Back to Journals » Drug Design, Development and Therapy » Volume 17
Investigation of the Mechanisms and Experimental Verification of Yulin Formula in the Treatment of Diminished Ovarian Reserve via Network Pharmacology
Authors Wang R, Zhao Y, Miao C, Chen Y , Ren N, Yang L, Cheng W, Zhang Q , Fang X
Received 5 April 2023
Accepted for publication 15 July 2023
Published 24 July 2023 Volume 2023:17 Pages 2147—2163
DOI https://doi.org/10.2147/DDDT.S413142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Ruye Wang,1,* Ying Zhao,2,* Chenyun Miao,3 Yun Chen,1 Ning Ren,1 Liuqin Yang,1 Wei Cheng,4 Qin Zhang,1,5 Xiaohong Fang1
1Department of TCM Gynecology, Hangzhou TCM Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2School of Public Health, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3School of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 4Department of Orthopedics, Hangzhou TCM Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 5Research Institute of Women’s Reproductive Health, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qin Zhang; Xiaohong Fang, Department of Medical TCM Gynecology, Hangzhou TCM Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310007, People’s Republic of China, Email [email protected]; [email protected]
Purpose: The aim of this study is to examine, using network pharmacology analysis and experimental validation, the pharmacological processes by which Yulin Formula (YLF) reduces cyclophosphamide-induced diminished ovarian reserve (DOR).
Methods: First, information about the active components, associated targets, and related genes of YLF and DOR was gathered from open-access databases. The primary targets and pathways of YLF to reduce DOR were predicted using studies of functional enrichment from the Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO), and Protein-Protein Interaction (PPI) networks. Second, we built a cyclophosphamide-induced diminished ovarian reserve (DOR) rat model to verify the primary target proteins implicated in the predicted signaling pathway to explore the mechanism of improve ovarian function of YLF.
Results: 98 targets met the targets of the 82 active ingredients in YLF and DOR after searching the intersection of the active ingredient targets and DOR targets. Fourteen targets, including AKT and Caspase-3 among others, were hub targets, according to the PPI network study. The PI3K/AKT pathway was revealed to be enriched by numerous targets by the GO and KEGG enrichment studies, and it was used as a target for in vivo validation. Animal studies showed that YLF administration not only reduced the number of atretic follicles, the proportion of TUNEL-positive ovarian cells, the rate of apoptosis of granulosa cells (GCs) and the proportion of abnormal mitochondria in DOR rats, but also reversed the high expression of Caspase-3, Caspase-9, BAX, cytochrome C, PI3K and P-AKT, improving the ovarian reserve in cyclophosphamide (CTX)-induced DOR rats.
Conclusion: Our research results predicted the active ingredients and potential targets of YLF-interfering DOR by an integrated network pharmacology approach, and experimentally validated some key target proteins participated in the predicted signaling pathway. A more comprehensive understanding of the pharmacological mechanism of YLF for DOR treatment was obtained.
Keywords: diminished ovarian reserve, Yulin Formula, network pharmacology, pharmacological mechanisms, apoptosis, PI3K/AKT
Introduction
Diminished ovarian reserve (DOR) is an ovarian disease associated with decreased quantity and quality of remaining oocytes in the ovary and insufficient secretion of ovarian granulosa cells, manifested by decreased fertility and sex hormone deficiency.1 The prevalence of DOR is increasing at a younger age,2 and 10% of women attending fertility clinics in the United States are diagnosed with DOR, which has a significantly lower success rate in women using assisted reproductive technology (ART).3 Moreover, the clinical features of DOR as a manifestation of ovarian aging, in addition to decreased fertility and reduced responsiveness to exogenous hormone stimulation, decreased live birth rates and clinical pregnancy, and increased miscarriage rates.4 Therefore, treating reduced ovarian reserve function is an urgent problem and an interesting topic in reproductive medicine.
Presently, there is no effective and recognized method for the treatment of DOR, and the main clinical applications are hormone replacement therapy (HRT), dehydroepiandrosterone (DHEA), and assisted reproductive technology (ART).5 Current research suggests that hormone supplementation can effectively improve the symptoms of patients with DOR; However, continued use may raise the chance of endometrial and breast cancer.6 Potential testosterone priming effect of DHEA is beneficial for pregnancy outcome and ovarian response in DOR patients;7 nevertheless, it is a type of androgen and has adverse effects such as hirsutism, acne, and obesity. ART can solve the fertility problem of DOR patients to a certain extent; however, it has problems such as low ovarian responsiveness to drugs, low number of eggs obtained, poor embryo quality, and poor pregnancy outcomes, which may lead to multiple treatment failures and cause economic drain and physical and psychological damage to DOR patients. Therefore, it can be said that the current treatments do not fundamentally address the ovarian function of DOR patients, and all have certain side effects.
