Back to Journals » Patient Preference and Adherence » Volume 17
Is Non-Adherence Associated with Adverse Outcomes in Kidney Transplant Recipients? The Role of Non-Adherence as a Risk and Predictor Factor for Graft Loss and Death
Authors Torres-Gutiérrez M, Lozano-Suárez N , Burgos-Camacho VA, Caamaño-Jaraba J, Gómez-Montero JA, García-López A , Girón-Luque F
Received 16 September 2023
Accepted for publication 2 November 2023
Published 11 November 2023 Volume 2023:17 Pages 2915—2925
DOI https://doi.org/10.2147/PPA.S436833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Manuel Torres-Gutiérrez,1 Nicolás Lozano-Suárez,2 Viviana A Burgos-Camacho,1 Jessica Caamaño-Jaraba,1 Julia Andrea Gómez-Montero,2 Andrea García-López,2 Fernando Girón-Luque2,3
1Department of Mental Health, Colombiana de Trasplantes, Bogotá, Colombia; 2Department of Transplant Research, Colombiana de Trasplantes, Bogotá, Colombia; 3Department of Transplant Surgery, Colombiana de Trasplantes, Bogotá, Colombia
Correspondence: Andrea García-López, Transplant Research, Colombiana de Trasplantes, Av Carrera, 30 No. 47 A-74, Bogota, 111311, Colombia, Email [email protected]
Introduction: Non-adherence in kidney transplants is diversely defined. Immunosuppression non-adherence (INA) is the most used definition and has been associated with graft loss and acute rejection. But INA assesses only one fraction of adherence. Therefore, we analyzed the association of a holistic non-adherence definition with transplant outcomes and compared its prediction performance with other definitions.
Methods: We retrospectively included 739 kidney recipients between 2019 and 2021. We evaluated holistic non-adherence (HNA), suboptimal-immunosuppressor levels (SIL), appointment non-adherence (ANA), procedure non-adherence (PNA) and INA. The main outcomes were graft loss, graft rejection, and mortality. A backward logistic regression was performed estimating adjusted and un-adjusted odds ratio (OR) for each outcome. Finally, we compared the non-adherence definitions’ prediction for the main outcomes using the area under the curve.
Results: HNA was present in 28.7% of patients. Non-adherent patients had an adjusted OR of 2.66 (1.37– 5.15) for mortality, 6.44 for graft loss (2.71– 16.6), and 2.28 (1.15– 4.47) for graft rejection. INA and PNA presented a moderate discrimination for graft loss and HNA and ANA mild-to-moderate discrimination for graft loss and death.
Conclusion: Holistic non-adherence was associated with worst outcomes in kidney recipients and had a significant prediction performance for graft loss and mortality.
Keywords: kidney transplantation, patient adherence, mortality, graft survival, patient outcome assessment
Introduction
Evidence supports that adherence improves health outcomes and quality of life.1–3 Even the World Health Organization (WHO) quoted “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments”.4 In kidney transplant recipients, adherence is a key factor for medium- and long-term outcomes.5,6 Therefore, the adherence definition is crucial in understanding kidney recipients’ outcomes. The most common definition used is immunosuppression non-adherence (INA), and some authors defend it for its simplicity.1,7 But for many others, adherence is a multifaceted construct that transcends the correct taking of the medication.1,8 The WHO defined it as “the extent to which a person’s behavior – taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a healthcare provider”.9
Nonetheless, immunosuppression non-adherence (INA) is the most studied definition in medical research for kidney recipients and has been associated with graft loss and graft acute rejection.10,11 Furthermore, INA has also been associated with mortality, displaying an HR of 3.07.12 Not only is INA associated with worst outcomes but non-adherence to appointments (ANA) is also a significant risk factor for graft rejection and graft loss, with an 8.2 OR for ≥2 rejection episodes13 and a 65% higher risk for graft loss. Health literacy has been studied in kidney recipients before and after the transplant, with evidence that marginal health literacy increases waiting list mortality and decreases patient survival following kidney transplantation.14 Some authors consider that the relation of non-adherence with graft loss is caused by an increased cellular rejection and less benefits from immunosuppressors.15
Therefore, even if adherence has been defined as the complete patient behavior regarding the treatment and disease, most evidence in kidney transplant reported the outcomes of a fragment of adherence, commonly INA or ANA. Consequently, there is a need to describe the outcomes of non-adherence in kidney recipients using a wide definition that assesses beyond INA and to compare these definitions’ predictive value for different kidney recipient outcomes.
