Back to Journals » Drug Design, Development and Therapy » Volume 17
Investigating the Mechanism of Action of Schisandra chinensis Combined with Coenzyme Q10 in the Treatment of Heart Failure Based on PI3K-AKT Pathway
Authors Wen S, Yang K, Bai Y, Wu Y, Liu D , Wu X, Zhang X, Sun J
Received 25 November 2022
Accepted for publication 16 March 2023
Published 27 March 2023 Volume 2023:17 Pages 939—957
DOI https://doi.org/10.2147/DDDT.S393995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Sihua Wen,1,* Kai Yang,1,* Yunfeng Bai,2 Yanan Wu,1 Ding Liu,1 Xu Wu,1 Xiaofei Zhang,1 Jing Sun1
1Department of Pharmaceutics, The Key Laboratory of Basic and New Drug Research of Traditional Chinese Medicine, Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China; 2Shaanxi Dongtai Pharmaceutical Co., Ltd, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofei Zhang; Jing Sun, Department of Pharmaceutics, College of Pharmacy, Shaanxi University of Chinese Medicine, Xianyang, People’s Republic of China, Tel +86 177 7003 7322, Fax +86 029-38185333, Email [email protected]; [email protected]
Purpose: To study the active components, drug targets and mechanism of Schisandra chinensis (S.chinensis) combined with coenzyme Q10 (CQ10) in the treatment of heart failure (HF).
Methods: Network pharmacology combined with the gene expression omnibus chip method to analyze the main pathways by which S.chinensis combined with CQ10 functioned to treat heart failure. Subsequently, the biological activities of the major pathway key proteins and their corresponding compounds were verified by molecular docking techniques. Finally, the molecular mechanism of S. chinensis combined with CQ10 for the treatment of heart failure was verified using a rat heart failure model induced by isoproterenol hydrochloride and using hematoxylin-eosin staining, TUNEL, immunohistochemistry and Western blot.
Results: Network pharmacology combined with experimental validation suggests that the mechanism of action of S.chinensis combined with CQ10 in the treatment of heart failure may involve CQ10, Citral, Schisandrone, Schisanhenol B, Gomisin O, Schisandrin C and other components, which may synergistically inhibit the PI3K-AKT signaling pathway and affect the expression of AKT1, PIK3CG and other targets on this pathway. In addition, S. chinensis combined with CQ10 could effectively improve the cardiac coefficients of rats with heart failure, reduce the area of myocardial fibrosis and lowered the serum levels of IL-1β and TNF-α in heart failure rats, as well as reduced cardiac myocyte apoptosis, increased Bcl-2 expression and decreased p-PI3K/PI3K, p-AKT/AKT, P65 and Bax expression in cardiac tissue. Comparison of the results showed that the combination of S.chinensis and CQ10 was more effective compared with CQ10 alone, ie, the ability of S.chinensis combined with CQ10 in improving cardiac function, inhibiting cardiomyocyte apoptosis and reducing inflammatory response lies in the synergistic effect of PI3K/AKT signaling pathway.
Conclusion: The therapeutic effect of S.chinensis combined with CQ10 on heart failure, which may occur through the inhibition of PI3K/AKT signaling pathway.