Furthermore, TCM is considered to have significant efficacy in the treatment of declining ovarian reserve, and a current systematic evaluation and meta-analysis8 suggest that TCM treatment can help DOR patients restore reproductive hormone balance, improve fertility, and increase IVF-ET success rates. Therefore, TCM offers a new approach to the therapy of DOR infertility. According to the theory of TCM diagnosis and treatment, fertility is directly related to kidney essence, and the fundamental pathogenesis of DOR is linked to kidney essence deficiency.9 Yulin Formula (YLF, with China Patent Application number 201710902472X) was derived from Zhejiang He’s School of Gynecology, an intangible cultural heritage of Zhejiang Province.10 YLF is a special herbal formula for improving ovarian function, ancestrally passed down from He’s School of Gynecology, which mainly consists of 10 herbs (Table 1) and has the effects of tonifying the kidney, strengthening the spleen, and regulating qi and blood. Recent studies have found that YLF can effectively improve clinical symptoms, serum hormone levels, and improve the pregnancy rate of DOR patient.11 Furthermore, animal experiments have shown that high-dose YLF can effectively improve the ultrastructure of granulosa cells in cyclophosphamide (CTX)-induced DOR rats, improve granulosa cell function, promote INHB secretion, inhibit follicular apoptosis, and improve the quantity and quality of oocytes in ovaries.12,13 Conclusively, these findings provide a theoretical basis for applying YLF in DOR treatment; however, the specific molecular mechanism of its action has not been studied.
 |
Table 1 Composition of YLF |
Recently, On the basis of systems biology theory, network pharmacology has recently been used to reveal the active components and possible mechanisms of medicinal herbs. This study aimed to provide more scientific evidence for the efficacy of YLF by combining network pharmacology with experiments to explore the basic mechanism and therapeutic theory of YLF. A flowchart of this study is shown in Figure 1.
Materials and Methods
Screening for Active Ingredients in YLF and Predicting Their Targets
The Traditional Chinese Medicine Systematic Pharmacology (TCMSP) database and analysis tool (http://tcmspw.com/TCMSP.php) was used to find the active ingredients in YLF.14 We used “Tu Si Zi”, “Gou Qi Zi”, “Dang Gui”, “Chuan Xiong”, “Shu Di Huang”, “Ba Ji Tian”, “Rou Cong Rong”, “Xian Lin Pi”, “She Chuang Zi” and “Fu Peng Zi”, to filter out as keywords to find targets associated with YLF. Oral bioavailability (OB) ≥ 30% and drug similarity (DL) ≥ 0.18 were the two criteria we used to evaluate potential ingredients in order to obtain completely active ingredients.15 In addition, the UniProt database (https://www.uniprot.org/) was also used to standardize all target names.16
Identification of Potential Targets for DOR
To identify therapeutic targets for DOR, we used “ovarian reserve hypofunction” as an index keyword and limited the species selection to “Homo sapiens”. We applied Genecards (https://www.genecards.org/)17 and Online Mendelian Inheritance in Man (OMIM, https://www.genecards.org/)18 to gain candidate genes for DOR. After excluding duplicates, potential targets for DOR were found.
Construction of Ingredient-Target- Disease Network
We collected active components in YLF and potential targets in DOR and entered them into Cytoscape 3.9.0 software to construct a component-target-disease interaction network that visualizes and integrates topological parameters. The importance of components and targets is measured by degree, which shows the total number of routes related to that node by other nodes. The higher the degree value, the higher its importance.
Construction of Protein–Protein Interaction (PPI) Network
We used the String database to integrate the collection and prediction of all known and protein functional associations to establish the functional connectivity of the protein network.19 In order to acquire the relevant protein interaction relationships, the identified potential proteins were also imported into String (https://String-db.org/), and the PPI network was created using Cytoscape 3.9.0 software. Furthermore, important proteins were identified using CytoNCA software based on closeness centrality (CC), betweenness centrality (BC), and degree centrality (DC). The BC, CC, and DC should be greater than or equal to the median value.19
Functional Enrichment Analyses
GO functional analysis using three ontologies was used to describe gene function: Biological Process (BP), Cellular Component (CC) and Molecular Function (MF). Common targets were analyzed for YLF processing using the “Pathview” package in R software and KEGG pathway enrichment analysis. Furthermore, enrichment analysis can be used to analyze common DOR targets, p < 0.05 was statistically significant in GO and KEGG analyses.20,21
Preparation of Yulin Formula
The pharmacy department of Hangzhou TCM Hospital provided the YLF formula for this research, and Zhejiang Traditional Chinese Medical University approved it. The medicinal ingredients should be soaked for 30 minutes, then boiled for 1 hour with distilled water, filtered, concentrated with a rotary evaporator to a concentration of 2g/mL, sealed, and kept cold at −20°C for later use. YLF consists of 10 herbs (Table 1) as follows: 23% Cuscuta chinensis Lam, 9% Lycium barbarum L., and 8% Angelica sinensis (Oliv.) diels; 8% Rehmannia glutinosa Libosch.; 8% Ligusticum chuanxiong Hort.; 8% Morinda officinalis How; 8% Cistanche salsa (C.A.Mey.) beck; 12% Epimedium brevicornum Maxim.; 4% Cnidium monnieri (L.) Cuss.; 12% Rubus chingii Hu.