Methods
Design and Objectives
A retrospective cohort study was conducted to determine the outcomes of non-adherence in kidney transplant recipients and to compare the association of different non-adherence definitions with the outcomes studied.
Population and Sample
We included all kidney transplant recipients attended by Colombiana de Trasplantes between January 2019 and July 2021. Follow-up was until death, graft loss, or after the first year of monitoring since the adherence assessment. Patients who did not complete the follow-up were excluded. The sample size was not calculated as we performed a convenience sample, including all patients available that fulfilled the selection criteria.
Data Collection and Variables Definition
A retrospective data collection from the clinical records was performed. Adherence was considered a holistic clinical impression assessed in a semi-structured interview by a mental health team member as part of routine clinical care. This interview explored sociodemographic, clinical, and transplant care variables in the pre-transplant, early, and late post-transplant periods. The semi-structured interview and definitions of the included variables are presented in Appendices 1 and 2. In addition, other adherence definitions were obtained, such as immunosuppression non-adherence (INA), suboptimal immunosuppressor levels (SIL), appointment non-adherence (ANA) and procedure non-adherence (PNA) (Table 1).
 |
Table 1 Non-Adherence Definition and Assessment Methods |
The outcomes were graft rejection, death, and graft loss. Graft rejection was confirmed by the pathology conclusion of acute rejection in a renal biopsy, and graft loss was considered the definitive renal replacement therapy requirement after the kidney transplant.
Statistical Analysis
We conducted a descriptive analysis based on each variable nature and distribution. First, we used the chi-square test to compare categorical variables and the Student’s t-test or Mann–Whitney U-test for quantitative variables, determined by their respective distributions. We deemed statistical significance by a p-value of less than 0.05. Next, we employed logistic regression to uncover the factors associated with each outcome. We calculated the crude and adjusted odds ratios (OR) alongside their corresponding 95% confidence intervals (CI) to pinpoint independent risk factors. An automatic backward variable selection method based on Akaike’s information criterion (AIC) was used as model variable inclusion. Variables that changed the estimates by 10% were considered cofounders and controlled by their inclusion in the model. The Hosmer–Lemeshow test and Nagelkerke’s R2 (Pseudo-R-square) were used to assess the reduced model. Finally, we evaluated multicollinearity using the Variance Inflation Factor (VIF), excluding any variable with a VIF greater than five.
To compare the prediction of the different non-adherence definitions (HNA, INA, ANA, PNA, SIL) we used a logistic regression prediction model following the steps described by Shipe ME and Steyerberg.16,17 We trained each model with 80% of the sample and tested the prediction in the remaining 20%; the train and test population selection were randomized. Following, we estimated non-adjusted ORs and confidence intervals for each outcome. Next, we calculated the Area Under the Curve (AUC), sensitivity, and specificity in the test sample. Understanding the AUC as a marker of the discrimination ability considering a prediction of a binary event, where 1 is a perfect discrimination between populations and 0.5 a failed discrimination.18,19 We understand the results using a common classification presented by de Hond et al,19 where categories assessed discrimination as failed or random (0.5–0.6), low or mild (0.6–0.7), moderate (0.7–0.8), good (0.8–0.9) and excellent (0.9–1); therefore, non-significant all AUC values were below 0.6. A sensitivity analysis was made comparing baseline characteristics between included and excluded patients. All analyses were conducted using R software version 4.2.2.
Ethical Statement
This study complied with national and international guidelines, such as the Declaration of Helsinki20 and the Colombian Resolution 8430 of 1993.21 Following the Declaration of Istanbul, all kidneys were donated voluntarily with written informed consent.22 The study was approved by the Dexa Diab ethics committee, and the written informed consent was waived by the Dexa Diab ethics committee, due to retrospective nature of the study and anonymized presentation of results.
Results
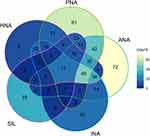
Between January 2019 and July 2021, we assessed 1031 patients, but 292 patients did not complete the follow-up and were excluded. Therefore, the study included 739 kidney transplant recipients that achieved the selection criteria, the median follow-up time was 26 months, baseline characteristics are presented in Table 2. Holistic non-adherence was presented in 28.7% of the patients, INA in 23%, SIL in 15.3%, ANA 41.1%, and PNA 37.2%. The distribution of non-adherent patients is presented in a Venn diagram (Figure 1) and of adherent patients in the Appendix 3 (Figure 1).