Keywords: Schisandra chinensis, coenzyme Q10, network pharmacology, molecular docking, mechanism validation, heart failure
Graphical Abstract:

Introduction
Heart failure (HF) is a clinical syndrome of cardiac insufficiency, which occurs due to abnormal structural remodeling of the heart from various causes.1 HF is characterized by the inability of the heart to maintain adequate blood output and represents an advanced stage in the progression of most cardiovascular diseases. The main clinical manifestations of HF are dyspnea and fatigue, as well as fluid retention.2 HF is associated with high rates of morbidity and mortality,3 and in China, the prevalence is as high as 0.9% and the number of patients has increased to 4.5 million, thus representing a major public health problem.4 The current treatment of HF is still based on western drugs, mainly including vasodilators, angiotensin-converting enzyme inhibitors and β-blockers. However, the morbidity and mortality rate of patients with HF remains high.5 Isoprenaline (ISO) is a major adrenergic receptor agonist that is widely used to induce heart failure model. Studies have shown that apoptosis plays an important role in cardiac dysfunction and structural changes in heart failure and is involved in left ventricular remodeling and the subsequent development of cardiac dysfunction up to symptomatic heart failure, and programmed cell death of cardiomyocytes (apoptosis) has been identified as an important process in the progression to heart failure.6 Currently, clinicians recognize that heart failure is associated with an inflammatory response characterized by the induction and activation of multiple pleiotropic cytokines and chemokines, leading to cardiac remodeling and functional deterioration.7 Therefore, suppression of the inflammatory response of the organism as well as anti-apoptosis is an attractive approach to intervene in heart failure. The use of Chinese Medicine in the treatment of HF has received much attention and research because of its long-lasting effects and few side effects.
Schisandra chinensis (Turcz.) Baill. is the dried, ripe fruit of Schisandra chinensis (S. chinensis), of the family Magnoliaceae. S. chinensis is a traditional northeastern authentic herb, mainly produced in Heilongjiang, Jilin, and Liaoning provinces, and is known as Schisandra because of its sweet, sour, and salty skin and pungent, bitter, and salty core.8 Numerous experimental studies have shown that,9–15 S.chinensis and its main chemical components schisandrin B, Schisanhenol, Schizandrin C and Schisandrol A can inhibit myocardial cell apoptosis, reduce myocardial cell inflammatory damage and have a cardioprotective effect.
Coenzyme Q10 (CQ10) is an over-The-counter nutritional supplement and a lipid-soluble molecule. CQ10 as a facilitator and activator of energy metabolism in cardiomyocytes can effectively improve myocardial energy supply deficiency and oxidative overstimulation in patients with HF, and activate hibernating or stagnant cardiomyocytes to subsequently improve cardiac function.16 At the same time, CQ10 acts as an electrical carrier in mitochondria and as a coenzyme of mitochondrial enzymes, which can promote the conversion of nutrients into energy in mitochondria and has a significant anti-lipid peroxidation effect. CQ10 can prevent CQ10 myocardial ischemia by inhibiting the reaction of nitric oxide and peroxide, reducing overall peripheral resistance, improving the ejection function of the heart, and increasing the relaxation of vascular smooth muscle.17
PI3K/Akt is a classical signal transduction pathway, which can directly regulate the expression of Bcl-2/Bax and plays an important role inregulating cardiomyocyte survival, myocardial remodeling, and inflammation.4 It has been shown18,19 that both CQ10 and S. chinensis mediate their cardioprotective effects through the PI3K/Akt pathway, where CQ10 exerts its pharmacological effects by inhibiting p-PI3K/PI3K, p-AKT/AKT targets to protect primary chicken cardiomyocytes. Schisandrin B has been shown to inhibit the proliferation of cardiac fibroblasts and collagen deposition in neonatal Sprague–Dawley (SD) rats by inhibiting PI3K and p-AKT targets. It was initially hypothesized that the combination of S. chinensis and CQ10 could exert a synergistic effect through the PI3K/Akt pathway for treating HF. Therefore, in this study, we sought to explore the efficacy and mechanism of action of the combination of these two drugs for treating HF and to provide a basis for their clinical application.
Network pharmacology is a discipline that reveals the effects of Chinese Medicine on the regulatory network of an organism at the systemic level; this systematic and holistic approach to research is consistent with the pluralistic, multi-effective, and synergistic nature of Chinese Medicine. However, traditional network pharmacology tends to focus on single herbs or compounds, while neglecting the extraction of functional components with positive effects. As a result, the basic research on active substances is insufficient and the functional components of Chinese Medicine require further elucidation.20 To obtain more data, we adopted an integrated research approach combining network and experimental pharmacology to reveal the efficacy and molecular mechanisms of the functional components of S. chinensis combined with CQ10 for treating HF, which provides theoretical support for developing novel drugs in the future.