Animals and Treatment
First, A total of 48 female Sprague Dawley rats (8-week-old, 220 ± 10 g) were purchased from Shanghai BK Biotechnology Co., Ltd. All animals were housed in a specific pathogen-free (SPF) classroom (20–26°C, 12 h light/dark cycle) at the Animal Center of Zhejiang Traditional Chinese Medical University (certification: SYXK (Zhejiang) 2021–0012). Moreover, all experiments were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (No. 202109-03), and all laboratory procedures were performed following the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China.
The rats were adjusted and fed in cages at the Experimental Animal Research Center for a week prior to the examinations, receiving regular food and tap water. Meanwhile, vaginal smears were obtained to determine the regularity of the estrous cycle, and 40 of the rats with normal estrous cycles were randomly classified into four groups (n=10 per group) as follows: the control group, the DOR model group, estradiol valerate group (estradiol group), and Chinese herbal compound fertility group (YLF group). Except for the control group, the rats in the other experimental groups were intraperitoneally injected with CTX (90 mg/kg body weight) to create the CTX-induced DOR model.9,22 Subsequently, a vaginal smear of rats was obtained to determine the regularity of the estrous cycle, and the model was considered established successfully if the rats were in metestrus.
Regarding the Chinese Pharmacopoeia and practical medicinal effects, the daily dose of YLF for an adult weighing 60 kg is 128 g. The equivalent dose ratio for rats and humans converted by the surface area was 6. The dose administered was determined based on our previous studies,12,13 we have found that the optimal dose for YLF administration in DOR rats was 25.6 g/kg/day, while the optimal dose for estradiol administration was 0.21 mg/kg/day. In this study, the rats in the YLF group received an equivalent dose of 25.6 g/kg/day for 21 days. Additionally, rats in the Estradiol group were constantly given estradiol valerate tablets (0.21 mg/kg/day) for 21 days, while rats in the control and DOR groups received saline in a quantity equivalent to that of the estradiol group by gavage. The rats received their treatment for 21 days before being put to death with an intraperitoneal injection of sodium pentobarbital. The experimental steps are shown in Figure 2.
 |
Figure 2 Study design of the project. |
Vaginal Smear Examination
A tiny cotton swab was moistened with saline, gently rotated for several circles in the rat vagina, and evenly smeared on the slide. After drying, the samples were fixed with methanol, and the vaginal smear was stained using the Giemsa staining method (Solarbio, Beijing. China). After washing with running water and drying in air, the samples were observed under an optical microscope. The specific stages and cells involved are shown in Figure 3B.
Histopathological and Electron Microscopic Observation
Ovarian tissues of the rats were fixed in 4% paraformaldehyde, paraffin-embedded, and serially sectioned. 4-μm-thick ovarian sections were stained with hematoxylin and eosin (H&E). Images of ovarian structures and follicle counts were obtained using a MOTIC AE2000 microscope. The ovarian tissue was immediately dehydrated, glutaraldehyde and osmolytic acid fixed, and then moved to a resin mixture. 100 nm-thick ovarian slices were stained with uranyl acetate and lead citrate. Using a Hitachi H7650 transmission electron microscope, the slices were taken in pictures.
TUNEL Staining of Ovarian Cells
The manufacturer’s directions for the TUNEL assay were followed. In order to remove the proteins from the nuclei, paraffin-embedded sections were deparaffinized and then treated with proteinase K. Following that, the sections were incubated for 2 hours at 37°C with a 1:9 combination of TdT and dUTPase reaction mixture. Furthermore, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Finally, digital images were recorded using a fluorescence microscope (Leica DM2500B), and the percentage of TUNEL-positive granulosa cells in the antral follicles was calculated.
Protein Expression Analysis
Total protein was extracted from rat ovarian tissues using a total protein extraction reagent (KeyGen Biotech, Jiangsu, China) according to the manufacturer’s protocol. First, protein concentrations were determined using a BCA protein analysis kit (KeyGen Biotech, Jiangsu, China), and protein quantification was performed using the Simple Western (WesProteinSimple, San Jose, CA, USA) protein quantification system (PI3K, P-AKT, AKT, BAX, Cytochrome C, Caspase-3, and Caspase-9). Among the antibodies used in the abovementioned appeal, Cytochrome C was obtained from Immunoway Biotechnology Company (Immunoway, Suzhou, China), while the rest were obtained from Cell Signaling Technology (CST, Danvers, MA, USA). Finally, the signal intensity was quantified and analyzed using Compass software.