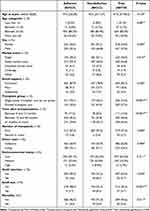 |
Table 2 Characterization of Adherent and Non-Adherent Patients and Their Outcomes |
Compared to adherent patients, non-adherents had more divided transplant care, longer time after transplantation, and less stable marital union. In the outcomes, non-adherent patients had more graft rejection (8.5% vs 4.6%, P value 0.055), higher incidence of graft loss (8.5% vs 1.7%, P value <0.001) and mortality (9% vs 4%, P value 0.011) (Table 2).
Multivariate Analysis
In the multivariate analysis, non-adherence was associated with all adverse outcomes. The non-adherent patients had an adjusted OR of 2.28 (IC95% 1.15–4.47) for graft rejection, 2.66 (IC95% 1.37–5.15) for mortality, and 6.44 for graft loss (IC95% 2.71–16.6).
The logistic regression for graft rejection exposed that each year growth decreases a 4% the risk of the outcome (OR 0.96, CI 95% 0.94–0.98) and 13 to 60 months after transplantation (OR 0.36, CI 95% 0.16–0.79) presented as a protective factor compared to less than one year after transplant. In the model for mortality age presented as a risk factor, with a 5% increased risk for each year’s growth (OR 1.05, CI 95% 1.02–1.08). Finally, the model for graft loss included age (OR 0.93, CI 95% 0.90–0.97), longer time after transplantation (Between 13 and 60 months OR 0.21, CI 95% 0.06–0.71), and a living donor (OR 0.27, CI 95% 0.08–0.74) as protective factors. All adjusted and non-adjusted OR with the respective p values are described in Table 3.
 |
Table 3 Multivariate Analysis of the Main and Secondary Outcomes |
The three outcome reduced models had goodness of fit by the Hosmer–Lemeshow test and no interaction or multicollinearity. The only confounding variable found was the marital status for graft loss which was controlled by its inclusion in the model. The Nagelkerke R2 was 0.10 for mortality, 0.20 for graft loss, and 0.11 for graft rejection.
Adherence Definitions Comparison
The different non-adherence definitions were compared for mortality, graft loss and acute rejection. The AUC and un-adjusted ORs are presented in Table 4 and Figure 2.
 |
Table 4 Adherence Definitions Non-Adjusted Odds Ratios (OR) and Area Under the Curve (AUC) for Each Outcome |
 |
Figure 2 ROC curves for (A) mortality, (B) graft loss and (C) graft rejection. |
For the three outcomes, only mild and moderate discrimination was found. For mortality, HNA and ANA presented a mild prediction discrimination, while PNA, SIL and INA had no prediction significance. For graft loss, HNA and ANA elicited a mild prediction discrimination, and INA and PNA a moderate prediction discrimination. Finally, for graft rejection, all the definitions had no prediction significance.
Sensitivity Analysis
We compared the baseline characteristics of the included and excluded patients and found no significant differences, except for donor type, time after transplant, and transplant care group. We conducted another analysis divided by donor type. We found a similar risk factor tendency for HNA for all outcomes. Still, it was only significant for mortality and graft rejection of living donors and graft loss of cadaveric donors. Finally, we analyzed adolescent patients who demonstrated a higher prevalence of all non-adherence definitions, graft loss, and graft rejection; the associated factors for each outcome could not be estimated because of the small number of patients. The detailed analysis is presented in Appendix 3.
Discussion
Main Findings
The main goal of our 739-kidney recipient’s retrospective cohort was to describe the outcomes of non-adherence using a wide definition proposed by the authors. Our results have undeniably confirmed that non-adherence is a significant risk factor for worst outcomes in kidney transplant recipients. This is consistent with previous literature that associated INA with adverse outcomes;12,23,24 but novel in the non-adherence definition of a behavioral pattern beyond INA. This definition was associated with mortality, graft rejection, and graft loss. Moreover, HNA performs as a relevant predictor for mortality and graft loss.