Materials and Methods
Materials and Reagents
S. chinensis was purchased from Zhashui Century Ecological Agriculture Co., Ltd. (number of code 2021622; Shaanxi, China) CQ10 was purchased from Haoxiang Biotechnology Co., Ltd. (number of code 2021-3-24; China) isoprenaline (ISO) was purchased from Shanghai Yuanye Biotechnology Co., Ltd (number of code S31064; China).
Screening of Chemical Composition
We conducted a literature review of China’s National Knowledge Infrastructure (CNKI) (https://www.cnki.net/) and Web of Science (https://www.webofscience.com/wos) and obtained 42 active compounds from S. chinensis.21–25 (Table 1)
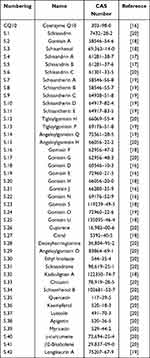 |
Table 1 42 Active Compounds of Schisandra chinensis and Coenzyme Q10 |
Acquisition of Chemical Composition Targets
The corresponding CAS numbers of all chemical components were obtained through the chemical source network, and then through the PubChem (https://pubchem.ncbi.nlm.nih.gov) database to obtain the corresponding 2D structures (Figure 1) and the Simplified Molecular Input Line Entry System (SMILES). The chemical structures of all compounds were entered into Swiss Target Prediction (https://www.swiss.target.prediction.ch/) and metaTarFisher (https://metatarget.scbdd.com/home/index/) databases to predict their potential targets. The targets obtained from the Swiss and meta databases were combined and duplicate values were removed to yield the chemical composition targets of S. chinensis combined with CQ10.
 |
Figure 1 Chemical structures of 43 active components. |
Acquisition of Disease Targets
The GeneCards (https://www.genecards.org) and OMIM (https://omim.org/) databases were used to screen the disease targets. After entering “Heart Failure” as the disease name in the search field, targets related to HF were collected and integrated. The results were output as an Excel spreadsheet and the disease targets were obtained from de-integration through the median value method. Next, by employing the correlation score method as a multiple screening indicator, disease indicators were obtained by de-integration.
Acquisition of Differentially Expressed Genes by the Gene Expression Omnibus (GEO) Chip Method
The targets of differentially expressed genes were obtained from the GEO database. Data were downloaded from GSE76701 and processed using the online analysis tool GEO 2R. To obtain differentially expressed genes between heart failure and normal tissues, the criteria for gene screening were P < 0.05 and |logFC| > 0.5. Heat maps were drawn using a heat map package in R language, and 710 differentially expressed genes were plotted in volcanoes based on multiple variations in gene expression in different experimental groups, such as horizontal coordinates and statistical significance of genomic sequences.
Cross Analysis of Wayne Drug and Disease Targets
The intersection of drug targets, disease targets, and differential genes was obtained using the online mapping tool (http://bioinformatics.psb.ugent.be/beg).
Construction and Analysis of the Protein-Protein Interaction (PPI) Network
The intersecting genes were imported into the STRING database, the species was set as humans to obtain the protein interaction map, and the results were output in the TSV format. The topology of the protein interaction network was studied with Cytoscape software, and the top ten protein targets were obtained.
Construction of the “Key Component-Core Target” Network
All of the core targets in the PPI network were selected, and the corresponding chemical components were used as key components to construct the “key component-core target” network using Cytoscape (3.7.2) software, where “nodes” represent components or targets, and “edges” represent interactions between nodes. The “Analyze Network” tool was used to analyze the topology of the target network, where the higher the degree value, the greater the role in the network.
Enrichment Analysis of Interselection Targets
The interselection targets were imported into the DAVID database for GO biofunctional enrichment and KEGG pathway enrichment analyses, where the GO biofunctional enrichment analysis includes three parts of the GO biological process (BP), GO cellular component (CC), and GO molecular function (MF), to establish possible pathways.