Real-Time PCR
The Takara MiniBEST Universal RNA Extraction Kit (TaKaRa, Shiga, Japan) was used to prepare the RNA samples, and the Takara PrimeScript RT Master Mix (TaKaRa, Shiga, Japan) was used to carry out the reverse transcriptase processes. Specific genes were detected using SYBR Premix Ex TaqTM II (Perfect Real Time) (TaKaRa, Shiga, Japan). Reverse transcription was performed at 37°C for 15 min and 85°C for 5s (with cooling to 4°C). The tubes used for the fluorescence quantification reaction received a reverse transcription reaction system, and the following settings were used for the amplification reaction: pre-denaturation at 95°C for 3 min; 40 cycles (95°C for 10s, 60°C for 30s); solubility curve, starting at 55°C and increasing by 0.5°C every 30s to 95°C for one cycle. The ABI StepOnePlusTM Real-Time PCR System was used to gather all reaction data. Normalized expression was then used to determine the relative fold change using the formula 2−ΔΔCT. The primer sequences utilized in quantitative real-time polymerase chain reaction studies are displayed in Table 2. The internal reference gene was GAPDH.
 |
Table 2 Primer Sequences of the Target Genes |
Statistical Analysis
Statistical analysis was performed by Prism GraphPad 8 software. The data distribution was evaluated using Shapiro–Wilk test and QQ plot. The result conforming to normal distribution were analyzed using one-way ANOVA tests, the Honestly Significant Difference (Tukey’s HSD) performed post hoc tests. All quantitative data were expressed as mean ± standard error of the mean (SEM). Statistical significance was defined as p <0.05, and the number of asterisks indicated the following levels of statistical significance: *p<0.05 or **p<0.01 represented significant difference and very significant difference, respectively.
Results
Active Ingredients in YLF and Target Prediction
By searching the database of traditional Chinese medicine and fulfilling the screening criteria (DL≥ 0.18, OB≥ 30%), 85 active ingredients in the Yulin Formula (YLF) were screened, including 16 kinds of Ba Ji Tian, 16 kinds of Gou Qi Zi, 6 kinds of Rou Cong Rong, 2 kinds of Shu Di Huang, 10 kinds of Tu Si Zi, 2 kinds of Dang Gui, 6 kinds of Chuan Xiong, 18 kinds of Chuang Zi, 23 kinds of Xian Lin Pi, and 6 kinds of Fu Pen Zi, of which there were 10 duplicate active ingredients. Subsequently, 1222 potential targets of YLF were retrieved from the TCMSP database, including 56 types of Ba Ji Tian, 196 types of Gou Qi Zi, 174 types of Rou Cong Rong, 30 types of Shu Di Huang, 213 types of Tu Si Zi, 51 types of Dang Gui, 29 types of Chuan Xiong, 73 kinds of She Chuang Zi, 219 types of Xian Lin Pi and 181 types of Fu Pen Zi. After eliminated duplicate targets, a total of 265 targets were retained for further study.
Potential Targets Prediction of DOR
1230 DOR targets were found in the GeneCards database by searching for DOR-related studies in two databases, and 168 DOR targets were gained from the OMIM database. After excluding duplicates, 617 targets were stored, closely associated with the development of DOR.
Ingredient-Target-Disease Network
To locate the point where the target genes for YLF and DOR meet, a Venn diagram analysis was done. As shown in Figure 4A, 98 target genes were identified, matching the relevant targets of the 83 active ingredients. Using the Cytoscape 3.9.0 software, we built the ingredient-target-disease network to clarify the connections between active ingredients, potential targets, and DOR (Figure 4B). The top ten key active ingredients of YLF for DOR are listed in Table 3.
 |
Table 3 The Top 10 Key Active Ingredients of Yulin Formula in the Treatment of DOR |
PPI Network Construction and Key Targets to Identify
STRING database conducted common targets PPI network analysis (Figure 4C). With 88 nodes and 716 edges, the PPI network performed the interaction of common targets and depicted the biological processes of TLF intervention DOR. After that, we screened the BC, CC, and DC limits for the core proteins using CytoNCA to determine the topological parameters. In the first step, DC was greater than or equal to 13. In the second step, the BC, CC, and DC were greater than or equal to 40, 0.428, and 26, respectively (Figure 4B). Thus, 14 core proteins closely related to DOR were identified, including AKT1, Caspase-3 (Figure 4C).
Functional Enrichment Analysis
Based on the possible targets of YLF, the GO and KEGG functional enrichment analyses were carried out to look into the potential signaling pathways or biological processes regulated by YLF. The GO terms included BP, CC, and MF. The BP terms mainly appear in the cellular response to lipids, organic cyclic compounds, organonitrogen compounds, positive regulation of cell migration, negative regulation of cell population proliferation, regulation of apoptotic signaling pathway, and cellular stress response. Furthermore, the CC terms mainly appeared in the membrane raft, vesicle lumen, transcription regulator complex, extracellular matrix, nuclear envelope, and serine protease inhibitor complex. Additionally, MF analysis revealed that the major possible targets were mainly cytokine receptor binding, DNA-binding transcription factor binding, DNA-binding transcription factor binding, and kinase binding. The first 10 entries for BP, CC, and MF are shown in Figure 5A.