Non-Adherence and Worst Outcomes
Our study found that almost one in three kidney recipients is non-adherent. This is consistent with the systematic review of Belaiche S et al,25 who described a non-adherence prevalence of 1.6 to 58.7% in kidney transplant patients. This variability is caused by diverse assessment methods, for example, electronic monitoring, immunosuppressant blood levels, interviews, self-reports, and refills, among others.25,26 Furthermore, our study found different non-adherence incidences upon the definition used. Therefore, the non-adherence definition and assessment are primordial for understanding results. Moreover, as non-adherent patients are a high-risk population, a wide definition permits a broader diagnosis of patients that may need special care to reduce the risk of worst outcomes.27
Graft loss was presented in 3.7% of our patients. Similar to the observational study published by Prihodova,12 who evidenced a graft loss prevalence of 4.4% in kidney transplant patients. However, this is lower than the previous graft loss incidence of 11.6% reported by Pinto in our same population28 and 13% reported in different populations by Gumabay.29 The incidence of this outcome can vary due to the numerous factors contributing to graft loss.30 Even so, this outcome is of paramount relevance due to the need for re-transplantation or dialysis. Therefore, multiple studies have evaluated the associated factors with graft loss, but they mainly include clinical variables.28,31 Therefore, there is a need to assess the relation of nonclinical factors such as non-adherence.
Following that course, one of our main results is the sixfold increased risk of graft loss for non-adherent patients. These results are comparable to Prihodova’s results, who found a similar HR of 6.03 (P < 0.05) for graft loss in poorly adherent patients12 and Butler´s systematic review that described a sevenfold greater risk for graft loss in non-adherent (OR 7.1 p < 0.001).32 A longitudinal cohort study presented similar evidence, describing ANA and INA as a risk factor for graft loss, and they also found that a joint view of non-adherence provides a higher association measure for graft loss.33 Conversely, other studies have concluded that non-adherence is not associated with graft loss; these authors explained that this might occur cause of insufficient statistical power, given the relatively small number of events.29
Some authors consider that the relation of graft loss and non-adherence is caused by an increased cellular rejection, the development of donor-specific antibodies, transplant glomerulopathy, and a reduced beneficial response to immunosuppressor.15 On the other side, the non-adherence consensus conference reported that the impact of non-adherence may be due to the consequences of comorbidities and lifestyle factors. But they clarified that it is a complex relationship that could be affected by several other mechanisms.34 Currently, there is little understanding of the relationship between graft loss and non-adherence. However, it is known that non-adherence is a relevant risk factor for graft loss, which may be aggravated in the acute rejection panorama.
Evidence suggests that non-adherence is accountable for 47–80% of late acute rejections.11,35,36 Our study confirms the association between non-adherence and graft rejection, based on a twofold increased risk (adjusted OR 2.28, p 0.016) for graft rejection compared to adherent patients. Furthermore, previous literature reported similar findings: ANA increased 1.5 times the risk,33 INA a 2.64 OR (p 0.012)15 and history of non-adherence by healthcare professional assessment had an HR 1.32 (p 0.250).29 This is relevant as these acute rejections affect the quality of life of the kidney recipients, increase caretaker stress, and intensify the therapies needed and the cost of the treatment.37–42
This study also provides compelling evidence of the significant association between mortality and non-adherence, increasing the risk of death twofold compared to adherent kidney recipients. This is comparable to previous evidence of a 31% higher risk for death in kidney transplant patients with appointment non-adherence.33 Moreover, even regular immunosuppression adherence has been reported as a risk factor, with a 54% higher risk of death compared to excellent adherence.43 Therefore, non-adherence plays a major role in kidney recipient care, associated with several worst outcomes, as suggested by previous evidence and confirmed by our results.
Non-Adherence as a Predictive Tool
In the light of the evidence of non-adherence as a risk factor for worst outcomes, our team tested five different non-adherent definitions for the four studied outcomes. The definitions were self-reported immunosuppressor non-adherence (INA), suboptimal immunosuppressor levels (SIL), appointment non-adherence (ANA), procedure non-adherence (PNA) and holistic non-adherence (HNA). None of the above provides an optimal prediction for graft rejection. However, HNA and ANA presented a mild-to-moderate predictive discrimination for graft loss and mortality, while INA and PNA presented only for graft loss.
