Back to Journals » Journal of Pain Research » Volume 9
Is blood glucose associated with descending modulation of spinal nociception as measured by the nociceptive flexion reflex?
Authors Terry E, Guereca Y, Martin S, Rhudy J
Received 2 December 2015
Accepted for publication 18 January 2016
Published 4 April 2016 Volume 2016:9 Pages 187—193
DOI https://doi.org/10.2147/JPR.S101720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Ellen L Terry, Yvette M Güereca, Satin L Martin, Jamie L Rhudy
Department of Psychology, The University of Tulsa, Tulsa, OK, USA
Objectives: Prior research has shown a relationship between blood glucose levels and some forms of self-regulation (eg, executive function), with low blood glucose levels associated with impaired self-regulation. Further, engagement in self-regulation tasks depletes blood glucose. Given these relationships, the present study examined whether blood glucose is associated with another form of self-regulation, ie, descending pain modulatory processes.
Methods: Forty-seven (32 female) pain-free participants were recruited and completed testing. Blood glucose was measured from finger sticks and a digital meter before and after experimental pain tests. Pain tests included the nociceptive flexion reflex (NFR) threshold to assess descending modulation of spinal nociception, but also electric pain threshold to assess perceptual pain detection. The Stroop color word naming test was also assessed before and after pain testing to examine changes in executive function.
Results: Results indicated that mean blood glucose levels decreased after pain testing, but Stroop performance did not significantly change. Importantly, changes in blood glucose were correlated with NFR threshold, such that decreases in blood glucose were associated with lower NFR thresholds (reduced descending inhibition). Changes in blood glucose were unrelated to pain threshold or executive function.
Conclusion: This study suggests that glucose depletion may impair performance of descending inhibitory processes, without impacting the perceptual detection of pain (pain threshold). Although findings need to be replicated, maintaining adequate glucose levels may be necessary to support inhibition of spinal nociception.
Keywords: pain, nociception, glucose, descending modulation, executive function
Introduction
Endogenous pain modulatory mechanisms are active processes that work to maintain a balance in pain transmission.1–3 These processes involve both supraspinal (eg, cerebral cortex, thalamus, periaqueductal gray, rostral ventromedial medulla) and spinal regions (eg, dorsal horn).2,3 These descending modulatory systems serve an important and functional role as they allow an organism to down- and upregulate pain to adaptively respond in its environment.4 For example, pain inhibition can be important when an organism is trying to escape a predator and injury is imminent. In contrast, pain facilitation promotes recuperation following an injury and protection from further injury.1,4,5 Therefore, the balance between descending inhibition and facilitation plays an important role in an organism’s survival.1 Research suggest that disruptions in this balance might be a risk marker for the development of chronic pain,6,7 as patients with chronic pain evidence disruptions in this descending balance (eg, hyperfacilitation, hypoinhibition).8–11
Descending modulation is mediated by active neural systems; therefore, modulation relies on a fuel source, namely glucose, to ensure appropriate function.12,13 This means, changes in blood glucose levels could affect these processes.13 However, to our knowledge, no study has assessed the relationship between normal fluctuations in blood glucose levels and descending pain regulatory processes in humans. Nonetheless, prior studies have established a relationship between blood glucose levels and other forms of self-regulation (eg, executive function, behavioral inhibition).14,15 For example, low blood glucose levels are associated with impaired executive function,16 and engagement in executive function tasks depletes blood glucose.15,17 Moreover, it appears that the change in blood glucose from a demanding task is a better predictor of performance on the task than blood glucose measured prior to the task.15 Given that pain modulation is an active, self-regulatory process and that there is an inverse relationship between pain and executive function,18–21 we reasoned that blood glucose levels may be associated with descending modulation of pain. Therefore, the present study contributes to the understanding of pain modulation by assessing the relationship between normal fluctuations in blood glucose levels and its relationship to engagement of descending systems to modulate spinal nociception.