With filters set to adjusted p-values <0.05, KEGG enrichment analysis was carried out to examine representative pathways connected to key targets. The pathway in cancer, the PI3K/AKT signaling pathway and the AGE-RAGE signaling pathway are among the other pathways on the final list of the 20 most significant KEGG signaling pathways closely associated with DOR (Figure 5B). Although the target genes are mostly enriched in pathway in cancer, it is a complex signaling pathway which contains such as PI3K signaling pathway, ERK signaling pathway, etc. Meanwhile, we found that PI3K signaling pathway as the second most abundant signaling pathway in gene enrichment, it was particularly rich in targets among these numerous pathways, was linked to apoptosis, and could be used as a target for in vivo experimental research.
YLF Ameliorated the Progression of CTX-Induced DOR Model Rats
We further confirmed the main pharmacological mechanisms for treating DOR that were anticipated by the network pharmacology analysis of YLF. In in vivo animal experiments, CTX intraperitoneal injection was applied to construct DOR model rats and administered intragastrically with YLF. These results imply that the administration of YLF greatly reduced the development of DOR.
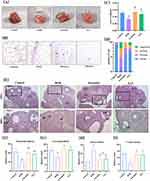
YLF Administration Increased Ovarian Weight and Improved the Estrous Cycle in DOR Rats
As shown in Figure 3A and B, the ovarian weight of rats in the DOR group was significantly lower than that in the control group (p=0.0095). In contrast, rats in the YLF-treated and estradiol groups were significantly higher than that in the DOR group (p=0.0471, p=0.0029). As shown in Figure 3C, the estrous cycle consisted of diestrus, proestrus, estrus, and metestrus. There are mainly leukocytes in diestrus, and nucleated epithelial cells in proestrus; we observed a large amount of anucleated keratinized epithelial cells in estrus and mainly leukocytes and keratocytes in diestrus. The estrous cycle was disordered in DOR model rats, but YLF reversed the estrous cycle to normal levels (Figure 3D).
YLF Administration Improved Histological Changes in the Ovaries of DOR Rats
Primordial follicles and growing follicles were significantly reduced in rats in the DOR group compared with those in the control group (p=0.0018, p=0.0227), and YLF administration significantly reversed this change (p=0.0165, Figure 3E–G). Significantly more atretic follicles were observed in the DOR group compared to the control group (p=0.0034). In contrast, the number of atretic follicles was significantly reduced in the YLF and estradiol groups compared with the DOR group (p=0.0181, p=0.0127; Figure 3H). The number of corpora lutea was significantly lower in the DOR group (p=0.0087) and significantly higher in the YLF and estradiol groups than in the control group (p=0.0149, p=0.0114; Figure 3I).
YLF Ameliorated Granulosa Cell Apoptosis in DOR Rats
The predicted results of the mentioned network pharmacological analysis suggested that YLF affects the process involved in apoptosis through the PI3K/AKT signaling pathway. Therefore, we first observed the mitochondrial morphology of granulosa cells by electron microscopy and apoptosis of ovarian granulosa cells by the TUNEL assay. We then detected the expression of the PI3K / AKT signaling pathway and apoptosis-related proteins and mRNA using Western blotting and PCR analysis.
YLF Administration Improved the Mitochondrial Morphology in Ovaries of DOR Rats
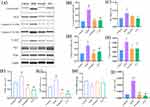
We used electron microscopy to examine ultrastructural changes in the ovaries (Figure 6A). We observed significant differences in the mitochondrial morphology among the four groups. The majority of the mitochondria in the control group had normal structures, such as round, oval, or rod-shaped shapes, and clear, complete mitochondrial cristae. However, in the ovaries of the DOR group, the mitochondria of granulosa cells showed abnormal structures in the form of vacuoles, accompanied by aggregation, extrusion, and fusion, in which the cristae of the mitochondria were barely visible. In addition, the percentage of abnormal mitochondria (blurred mitochondrial cristae and vacuolated mitochondria) was significantly lower in the YLF group than that in the DOR group (P < 0.0001, Figure 6B).
YLF Administration Attenuated Granulosa Cell Apoptosis in DOR Rats
As shown in Figure 6C, there were relatively few TUNEL-positive cells in the control group. In contrast, apoptotic cells were present in large amounts in the DOR group. The YLF and estradiol treatment groups had significantly fewer TUNEL-positive cells than the DOR group. Conversely, quantitative analysis showed that the proportion of tunel-positive cells was higher in the DOR group than in the control group (p=0.0002). The proportion of TUNEL-positive cells was significantly lower in the YLF and estradiol groups than in the DOR group (p < 0.0001; Figure 6D).