Currently, multiple studies have reported novel prediction models for graft loss. A systematic review of risk prediction models for kidney transplantation found more than 39 articles presenting or validating prediction models in this population. The main factors addressed were related to the transplantation (ex. HLA mismatch, acute rejection, cold ischemia time), donor clinical variables (ex. age, gender, BMI, diabetes, dialysis duration, creatinine), and recipient clinical variables (ex. age, donor type, gender, comorbidities).44 These proposed models have different prediction methods: decision tree, random forest, artificial neural network, support vector machine, adaptive boosting, Cox model, deep learning, and logistic regression.28,44–47 Therefore, in different prediction performances, Naqvi46 reported an AUC of 69% for five years of graft loss prediction and Yoo K45 an 70% AUC for ten years of graft survival. In our population, Pinto et al28 have proposed a prediction model for graft loss and death, reporting a c-index of 0.6 and 0.72, respectively. Both models, ours and Pinto’s, had mild performance, but ours considered only non-adherence, while Pinto’s included mainly clinical factors. Therefore, we believe that the conjunction of clinical factors and adherence to new prediction tools may improve the performance of the models.
Study Limitations
First, a main limitation is the subjectivity of the non-adherence definition that could restrain the reproducibility of this methodology, to reduce this limitation and increase the reproducibility the semi-structured interview was presented in the Supplemental Material. Also, non-adherence was presented only in the first assessment and not as process across time. Second, there was an exclusion of a third of patients due to incomplete follow-up, who may be also non-adherent patients, to address this limitation a sensitivity analysis was made comparing baseline characteristics between included and excluded, but the outcome information could not be retrieved. Third, previous literature evidence has supported higher non-adherence prevalence in the Covid-19 pandemic.48 Therefore, these results must be considered in the global pandemic context and reinforced by future research in post-pandemic. Finally, the generalization of these findings needs validation in other populations, as these descriptions were made in a single-center institution with a convenience sample.
Conclusion
Finally, we have described that a wide definition of non-adherence is a significant risk factor for worst outcomes in kidney transplant recipients. Compared to other non-adherence definitions, ours and appointment non-adherence have mild-to-moderate discrimination in predicting graft loss and death. This may open the discussion for the usage of different non-adherence definitions above immunosuppression noncompliance. Also, these results may be the base for further research in kidney recipient’s outcome prediction that includes clinical considerations and non-adherence in the post-pandemic era. In addition, this study supports the clinical consideration of non-adherence as a fundamental risk factor in this population. Therefore, we encourage healthcare professionals to assess non-adherence in kidney transplant recipients and consider early interventions to prevent worst outcomes.
Acknowledgments
The authors thank Colombiana de Trasplantes for its support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive a specific grant from any funding agency.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Ahmed R, Aslani P. What is patient adherence? A terminology overview. Int J Clin Pharm. 2014;36(1):4–7. doi:10.1007/s11096-013-9856-y
2. Shafiekhani M, Shahabinezhad F, Tavakoli Z, et al. Quality of life associated with immunosuppressant treatment adherence in liver transplant recipients: a cross-sectional study. Front Pharmacol. 2023;14.
3. Hooper DK, Varnell CD, Rich K, et al. A Medication Adherence Promotion System to Reduce Late Kidney Allograft Rejection: a Quality Improvement Study. Am J Kidney Dis. 2022;79(3):335–346. doi:10.1053/j.ajkd.2021.06.021
4. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proce. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
5. Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transplant Int. 2005;18(10):1121–1133. doi:10.1111/j.1432-2277.2005.00176.x
6. Nevins TE, Matas AJ. Medication noncompliance: another iceberg’s tip. Transplantation. 2004;77(5):776–778. doi:10.1097/01.TP.0000110409.71847.6F
7. Caisley H, Müller U. Adherence to medication in adults with attention deficit hyperactivity disorder and pro re nata dosing of psychostimulants: a systematic review. Eur Psychiatry. 2012;27(5):343–349. doi:10.1016/j.eurpsy.2012.01.002
8. Meichenbaum D, Turk DC. Facilitating Treatment Adherence a Practitioner’s Guidebook.
9. World Health Organization. Adherence to long-term therapies: evidence for action. World Health Org. 2003;194.
10. Kiley Deborah J, Lam CS, Pollak R. A study of treatment compliance following kidney transplantation. Transplantation. 1993;55(1):51–56. doi:10.1097/00007890-199301000-00010
11. Rovelli M, Palmeri D, Vossler E, Bartus S, Hull D, Schweizer R. Noncompliance in organ transplant recipients. Transplant Proc. 1989;21(1 Pt 1):833–834.