To assess descending modulation in the current study, the nociceptive flexion reflex (NFR) was measured. The NFR is a withdrawal reflex that occurs in response to noxious stimuli (ie, the withdrawal response that happens when stepping on a tack) and it is used as a physiologic measure of spinal nociception.22,23 The NFR does not require supraspinal input (it involves a simple polysynaptic loop: primary afferents → dorsal horn interneurons → motoneurons), because it can be evoked in spinally transected individuals.24 Despite this, the spinal circuitry mediating the NFR can be inhibited or facilitated by descending modulation from supraspinal centers.22,25 Interestingly, the NFR appears to be under tonic descending inhibition in humans, because it is easier to evoke this reflex in persons who have disrupted descending communication from the brain (eg, those with a spinal transection).24
The goal of the present study was to determine the relationships between blood glucose levels and NFR threshold. Further, pain threshold was measured to assess a perceptual measure of pain detection, and executive function was assessed using the Stroop test (ie, color word naming test). Blood glucose was measured before and after pain testing. It was predicted that reductions in blood glucose levels over the course of pain testing would be associated with: lower NFR thresholds (ie, reduced descending inhibition), lower pain thresholds (ie, enhanced pain perception), and impaired executive function.
Materials and methods
Participants
Participants were healthy, pain-free individuals recruited from the University of Tulsa psychology subject pool and from the Tulsa, OK, community. Subject pool participants received research credit, whereas community participants received a $50.00 honorarium. Participants were excluded for the following self-reported conditions: neurological, cardiovascular, or circulatory problems; chronic pain; any type of blood glucose dysregulation, including diabetes, hypo- or hyperglycemia; persons who used tobacco products in the 2 hours prior to testing; color blindness (due to Stroop test); recent psychological trauma; use of over-the-counter pain medication within 24 hours, or prescription pain medication within 2 weeks of participation; use of antidepressant, anxiolytic, or high blood pressure medications; having a body mass index of 35 kg/m2 or above (due to difficulties obtaining an NFR in persons with high adiposity); or <18 years of age. Additionally, participants were asked to refrain from eating or drinking anything (other than water) for 2 hours prior to testing in order to allow blood glucose levels to stabilize so as to reduce extraneous variance.15
Fifty participants were targeted with completed data because this would provide power ≥0.70 to identify correlations ≥0.35 at α =0.05. Fifty-three participants consented to participate, but four were found to not meet inclusion criteria and two did not complete testing (one reached 50 mA maximum stimulation before NFR threshold was reached and one found the stimulations too painful). Thus, data from 47 participants were available for analysis. The majority of completers were female (70%, n=32), White (78%, n=36), single (83%, n=38), and employed (28%, n=61). They had an average of 15.5 years of education (standard deviation [SD] =2.2) and averaged 23 years of age (SD =6.1). All participants provided verbal and written informed consent. All participants were informed that they could withdraw from the study at any time.
Apparatus and signal acquisition
Self-report ratings and physiological signals were collected by a computer with dual-monitor capacity, A/D board (PCI – PCI-6071E; National Instruments, Austin, TX, USA), and LabVIEW software (National Instruments). One computer monitor was used by the experimenter to monitor physiological signals, and the second monitor was used by the participant to complete electronic questionnaires/ratings. Testing was completed in a sound-attenuated and electrically-shielded room. Participants were monitored from an adjacent control room via a video camera connected to a television. Participants wore sound-attenuating headphones that allowed them to hear the experimenter’s instructions and they could speak to the experimenter via the microphone on the video camera.
Electric pain stimuli were generated by a Digitimer stimulator (DS7, Digitimer; Hertfordshire, England, UK) and delivered using a bipolar surface stimulating electrode (Nicolet, Madison, WI, USA; 30 mm interelectrode distance) attached to the left leg over the retromalleolar pathway of the sural nerve. The computer controlled the timing of the stimulations, and the maximum stimulation intensity was set at 50 mA. Biceps femoris electromyogram (EMG) for NFR assessment was amplified and bandpass filtered (10–300 Hz) online using a Grass Technologies (West Warwick, RI, USA) Model 15LT amplifier (with AC Module 15A54). The signal was sampled at 1,000 Hz.