Western blotting showed that the expression of Cytochrome C, caspase-3, Caspase-9 and BAX was considerably higher in the DOR group compared to the control group (p=0.0141, p<0.0001, p=0.0102, p=0.0386, Figure 7A–E). However, the detected reduction in the expression of these apoptosis-related proteins demonstrated that YLF administration inhibited CTX-induced apoptosis in the ovaries of DOR rats (p=0.0213, p=0.0011, p=0.0442, p=0.0336).
YLF Attenuated CTX-Induced Ovarian Damage by Regulating the PI3K/AKT Signaling Pathway
PI3K and AKT mRNA levels were considerably higher in the DOR group compared to the control group (p=0.0054, p=0.004), while YLF decreased PI3K and AKT mRNA expression, as did estradiol (p < 0.0001, Figure 7F–G). Furthermore, YLF did not affect PTEN mRNA expression (p > 0.05; Figure 7H). Meanwhile, the ratio of p-AKT/AKT protein expression was significantly higher in the DOR group than in the control group (p < 0.0001). In contrast, the ratio was significantly decreased in the YLF treatment group compared to that in the DOR group (p < 0.0001, Figure 7I).
Discussion
DOR is characterized by a decrease in the number and quality of oocytes, accompanied by a decrease in clinical pregnancy and live birth rates, and an increase in the rate of miscarriage.2 The incidence of DOR has been gradually increasing recently and has become an interesting topic of current research in the field of reproduction. Estradiol is used as a hormonal supplement to improve symptoms effectively; however, its long-term use can potentially increase the risk of breast cancer and endometrial cancer.23 Interestingly, traditional Chinese medicine is a very effective method,5 which has been used for a century and has proven effective in treating DOR because of its obvious clinical efficacy. However, the molecular mechanism by which YLF acts on DOR is still mainly unknown, which severely restricts its use and promotion. Network pharmacology is a cutting-edge method for methodically analyzing the mechanisms of action of herbal remedies in a range of illnesses. Here, we compared the effects of YLF and estradiol valerate supplementation on DOR and investigated the effects and potential pharmacological mechanisms of YLF on DOR. Interestingly, Network analysis revealed specific signaling pathways (most notably the PI3K/AKT pathway) and pertinent potential targets (Caspase-3 and AKT). Further experiments in a CTX-induced DOR rat model confirmed that YLF reversed the induced CTX.
Network pharmacological analysis was used to identify core targets and signaling pathways in order to elucidate the fundamental mechanism of action of YLF against DOR. In this study, the core targets of YLF active components included Caspase-3, and AKT1, which are closely related to apoptosis. The KEGG enrichment analysis showed that the PI3K/AKT signaling pathway, along with the apoptotic pathway, is a potential way for YLF to interfere with DOR. Moreover, Caspase-3 is required for apoptosis in ovarian granulosa cells24 and is a key target for DOR therapy. Previous studies have found high expression of Caspase-3 in CTX-treated ovarian granulosa cells, as detected by IHC staining, and the upregulation of Caspase-3 and BAX protein expression also confirmed the presence of CTX-induced apoptosis in ovarian granulosa cells in the DOR rat model.9 Granulosa cell (GC) apoptosis is a physiological phenomenon in follicles that induces follicular atresia and decreases follicle number.25,26 Apoptosis occurs through two different pathways as follows: extrinsic and intrinsic pathways.27 The extrinsic pathway is triggered by the binding of a ligand to a transmembrane death receptor, which, in turn, binds to the ligand and recruits an articulated protein containing the corresponding death domain to activate downstream Caspase-3 to execute cell death. Furthermore, the intrinsic pathway, also known as the mitochondrial pathway, involves the release of Cytochrome C from the mitochondria into the cytoplasm in response to cellular stress. The mitochondrial pathway is primarily regulated by the Bcl-2 family of proteins, a family of pro-apoptotic proteins such as BAX, which produce pores in the mitochondrial membrane in response to apoptotic stimuli and promote the release of Cytochrome C. Cytochrome C binds to apoptotic protease-activating factor-1 (Apaf-1) to form apoptotic vesicles, and in the apoptotic body, procaspase-9 is activated to form Caspase-9, which causes cell death by activating downstream caspase-3.27,28 Therefore, apoptosis of granulosa cells is closely related to Caspase-3 and is closely associated with the pathogenesis of follicular atresia and the improvement of DOR.25 PI3K/Akt is the main signaling pathway that regulates primordial follicle recruitment and growth. This pathway also contributes to cell growth, survival, metabolism, and the maintenance of genomic integrity.29 It has been observed that high PI3K/Akt activity is associated with reduced primordial follicle numbers and ovarian aging and that PTEN, a central negative regulatory molecule of this signaling pathway, activates primordial follicles in a range of species and is closely associated with the development of DOR.29 CTX, a chemotherapeutic agent, is often used in animal models to induce DOR.9,30–32 Chemotherapeutic agents cause ovarian insufficiency mainly by decreasing oocyte PTEN expression, inducing accelerated activation of primordial follicles, and increasing atresia of growing follicles, wherein apoptotic changes in granulosa cells are the mechanisms leading to follicle loss.33 Previous studies have shown that CTX treatment leads to excessive activation of primordial follicles through upregulation of PI3K and AKT protein phosphorylation, whereas activation of the PI3K/AKT pathway promotes excessive loss of primordial follicles, leading to abnormal growth and development of growing follicles and apoptosis.9
Furthermore, the outcomes of animal studies demonstrated that YLF administration decreased the percentage of atretic follicles, abnormal mitochondria in granulosa cells (GCs), and TUNEL positivity in granulosa cells in addition to increasing the number of primordial and growing follicles and corpora lutea. Unlike CTX, YLF administration reversed the high expression of PI3K and P-AKT/AKT and downregulated the expression of apoptosis-related proteins such as Caspase-3, Caspase-9, BAX, and Cytochrome C. This is consistent with our previous findings that high doses of YLF can effectively improve cyclophosphamide (CTX)-induced granulosa cell function, promote INHB secretion, inhibit follicular apoptosis, and increase the number and quality of oocytes in the ovary in DOR rats. This experiment illustrates from a deeper molecular mechanism that a possible mechanism for the therapeutic effect of YLF on DOR is the downregulation of PI3K/AKT-related apoptotic signaling pathway. YLF can treat DOR through a multi-component, multi-target and multi-pathway model.