12. Prihodova L, Nagyova I, Rosenberger J, et al. Adherence in patients in the first year after kidney transplantation and its impact on graft loss and mortality: a cross-sectional and prospective study. J Adv Nurs. 2014;70(12):2871–2883. doi:10.1111/jan.12447
13. Mohamed M, Soliman K, Pullalarevu R, et al. Non-Adherence to Appointments is a Strong Predictor of Medication Non-Adherence and Outcomes in Kidney Transplant Recipients 2021. Available from: www.amjmedsci.com.
14. Lorenz EC, Petterson TM, Schinstock CA, et al. The Relationship between Health Literacy and Outcomes before and after Kidney Transplantation. Transplant Direct. 2022;8(10):E1377. doi:10.1097/TXD.0000000000001377
15. Al-Sheyyab A, Binari L, Shwetar M, et al. Association of medication non-adherence with short-term allograft loss after the treatment of severe acute kidney transplant rejection. BMC Nephrol. 2019;20(1). doi:10.1186/s12882-019-1563-z
16. Steyerberg EW. Applications of prediction models. In. 2009;9(1):11–31. doi:10.1007/s10158-008-0085-3
17. Shipe ME, Deppen SA, Farjah F, Grogan EL. Developing prediction models for clinical use using logistic regression: an overview. J Thorac Dis. 2019;11(S4):S574–S584. doi:10.21037/jtd.2019.01.25
18. Martínez-Camblor P, Pérez-Fernández S, Díaz-Coto S. The area under the generalized receiver-operating characteristic curve. Int J Biostatistics. 2022;18(1):293–306. doi:10.1515/ijb-2020-0091
19. de Hond AAH, Steyerberg EW, van Calster B. Interpreting area under the receiver operating characteristic curve. Lancet Digital Health. 2022;4(12):e853–5. doi:10.1016/S2589-7500(22)00188-1
20. Declaration of Helsinki. 64th World Medical Association General Assembly. Fortaleza, Brazil: Declaration of Helsinki; 2013
21. Ministerio de Salud de Colombia. Resolución 8430 de; 1993.
22. Abboud O, Abbud-Filho M, Abdramanov K, et al. The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Clin J Am Soc Nephrol. 2008;3(5):1227.
23. Gandolfini I, Palmisano A, Fiaccadori E, Cravedi P, Maggiore U. Detecting, preventing and treating non-adherence to immunosuppression after kidney transplantation. Clinical Kidney J Oxford Univ Press. 2022;15(7):1253–1274. doi:10.1093/ckj/sfac017
24. Gokoel SRM, Gombert-Handoko KB, Zwart TC, van der Boog PJM, Moes DJAR, de Fijter JW. Medication non-adherence after kidney transplantation: a critical appraisal and systematic review. Transplant Rev. 2020;34:100511. doi:10.1016/j.trre.2019.100511
25. Belaiche S, Décaudin B, Dharancy S, Noel C, Odou P, Hazzan M. Factors relevant to medication non-adherence in kidney transplant: a systematic review. Int J Clin Pharm. 2017;39(3):582–593. doi:10.1007/s11096-017-0436-4
26. Lieb M, Hepp T, Schiffer M, Opgenoorth M, Erim Y. Accuracy and concordance of measurement methods to assess non-adherence after renal transplantation - A prospective study. BMC Nephrol. 2020;21(1). doi:10.1186/s12882-020-01781-1
27. Torres-Gutiérrez M, Burgos-Camacho V, Caamaño-Jaraba J, Lozano-Suárez N, García-López A, Girón-Luque F. Prevalence and Modifiable Factors for Holistic Non-Adherence in Renal Transplant Patients: a Cross-Sectional Study. Patient Prefer Adherence. 2023;17:2201–2213. doi:10.2147/PPA.S419324
28. Pinto-Ramirez J, Garcia-Lopez A, Salcedo-Herrera S, et al. Risk factors for graft loss and death among kidney transplant recipients: a competing risk analysis. PLoS One. 2022;17(7):e0269990. doi:10.1371/journal.pone.0269990
29. Gumabay FM, Novak M, Bansal A, et al. Pre-transplant history of mental health concerns, non-adherence, and post-transplant outcomes in kidney transplant recipients. J Psychosom Res. 2018;105:115–124. doi:10.1016/j.jpsychores.2017.12.013