Molecular Docking Validation
The protein structure of the key target was downloaded from the Protein Data Bank (PDB, https://www.rcsb.org/) database, and we download the SDF file for the ligand (active component and positive drug corresponding to the target) from the Pubchem database. The LibDock tool of Discovery Studio 4.0 software (Neo Trident Technology LTD, Beijing, China) was used to dock the key target proteins with their corresponding active ingredients and positive drugs for the score analysis.
Experimental Methods
Experimental Animals
Healthy male SD rats (n=42), weighing 180–220 g, free of specific pathogens, were purchased from Chengdu Dashuo Experimental Animal Co., Ltd. according to SCXK (Sichuan) 2020–030 Laboratory Animal License. Throughout the study, animals were maintained under standard laboratory temperature and humidity conditions, and food and water were freely available. Animal welfare and experimental procedures were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996). The experiment was approved by the Animal Ethics Committee of Shaanxi University of Traditional Chinese Medicine (No. SUCMDL20220401007; Xianyang, China).
Animal Grouping and Intervention
Adaptively feed rats for 7 days and divided into the following seven groups (n=6/group) according to random numbering: Normal, Model, Positive drug group (Positive), Low dose group (Low), Middle dose group (Middle), High dose group (High), and single Coenzyme Q10 group (Coenzyme Q10). After grouping, each group of rats was housed once a day in a separate cage, where the normal group (rats treated with sodium CMA, 10 mL/kg/day, P.O.); the model group (rats modeled with ISO 5 mg/kg, i.h, sodium CMA treated rats, 10 mL/kg/day, P.O.); the positive drug (rats modeled with ISO 5 mg/kg/day, i.h, rats treated with propranolol, 8.4 mg/kg/day, P.O.); low-dose group (rats modeled with ISO 5 mg/kg/day, i.h, rats treated with S. chinensis 1.2 g/kg/day and CQ10 15 mg/ kg/day, P.O.); medium-dose group (rats were modeled with ISO 5 mg/kg/day, i.h, treated with S. chinensis 2.4 g/kg/day and CQ10 15 mg/kg/day, P.O.); high-dose group (rats were modeled with ISO 5 mg/kg /day, i.h, rats treated with S. chinensis 4.8 g/kg/day and CQ10 15 mg/kg/day, orally); and coenzyme Q10 group (rats were modeled with ISO 5 mg/kg/day, i.h, rats treated with CQ10 15 mg/kg/day dose, P.O.). Subcutaneous injections of ISO for modeling were performed daily, and the groups were treated with dosing on the same day for a total of 7 days.
Sample Collection
After the last administration intervention, blood was collected from the abdominal aorta of each group of rats after anesthesia with 40 mg/kg pentobarbital sodium intraperitoneally. The sera were centrifuged at 3500 rpm at 4°C for 20 minutes. The serum was separated and stored in a low-temperature refrigerator at −80°C for future use. Immediately after blood collection, heart tissue was appropriated, rinsed with saline, and partly fixed in 4% paraformaldehyde solution for hematoxylin-eosin staining, TUNEL and immunohistochemical, and partly frozen in a −80°C cryofreezer for Western blotting.
Measurement of the Cardiac Coefficients in Rats
Following the completion of treatment, each group of rats was weighed before sampling, and the hearts were removed after careful separation of the connective tissue around the heart. After absorbing the residual blood on the surface of the organ with absorbent paper, the heart was weighed immediately with an analytical balance. The cardiac coefficients was calculated as follows: cardiac coefficients = (heart weight (mg)/(experimental rat weight (g)). Each group was compared to the control group and the differences were calculated.
Enzyme-Linked Immunosorbent Assay (ELISA)
In this study, the rat TNF- α (Product No.: MM-0180R1) and IL-1β (Product No.: MM-0047R1) ELISA kit purchased form Jiangsu Enzyme Immunoassay Industrial Co., Ltd. The concentration of TNF-α and IL-1β in the serum of each group of rats was detected by ELISA, and a standard curve was drawn according to the instructions of the kit. The serum cytokine content of each group was detected by an enzyme marker and the absorbance was measured at 450 nm. Among them, TNF- α Limit of quantitation is 10ng/L-360ng/L, IL-1β Limit of quantitation is 1pg/mL - 40pg/mL.
