ALNDs in three cases. Without the usage of iFS, such extra interventions may have been avoided with reduction of hesitancy in surgeons who do not specialize in breast cancer surgery.
A study conducted at Memorial Sloan-Kettering Cancer Center (MSKCC) found that age, tumor size, tumor type, lymphovascular invasion, tumor location, multifocality, estrogen receptor and progesterone receptor status were associated with sentinel lymph node metastasis.33 The MSKCC model has been validated throughout the world; it has proven to be more accurate in American and Australian studies with the area under the ROC curve ranging from 0.75 to 0.86.34,35 On the other hand, it demonstrated a lower accuracy in the European population with a range of 0.53 to 0.68.36,37 A Thai study concluded that tumor size, type and location, lymphovascular invasion, multifocality and progesterone receptor status were significant indicators for predicting nodal metastasis. Area under the ROC was 0.73, deemed an accurate tool in Thai patients.38 Our research supported these claims; we found that tumor staging, node staging, Nottingham histologic grading and lymphovascular invasion are significant predictors of SLN metastasis. Limitations of our epidemiological interpretation include a limited number of patients found with positive lymph nodes. We encourage a multicenter nationwide validation of the MSKCC model in Asian populations.
This study suggests that only a few re-operations were prevented by the addition of SLN iFS and even with their utilization, a second operation may be needed regardless. Our study suggests iFS may be avoided in most cases of early-stage clinically and radiographically node-negative breast cancer patients; however, correlation with clinical and radiological findings is advised for each individual case. Doing so will increase the cost-effectiveness of breast cancer treatment as well as reduce the financial burden on patients without impacting the overall quality of treatment. Moreover, a reduction in the total operative time and secondary hospital costs is expected.17,39 In resource poorer settings that do not have the luxury of sending SLN iFS, we have routinely observed complete omission of SLNB in favor of routine ALND because iFS was unavailable. Based on our data, we recommend instead performing the SLNB for permanent section and omitting iFS in this setting for patients with early-stage cancer and clinically and radiographically negative nodes. Long-term re-operative rates and cancer recurrence between the two groups must be further explored.
Conclusion
This 6-year retrospective study conducted in Thailand found that sending iFS in early-stage breast cancer patients with clinically negative axillae had low yield in terms of changing treatment plan. Five re-operations were prevented from the usage of 214 extra iFS procedures. There was also a non-negligible discordance rate between iFS and final pathology results, sometimes resulting in a second operation even though iFS was initially negative. Our study suggests iFS may be avoided in most cases of early-stage clinically and radiographically node-negative breast cancer patients. Doing so may reduce surgical costs and total operative time without a significant impact on the overall quality of treatment and standard of care.
Ethics Approval and Informed Consent
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Chulalongkorn University Institutional Review Board (IRB number 555/64). Individual consent was waived in this study as deemed by the Chulalongkorn University Institutional Review Board with regards to the study design as a retrospective study and the usage of anonymous case report form, ensuring patient data confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was not supported by any external funding sources.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Winters S, Martin C, Murphy D, et al. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1–32. doi:10.1016/bs.pmbts.2017.07.002
2. Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician. 2010;81(11):1339–1346.
3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323
4. Magnoni F, Galimberti V, Corso G, et al. Axillary surgery in breast cancer: an updated historical perspective. Semin Oncol. 2020;47(6):341–352.
5. Bromham N, Schmidt-Hansen M, Astin M, et al. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Re. 2017;1(1):CD004561. doi:10.1002/14651858.CD004561.pub3
6. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575.
7. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi:10.1001/jama.2017.11470
8. Galimberti V, Cole BF, Zurrida S, et al.; International Breast Cancer Study Group Trial 23-01 investigators. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a Phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305. doi:10.1016/S1470-2045(13)70035-4
9. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi:10.1016/S1470-2045(14)70460-7
10. Caudle AS, Hunt KK, Tucker SL, et al. American college of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012;19(10):3144–3151. doi:10.1245/s10434-012-2531-z
11. Zahoor S, Haji A, Battoo A, et al. Sentinel lymph node biopsy in breast cancer: a clinical review and update. J Breast Cancer. 2017;20(3):217. doi:10.4048/jbc.2017.20.3.217
12. Songtish D, Praditsitthikorn N, Teerawattananon Y. A cost-utility analysis comparing standard axillary lymph node dissection with sentinel lymph node biopsy in patients with early stage breast cancer in Thailand. Value Health Reg Issues. 2014;3:59–66. doi:10.1016/j.vhri.2014.01.003
13. Mysorekar VV. sentinel lymph node biopsy in breast cancer. World J Oncol. 2010;1(1):1–6. doi:10.4021/wjon2010.01.1206
14. St John ER, Al-Khudairi R, Ashrafian H, et al. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: a meta-analysis. Ann Surg. 2017;265(2):300–310.