The NFR was assessed from biceps femoris EMG recorded from two active Ag-AgCl electrodes placed 10 cm superior to the popliteal fossa. A ground electrode was placed over the lateral epicondyle of the femur. Before the stimulating and recording electrodes were applied, the skin was cleaned with alcohol and exfoliated using an abrasive paste (Nuprep; Weaver and Company, Aurora, CO, USA) to reduce impedances below 5 kΩ. All electrodes were then attached after conductive gel (EC60; Grass Technologies) was applied.
Questionnaires
Demographics/health status
A custom-built questionnaire designed to obtain demographic information and health problems was administered. Questions regarding health problems asked specifically about exclusionary criteria (chronic pain, medication use, etc).
Subjective pain ratings
To assess pain intensity in response to electric pain stimuli, participants used a computer-presented numerical rating scale that ranged from 0 to 100 with the following labels: 0 (no pain), 50 (painful), and 100 (most intense pain imaginable). Participants responded by moving an indicator to a position along the line that corresponded to their rating using a computer mouse. A mouse button press was used to submit the rating and return the scale to zero before the next rating.
Blood glucose levels
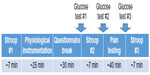
A finger prick test was administered by the researcher to measure blood glucose levels (eg, Accu-Check® Compact Plus monitoring device). Blood glucose levels were measured according to the procedure outlined in the user manual of the glucose meter. Prior to each finger prick, participants’ fingers were cleaned with an alcohol swab and then a disposable lancet was used to prick the finger to obtain a droplet of blood. The test strip, which is ejected from the monitoring device, was used to draw up the blood to be analyzed. Blood glucose was tested on a different finger each time to avoid sensitization of the test site. The finger prick procedure was completed three times; two assessments before pain testing and one assessment after pain testing (Figure 1).
  | Figure 1 Experimental procedures. |
Executive function assessment
Stroop color and word test
The Stroop color and word test (adult version) is a paper test that measures reaction time and inhibitory responses.26–28 This test provides three sets of stimuli, color words printed in black, Xs printed in different color hues (eg, red, blue, green), and color words printed in incongruent colored font (eg, the word “red” presented in blue ink). Participants were instructed to name the color in which a word is presented, while ignoring the printed word. Thus, incongruence between the word’s hue and the actual word requires inhibition and response selection.26 The interference T-score was calculated for each participant and used to determine a participant’s performance on the task, with higher values representing better inhibition.26,29,30 Participants completed the Stroop color and word test on three occasions, two administrations before pain sensitivity testing and one administration after pain sensitivity testing.
Pain testing
Pain outcomes included NFR threshold (a physiological correlate of spinal nociception) and electrocutaneous pain threshold. In addition, participants completed a 3-pulse threshold (which was used to determine the stimulus intensity for a subsequent testing procedure not presented in this manuscript).
NFR threshold assessment
NFR threshold was assessed using three ascending–descending staircases of electric stimuli. The first ascending–descending staircase started at 0 mA and increased in 2 mA steps until an NFR was detected. Each electric stimulus consisted of a train of five 1 ms rectangular wave pulses at 250 Hz. NFR was defined as a mean biceps femoris EMG response in the 90–150 ms poststimulus interval that exceeded the mean biceps femoris EMG activity during the 60 ms prestimulus baseline interval by at least 1.4 SD.31,32 After an NFR was obtained, the current was decreased in 1 mA steps until an NFR was no longer detected. The second and third ascending– descending staircases used 1 mA steps. The interval between electric stimulations varied randomly between 8 and 12 seconds to reduce predictability and habituation. The numerical pain rating scale was administered immediately following each stimulus. The stimulus intensity (mA) of the two peaks and two troughs of the last two ascending–descending staircases were averaged and used to define NFR threshold. The average NFR threshold for the present study was 17.41 mA (SD =10.63).
3-pulse threshold
To assess 3-pulse threshold, several series of three electrical stimulations at 2.0 Hz (0.5-second interstimulus interval) were delivered. The stimulus intensity of the first series started at 80% NFR threshold and increased by 1 mA until the third stimulus in the series evoked an NFR according to the definition used in NFR threshold assessment. Following each stimulus series, participants rated their pain on three pain rating scales (one for each stimulus in the series). This procedure was only included to set the stimulation intensity for the emotional controls of nociception procedure that was assessed at the end of the testing included in the present report (emotional controls of nociception data reported elsewhere33).