However, there are some limitations on this research. First, by concentrating on the elements and objectives at the top of the list and ignoring those at the bottom, we may slightly skew our findings. Second, experimental validation of targets and predicted pathways is relatively limited. We chose to validate only the key roles of PI3K/AKT and related apoptotic pathways in YLF treatment of DOR, while other predicted key targets and pathways needed additional validation in the future.
Conclusions
In conclusion, to investigate the pharmacological mechanism of action of YLF against DOR, network pharmacology analysis and in vivo experimental validation were done. Network pharmacology analysis predicted that YLF modulates numerous components, targets, and pathways to exercise therapeutic effects on DOR. Furthermore, animal experiments confirmed that the mechanism of YLF in DOR treatment inhibited the apoptosis of ovarian granulosa cells in DOR rats by regulating the PI3K/AKT signaling pathway and key proteins like Cytochrome C, Caspase-3, Caspase-9 and BAX, thereby delaying the progression of DOR. As a result, this research supplies a more effective method for understanding the pharmacological mechanism of YLF and an innovative target for the treatment of DOR.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
All human sample data were approved by the Ethics Committee of Hangzhou TCM Hospital (NO.2020KY153); all animal experiments were approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (No. 202109-03).
Funding
This work was financially supported through grants from Zhejiang Basic Public Welfare Research Program (LGF20H270006), China Zhang Qin Famous Old Chinese Medicine Experts Inheritance Studio Construction Project (GZS202202), Zhejiang Provincial Administration of Traditional Chinese Medicine Program (2022ZB249) and Zhejiang TCM modernization project (2021ZX012).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Scantamburlo VM, Linsingen RV, Centa LJR, et al. Association between decreased ovarian reserve and poor oocyte quality. Obstet Gynecol Sci. 2021;64(6):532–539. doi:10.5468/ogs.20168
2. Hu S, Xu B, Jin L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertil Steril. 2020;114(1):118–24.e1. doi:10.1016/j.fertnstert.2020.02.112
3. Tal R, Seifer DB. Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol. 2017;217(2):129–140. doi:10.1016/j.ajog.2017.02.027
4. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi:10.1093/humupd/dml034
5. Zhang QL, Lei YL, Deng Y, et al. Treatment progress in diminished ovarian reserve: Western and Chinese medicine. Chin J Integr Med. 2022;29(4):361–367. doi:10.1007/s11655-021-3353-2
6. D’Alonzo M, Bounous VE, Villa M, Biglia N. Current evidence of the oncological benefit-risk profile of hormone replacement therapy. Medicina. 2019;55(9):573. doi:10.3390/medicina55090573
7. Neves AR, Montoya-Botero P, Polyzos NP. Androgens and diminished ovarian reserve: the long road from basic science to clinical implementation. A comprehensive and systematic review with meta-analysis. Am J Obstet Gynecol. 2022;227(3):401–13.e18. doi:10.1016/j.ajog.2022.03.051
8. Xia T, Ma R-H, Mu W, et al. Traditional Chinese medicine for diminished ovarian reserve: a systematic review and meta-analysis. Chin Herb Med. 2014;6(02):93–102.
9. Liu W, Chen Q, Liu Z, et al. Zihuai recipe alleviates cyclophosphamide-induced diminished ovarian reserve via suppressing PI3K/AKT-mediated apoptosis. J Ethnopharmacol. 2021;277:113789. doi:10.1016/j.jep.2021.113789
10. Zou LSC, Chen B, Gao W, Weijiang C. Research on the construction status and suggestions of the first batch of “national academic school of traditional Chinese medicine inheritance studio construction units” in Zhejiang Province. J Zhejiang Univ Tradit Chin Med. 2020;2020:44.