15. Chao C, Wong SL, Ackermann D, et al. Utility of intraoperative frozen section analysis of sentinel lymph nodes in breast cancer. Am J Surg. 2001;182(6):609–615. doi:10.1016/s0002-9610(01)00794-2
16. Poling JS, Tsangaris TN, Argani P, et al. Frozen section evaluation of BREAST carcinoma sentinel lymph nodes: a retrospective review OF 1940 cases. Breast Cancer Res Treat. 2014;148(2):355–361. doi:10.1007/s10549-014-3161-x
17. Jung SM, Woo J, Ryu JM, et al. Is the intraoperative frozen section analysis of sentinel lymph nodes necessary in clinically negative node breast cancer? Ann Surg Treat Res. 2020;99(5):251. doi:10.4174/astr.2020.99.5.251
18. Cipolla C, Graceffa G, Cabibi D, et al. Current role of intraoperative frozen section examination of sentinel lymph node in early breast cancer. Anticancer Res. 2020;40(3):1711–1717.
19. Ratanawichitrasin A, Rojananin S, Bhothisuwan K, et al. Lymphatic mapping with isosulfan blue and sentinel lymph node biopsy for breast cancer patients. Thai J Surg. 1999;20(3):93–96.
20. Chottanapund S. Rate of sentinel lymph node identification using isosulfan blue dye in breast cancer patients at Charoenkrung Pracharak Hospital, Thailand. Asian Biomed. 2017;8(4):517–524.
21. Joyce DP, Lowery AJ, McGrath-Soo LB, et al. Management of the axilla: has Z0011 had an impact? Ir J Med Sci. 2016;185(1):145–149.
22. Morigi C, Peradze N, Galimberti V, et al. Feasibility and surgical impact of Z0011 trial criteria in a single-Institution practice. Breast J. 2020;26(7):1330–1336.
23. Huang TW, Su CM, Tam KW. Axillary management in women with early breast cancer and limited sentinel node metastasis: a systematic review and metaanalysis of real-world evidence in the post-ACOSOG Z0011 era. Ann Surg Oncol. 2021;28(2):920–929.
24. Sakorafas GH, Peros G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15(3):153–165.
25. Motomura K, Sentinel node biopsy for breast cancer: past, present, and future. Breast Cancer. 2015;22(3):212–220. doi:10.1007/s12282-012-0421-7
26. van de Vrande S, Meijer J, Rijnders A, et al. The value of intraoperative frozen section examination of sentinel lymph nodes in breast cancer. Eur J Surg Oncol. 2009;35(3):276–280.
27. Wada N, Imoto S, Hasebe T, et al. Evaluation of intraoperative frozen section diagnosis of sentinel lymph nodes in breast cancer. Jpn J Clin Oncol. 2004;34(3):113–117.
28. Treeratanapun N, Lerttiendamrong B, Vacharathit V, et al. Is sentinel lymph node biopsy without frozen section in early stage breast cancer sufficient in accordance with ACOSOG-Z0011? A retrospective review from King Chulalongkorn Memorial Hospital. BMC Surg. 2022;22(1):261. doi:10.1186/s12893-022-01709-6
29. Godazande G, Moradi S, Naghshvar F, et al. Is necessary intraoperative frozen section in sentinel lymph node biopsy for breast cancer patients? Asian Pac J Cancer Prev. 2020;21(3):647–651.
30. Loh ZJ, Lee KT, Chen YP, et al. False-negative frozen section of sentinel nodes in early breast cancer (cT1-2N0) patients. World J Surg Oncol. 2021;19(1):183.
31. Wong J, Yong WS, Thike AA, et al. False negative rate for intraoperative sentinel lymph node frozen section in patients with breast cancer: a retrospective analysis of patients in a single Asian institution. J Clin Pathol. 2015;68(7):536–540.
32. Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91(4):368–373.
33. Bevilacqua JL, Kattan MW, Fey JV, et al. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25(24):3670–3679. doi:10.1200/jco.2006.08.8013
34. Cripe MH, Beran LC, Liang WC, et al. The likelihood of additional nodal disease following a positive sentinel lymph node biopsy in breast cancer patients: validation of a nomogram. Am J Surg. 2006;192(4):484–487.
35. Soni NK, Carmalt HL, Gillett DJ, et al. Evaluation of a breast cancer nomogram for prediction of non-sentinel lymph node positivity. Eur J Surg Oncol. 2005;31(9):958–964.
36. Syed A, Eleti S, Kumar V, et al. Validation of Memorial Sloan Kettering Cancer Center nomogram to detect non-sentinel lymph node metastases in a United Kingdom cohort. G Chir. 2018;39(1):12–19.
37. Klar M, Jochmann A, Foeldi M, et al. The MSKCC nomogram for prediction the likelihood of non-sentinel node involvement in a German breast cancer population. Breast Cancer Res Treat. 2008;112(3):523–531.
38. Nimboriboonporn A, Sa-nguanraksa D, Samarnthai N, et al. Predictive factors for sentinel lymph node metastasis and validation of memorial Sloan–Kettering cancer center nomogram in Thai breast cancer patients. Thai J Surg. 2020;41(2):29–39.
39. Ahn SK, Kim MK, Kim J, et al. Can we skip intraoperative evaluation of sentinel lymph nodes? Nomogram predicting involvement of three or more axillary lymph nodes before breast cancer surgery. Cancer Res Treat. 2017;49(4):1088–1096.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