Pain threshold assessment
Similar to assessment of NFR threshold, pain threshold was assessed using three ascending–descending staircases of electric stimuli with a varying interstimulus interval of 8–12 seconds. The first ascending–descending staircase started at 0 mA and increased in 4 mA steps until pain threshold was reached (rating ≥50 on the pain rating scale). The intensity was then decreased in 2 mA steps until the participant rated a stimulus as ≤40 on the pain rating scale. The second and third ascending–descending staircases continued with 2 mA steps. Pain threshold was defined as the average intensity (mA) of the four stimuli first rated immediately above and immediately below 50 on the last two ascending and descending staircases. The average pain threshold for the present study was 13.50 mA (SD =8.48).
Procedure
All procedures were fully approved by the University of Tulsa ethics review board. Interested participants were administered a brief phone screen to initially evaluate inclusion/exclusion criteria. Potentially eligible participants were invited to attend a laboratory visit during which a thorough overview of the study was provided, informed consent was obtained, and then a comprehensive assessment of inclusion/exclusion criteria was conducted using a health status questionnaire and a brief interview. Afterward, participants received training in the use of the computer-presented numerical pain ratings scale that was used throughout pain testing. The participants then completed the first administration of the Stroop color and word test to familiarize them with the task (Figure 1). Next, psychophysiological sensors were applied, and then the participant was seated in a comfortable reclining chair with their knee angle kept at 160°. Before pain testing began, there was a 30-minute acclimation period during which participants were asked to complete several questionnaires unrelated to the current study. Then, the first blood glucose level was obtained, followed by the second administration of the Stroop color and word test, and then the second blood glucose level was obtained. Next, NFR threshold, 3-pulse threshold, and pain threshold were assessed. This pain testing lasted approximately 40 minutes. After the pain tests, blood glucose level was assessed for the third time, followed by the third administration of the Stroop color and word test.
Data analysis
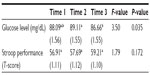
Preliminary data screening found several variables had marked skewness, outliers, and/or heteroscedasticity. To systematically address outliers, data were trimmed at ±3 SD from the mean for all variables (ie, glucose levels, Stroop performance, NFR threshold, and pain threshold), as described by Wilcox.34 Two repeated-measures linear–mixed analysis of variances with three levels (Time 1, Time 2, Time 3) were conducted to evaluate blood glucose levels and Stroop performance across time using SPSS MIXED 20.0. To examine the relationships between changes in blood glucose levels and changes in Stroop performance with pain variables, change scores were created for blood glucose (Glucose #3 minus Glucose #2) and Stroop performance (Stroop #3 minus Stroop #2) that occurred during pain testing. Pearson’s correlations were used to assess relationships among the variables. Significance was set at P<0.05 (two tailed).
Results
Mean changes in blood glucose levels and Stroop performance
Table 1 presents mean, standard error of the mean, and F-tests for blood glucose levels and Stroop performance. Glucose levels were significantly lower at time 3 compared to time 2 (ie, reduced following pain testing), but all other mean comparisons were nonsignificant. By contrast, Stroop performance did not significantly change over time. Sex was not a significant factor, nor did it moderate the effects of time, in either model (all P-values>0.212).
Relations between pain outcomes and changes in glucose levels and Stroop performance
Change in glucose was positively associated with NFR threshold (r=0.31, P=0.040), but not pain threshold (r=−0.07, P=0.635; Figure 2). By contrast, change in Stroop performance was not associated with NFR threshold (r=−0.07, P=0.659) or pain threshold (r=0.01, P=0.942). Further, changes in blood glucose levels were not associated with changes in Stroop performance (r=−0.133, P=0.400). Sex was not a significant predictor, nor did it moderate any of the relationships in any model (all P>0.141).