11. Jialin H, Jia Y. Efficacy of Yulin Formula with addition and reduction in the treatment of 30 cases of decreased ovarian reserve function in women of different ages. Chin J Tradit Med Sci Technol. 2011;18(06).
12. Bin-bin C, Jialin H, Su-xia W. Effects of Yulin decoction on secretion function of granulosa cells of ovarian reserve declined rats. Chin J Exp Tradit Med Formulae. 2015;21(15):1.
13. Binbin C, Su-xia W, Yu-tian Z, Jialin H, Xiaohong F. Effects of Yulin Formula on ultra structure of ovarian granulosa cells in diminished ovarian reserve rats. China J Traditi Chin Med Pharm. 2020;35(6):1.
14. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi:10.1186/1758-2946-6-13
15. Yang L, Zhao Y, Xu H, et al. Network pharmacology-based prediction and verification of the potential mechanisms of He’s Yangchao Formula against diminished ovarian reserve. Evid Based Complement Alternat Med. 2022;2022:8361808. doi:10.1155/2022/8361808
16. Bateman A, Martin M-J, Orchard S. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–D489. doi:10.1093/nar/gkaa1100
17. Stelzer G, Dalah I, Stein TI, et al. In-silico human genomics with GeneCards. Hum Genomics. 2011;5(6):709–717. doi:10.1186/1479-7364-5-6-709
18. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–D798. doi:10.1093/nar/gku1205
19. Zhang H, Yao S, Zhang Z, et al. Network pharmacology and experimental validation to reveal the pharmacological mechanisms of liuwei dihuang decoction against intervertebral disc degeneration. Drug Des Devel Ther. 2021;15:4911–4924. doi:10.2147/DDDT.S338439
20. Zhang YY, Ma JX, Zhu YT, et al. Investigation of the mechanisms and experimental verification of Cuscuta-Salvia in the treatment of polycystic ovary syndrome (PCOS) via network pharmacology. J Ovarian Res. 2022;15(1):40. doi:10.1186/s13048-022-00964-8
21. Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29(14):1830–1831. doi:10.1093/bioinformatics/btt285
22. Jiang M, Wang W, Zhang J, et al. Protective effects and possible mechanisms of actions of bushen cuyun recipe on diminished ovarian reserve induced by cyclophosphamide in rats. Front Pharmacol. 2020;11:546. doi:10.3389/fphar.2020.00546
23. Kesharwani DK, Mohammad S, Acharya N, Joshi KS. Fertility with early reduction of ovarian reserve. Cureus. 2022;14(10):e30326. doi:10.7759/cureus.30326
24. Glamoclija V, Vilovic K, Saraga-Babic M, Baranovic A, Sapunar D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril. 2005;83(2):426–431. doi:10.1016/j.fertnstert.2004.06.075
25. Bulgurcuoglu Kuran S, Altun A, Karamustafaoglu Balci B, Keskin I, Hocaoglu M. Expression of pro-apoptotic and anti-apoptotic proteins in granulosa cells of women with diminished ovarian reserve. J Assist Reprod Genet. 2022;39(3):765–775. doi:10.1007/s10815-022-02422-2
26. Regan SLP, Knight PG, Yovich JL, et al. The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol Cell Endocrinol. 2018;470:219–227. doi:10.1016/j.mce.2017.11.002
27. Nirmala JG, Lopus M. Cell death mechanisms in eukaryotes. Cell Biol Toxicol. 2020;36(2):145–164. doi:10.1007/s10565-019-09496-2
28. Santagostino SF, Assenmacher CA, Tarrant JC, Adedeji AO, Radaelli E. Mechanisms of regulated cell death: current perspectives. Vet Pathol. 2021;58(4):596–623. doi:10.1177/03009858211005537
29. Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt Signalling and DNA damage in the oocyte: implications for primordial follicle activation, oocyte quality and ageing. Cells. 2020;9(1):200. doi:10.3390/cells9010200
30. Zhao Y, Chen Y, Miao C, et al. He’s yangchao recipe ameliorates ovarian oxidative stress of aging mice under consecutive superovulation involving JNK- and P53-related mechanism. Evid Based Complement Alternat Med. 2022;2022:7705194. doi:10.1155/2022/7705194
31. Liu H, Yang H, Qin Z, et al. Exploration of the danggui buxue decoction mechanism regulating the balance of ESR and AR in the TP53-AKT signaling pathway in the prevention and treatment of POF. Evid Based Complement Alternat Med. 2021;2021:4862164. doi:10.1155/2021/4862164
32. Luan Y, Edmonds ME, Woodruff TK, Kim SY. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol. 2019;240(2):243–256. doi:10.1530/JOE-18-0370
33. Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenomenon? Cell Cycle. 2013;12(20):3245–3246. doi:10.4161/cc.26358
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.