30. Betjes MGH, Roelen DL, van Agteren M, Kal-van Gestel J. Causes of Kidney Graft Failure in a Cohort of Recipients With a Very Long-Time Follow-Up After Transplantation. Front Med. 2022;9. doi:10.3389/fmed.2022.842419
31. Foroutan F, Friesen EL, Clark KE, et al. Risk factors for 1-year graft loss after kidney transplantation systematic review and meta-analysis. Clinical J Am Soc Nephrol. 2019;14(11):1642–1650. doi:10.2215/CJN.05560519
32. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–776. doi:10.1097/01.TP.0000110408.83054.88
33. Taber DJ, Fleming JN, Fominaya CE, et al. The Impact of Health Care Appointment Non-Adherence on Graft Outcomes in Kidney Transplantation. Am J Nephrol. 2017;45(1):91–98. doi:10.1159/000453554
34. Fine RN, Becker Y, De Geest S, et al. Nonadherence Consensus Conference Summary Report. Am J Transpl. 2009;9(1):35–41. doi:10.1111/j.1600-6143.2008.02495.x
35. Sellarés J, de Freitas DG, Mengel M, et al. Understanding the Causes of Kidney Transplant Failure: the Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am J Transpl. 2012;12(2):388–399. doi:10.1111/j.1600-6143.2011.03840.x
36. Zhu Y, Zhou Y, Zhang L, Zhang J, Lin J. Efficacy of Interventions for Adherence to the Immunosuppressive Therapy in Kidney Transplant Recipients: a Meta-Analysis and Systematic Review. J Investigative Med. 2017;65(7):1049–1056. doi:10.1136/jim-2016-000265
37. Jalalzadeh M, Mousavinasab N, Peyrovi S, Ghadiani MH. The Impact of Acute Rejection in Kidney Transplantation on Long-Term Allograft and Patient Outcome. Nephrourol Mon. 2015;7(1). doi:10.5812/numonthly.24439
38. Łabuś A, Mucha K, Kulesza A, Fliszkiewicz M, Pączek L, Niemczyk M. Costs of Treatment of Acute Antibody-Mediated Rejection in Kidney Transplant Recipients. Transplant Proc. 2022;54(4):968–971. doi:10.1016/j.transproceed.2021.11.039
39. Diseth TH, Tangeraas T, Reinfjell T, Bjerre A. Kidney transplantation in childhood: mental health and quality of life of children and caregivers. Pediatric Nephrol. 2011;26(10):1881–1892. doi:10.1007/s00467-011-1887-9
40. Andrew M. The Experience of Mothering a Child with a Kidney Transplant and the Implications of Illness-Related Uncertainty. Nat Kidney Foundation J Nephrol Social Work. 2014;38(1):67.
41. Antunes AV, Mota Sousa LM, Justo C, et al. Evaluación de la calidad de vida percibida de un paciente de trasplante de riñón. Enfermería Nefrológica. 2018;21(2):138–144. doi:10.4321/S2254-28842018000200005
42. Passoni Dos Santos R, Lais Brandalize Rocha D. Qualidade de vida pós-transplante renal: revisão integrativa. Enfermería Nefrológica. 2014;17(1):51–58. doi:10.4321/S2254-28842014000100009
43. Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transpl. 2009;9(11):2597–2606. doi:10.1111/j.1600-6143.2009.02798.x
44. KaborÉ R, Haller MC, Harambat J, Heinze G, Leffondré K. Risk prediction models for graft failure in kidney transplantation: a systematic review. Nephrol Dialysis Transplantation. 2017;32(suppl_2):ii68–76. doi:10.1093/ndt/gfw405
45. Yoo KD, Noh J, Lee H, et al. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: a Multicenter Cohort Study. Sci Rep. 2017;7(1):8904. doi:10.1038/s41598-017-08008-8
46. Naqvi SAA, Tennankore K, Vinson A, Roy PC, Abidi SSR. Predicting Kidney Graft Survival Using Machine Learning Methods: prediction Model Development and Feature Significance Analysis Study. J Med Internet Res. 2021;23(8):e26843. doi:10.2196/26843
47. Kaboré R, Ferrer L, Couchoud C, et al. Dynamic prediction models for graft failure in paediatric kidney transplantation. Nephrol Dialysis Transplantation. 2021;36(5):927–935. doi:10.1093/ndt/gfaa180
48. Chang J, Diop M, Burgos YL, et al. Telehealth in outpatient management of kidney transplant recipients during COVID‐19 pandemic in New York. Clin Transplant. 2020;34(12). doi:10.1111/ctr.14097
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.