  | Figure 2 Relationship of glucose and Stroop changes for NFR threshold and pain threshold. |
Discussion
Blood glucose and pain outcomes
The current study found that mean blood glucose levels were significantly reduced over the 40 minutes of pain testing. This is consistent with the notion that pain regulation results in increased energy demand that in turn leads to increased blood glucose consumption.35 However, this conclusion is tentative given that we did not have a control group that did not receive pain testing to control for changes in blood glucose due to natural history. Nonetheless, one of our primary hypotheses was supported; reductions in blood glucose were associated with lower NFR thresholds. This suggests that those persons who had lower blood glucose had greater difficulty engaging descending inhibitory processes to dampen spinal nociception. Thus, less intense stimuli were able to elicit the reflex in these individuals. This is consistent with a study by Silvestrini and Rainville20 that used a within-subjects design to examine the impact of a cognitively demanding task on pain and NFR. Pain and NFR were tested immediately after 2 minutes of a cognitively demanding task or after 2 minutes of a low demanding task. Although they did not measure glucose levels, they found pain and NFR were enhanced following the demanding task, suggesting the task depleted glucose resources for maintaining inhibition. Interestingly, a few individuals in our study experienced an increase in blood glucose levels during pain testing (see x-axis of Figure 2), and these individuals benefitted from greater descending inhibition (higher NFR thresholds). Thus when taken together, it appears that glucose is necessary to maintain descending inhibition.
Interestingly, pain threshold was not associated with blood glucose levels. This is consistent with previous findings showing that regulation of pain and spinal nociception do not always parallel one another, likely due to separate modulatory mechanisms responsible for each.36,37 The lack of correlation with pain threshold may reflect that evaluation of subthreshold- and threshold-level stimuli does not require the same energy demands as ongoing inhibition of spinal nociception. Future studies should examine the relationship between blood glucose levels and a task that requires greater self-regulation and energy demands. For example, pain perception tasks that involve prolonged exposure to a suprathreshold stimulus (eg, cold pressor tolerance) might produce a more robust correlation with glucose levels.
Blood glucose and executive function
Prior research has found that reductions in blood glucose impair performance on cognitively demanding tasks.14–16 Given this, it was surprising that Stroop performance did not change in parallel with blood glucose levels regardless of whether analyses examined changes in mean levels (group-level change) or whether analyses examined correlations (interindividual change). Although it is unclear at this time why we did not observe these effects, it is possible that the inclusion of pain testing somehow disrupted the relationship observed by other nonpain studies.15
Executive function and pain outcomes
Prior research has shown that chronic pain impairs executive function,18,38 and intact executive function is important for pain regulation.21 By contrast, we did not find a connection between Stroop performance and our pain outcomes. Future studies are needed to determine why we failed to find this relationship, but it could stem from our study design. We did not test pain outcomes on multiple occasions to see whether they changed as executive function changed. Alternatively, other executive function tasks or other pain measures may be more sensitive to detecting the relationship. It is noteworthy that our null findings are not likely due to Type II error though, because the effect sizes were small (r=−0.07 and r=0.01).
Study limitations
This present study had a number of strengths. For example, it is the only study to examine the experimental linkages between pain processing, blood glucose, and executive function. Further, we assessed a physiological measure of spinal nociception (NFR), as well as pain perception. However, there are a few limitations worth noting. First, this study involved young, healthy participants, and so it is not clear whether our results will generalize to other populations. Further research is necessary to extend our findings to clinical populations (eg, patients with chronic pain and/or diabetes). Second, the study was cross sectional, and pain outcomes were only tested on one occasion. As a result, we cannot determine the direction of the glucose and NFR threshold relationship. It is possible that persons with higher NFR thresholds (those that had to be exposed to higher stimulus intensities to evoke the reflex) may have experienced increased stress which in turn produced a release of energy storages causing blood glucose to go up. Additional research is needed to verify the direction of the effects. Third, there were too few male participants in this study to adequately measure differences in response between males and females. And finally, it is possible that practice effects might have rendered the Stroop task less challenging, which might have affected relationships with executive functioning.
Conclusion
This study examined the relationship between blood glucose levels, pain regulation (pain threshold, NFR threshold), and executive function (Stroop test). Results suggest glucose depletion may impair performance of descending inhibition of spinal nociception, without impacting the perceptual detection of pain (ie, pain threshold). This means that maintaining blood glucose levels may help support descending inhibition.
Disclosure
The authors report no conflicts of interest in this work.
References
Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27(8):729–737. | |
Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. | |
Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh, Scotland: Churchill Livingston; 1999:309–329. | |
Walters ET. Injury related behavior and neural plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol. 1994;36:325–426. | |
Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci. 1980;3(2):291–323. | |
Palit S, Kerr KL, Kuhn BK, et al. Exploring pain processing differences in Native Americans. Health Psychol. 2013;32(11):1127–1136. | |
Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138(1):22–28. | |
Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41. | |
Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13(3):189. | |
Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319. | |
Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118(1):215–223. | |
Laughlin SB. The implications of metabolic energy requirements for the representation of information in neurons. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 3rd ed. Cambridge, MA: MIT Press; 2004:187–196. | |
Magistretti P, Allaman I. Brain energy metabolism. In: Pfaff D, editor. Neuroscience in the 21st Century. New York, NY: Springer; 2013:1591–1620. | |
Benton D, Owens DS, Parker PY. Blood glucose influences memory and attention in young adults. Neuropsychologia. 1994;32(5):595–607. | |
Gailliot MT, Baumeister RF, DeWall CN, et al. Self-control relies on glucose as a limited energy source: willpower is more than a metaphor. J Pers Soc Psychol. 2007;92(2):325–336. | |
Fairclough SH, Houston K. A metabolic measure of mental effort. Biol Psychol. 2004;66(2):177–190. | |
Sünram-Lea SI, Foster JK, Durlach P, Perez C. Investigation into the significance of task difficulty and divided allocation of resources on the glucose memory facilitation effect. Psychopharmacology. 2002;160(4):387. | |
Solberg Nes L, Roach AR, Segerstrom SC. Executive functions, self-regulation, and chronic pain: a review. Ann Behav Med. 2009;37(2):173–183. | |
Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Q J Exp Psychol. 2012;65(3):565–586. | |
Silvestrini N, Rainville P. After-effects of cognitive control on pain. Eur J Pain. 2013;17(8):1225–1233. | |
Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149(1):19–26. | |
Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77(6):353–395. | |
Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans – review article. Pain. 2002;96:3–8. | |
Sandrini G, Milanov I, Willer JC, Alfonsi E, Moglia A, Nappi G. Different effect of high doses of naloxone on spinal reflexes in normal subjects and chronic paraplegic patients. Neurosci Lett. 1999;261(1–2):5–8. | |
Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): do affective valence and arousal play a role? Pain. 2008;136(3):250–261. | |
Golden CJ. Stroop Color and Word Test. Wood Dale, IL: Stoelting Co; 1978. | |
Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. | |
Stroop JR. Studies of interference in serial verbal reactions [reprint]. J Exp Psychol. 1992;121(1):15–23. | |
Chafetz MD, Matthews LH. A new interference score for the Stroop test. Arch Clin Neuropsychol. 2004;19(4):555–567. | |
Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York, NY: Oxford University Press; 1991. | |
France CR, Rhudy JL, McGlone S. Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: further evaluation of standardized scoring criteria. Pain. 2009;145:211–218. | |
Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria Pain. 2007;128:244–253. | |
Rhudy JL, Martin SL, Terry EL, DelVentura JL, Kerr KL, Palit S. Using multilevel growth curve modeling to examine emotional modulation of temporal summation of pain (TS-pain) and the nociceptive flexion reflex (TS-NFR). Pain. 2012;153:2274–2282. | |
Wilcox RR. Applying Contemporary Statistical Techniques. San Diego, CA: Academic Press; 2003. | |
Holland-Fischer P, Greisen J, Grøfte T, Jensen TS, Hansen PO, Vilstrup H. Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol. 2009;26(4):311–317. | |
Rhudy JL, Williams AE, McCabe KM, Rambo PL, Russell JL. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain. 2006;126:221–233. | |
Roy M, Piche M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci USA. 2009;106(49):20900–20905. | |
Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.