Hematoxylin-Eosin (HE) Staining
The tissue specimens were fixed with 4% paraformaldehyde for 48 h, dehydrated with gradients of 50%, 70%, 85%, and 95% anhydrous ethanol until the tissues were transparent and then embedded in paraffin, cut into tissue sections of 5 µm thickness, Tissue sections were unfolded in a water bath at 43°C and dried in a blast oven at 60°C for 2–4 h. After dewaxing, tissue sections were immersed in graded alcohol and washed in distilled water for a few minutes in water. Tissue sections were stained with hematoxylin and eosin. After dehydration, tissue sections were sealed with xylene and changes were observed under a microscope (MD1000).
Terminal Deoxynucleotidyl Transferase-Mediated Deoxy-UTP-Fluorescein Nick End Labeling (TUNEL) Assay
The sections were inactivated with 30% hydrogen peroxide for 10 min at room temperature, before digesting dropwise with freshly diluted Proteinase K for 10 min at room temperature. Next, labeling buffer was added and the samples were placed in a wet box, labelled at 37°C for 2 h, and washed three times with TBS for 5 min each. The droplets of closure solution were incubated at 37°C for 30 min. The biotinylated anti-digoxin antibody was diluted with antibody diluent, mixed well, and added to the specimen slides, and the samples were incubated for 30 min at 37°C in a wet box and washed three times with TBS for 5 min each. The slices were added to diluted StreptAvidin—Biotin Complex, allowed to react at 37°C for 30 min, and washed four times with TBS for 5 min each time. The tissue was uniformly covered with DAB color development solution, rinsed with tap water, and re-stained with hematoxylin. Finally, the pathological changes were observed under a microscope.
Immunohistochemistry
The sections were placed in EDTA antigen repair solution, heated in a microwave oven on high for 8 min, naturally cooled, and then cooled to room temperature after repeated heating before incubating with 5% BSA blocking solution for 30 min at 37°C. Excess liquid was shaken off, primary antibody was added dropwise, and the tissue was incubated at 4°C overnight. After removal and rewarming at 37°C for 30 min, the tissue was washed thrice with phosphate buffered saline for 5 min each. Next, polymerized HRP anti-rabbit IgG secondary antibody was added and incubated for 30 min at 37°C. The tissues were uniformly covered with DAB chromogenic solution, gradient dehydrated, subjected to transparency, rinsed with tap water, and re-stained with hematoxylin. Finally, the pathological changes were observed under the microscope.
Western Blot Analysis
The heart tissue from the above groups was collected, lysed in RIPA buffer, and placed on ice for 5 min. The supernatant was centrifuged at 12,000 g for 10 min at 4°C in a cryopreserved centrifuge. The supernatant was isolated as a protein extract. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were sealed with 5% skim milk powder and then incubated with p-PI3K (Affinity, AF3241, China), PI3K (Huabio, ET1608-70, China), P65 (Huabio, ER0815, China), AKT (Invitrogen, 50599-2-Ig, China), p-AKT (Bioss, bs-0876R, China), Bcl-2 (Bioss, bs-4563R, China), and Bax (Invitrogen 50599-2-Ig, China) antibodies 4°C for 3 h (slow rotation). The membranes were then washed with Tris buffered saline + Tween (TBST), and the secondary antibody was diluted 5000 times with TBST and incubated for 30 min at room temperature. The membranes were then washed six times in TBST, uniformly sprayed with electrochemiluminescence (ECL) photoluminescence solution, and transferred to a dark box for exposure. The films were scanned and the optical density was calculated using Gel-Pro analyzer software. The relative expression value of the target gene was determined using GAPDH as an internal reference (grayscale value of the target gene / internal reference grayscale value of GAPDH).
Statistical Analysis
Data are expressed as mean ± standard error. Statistical differences were determined by one-way ANOVA and Tukey’s post hoc test using GraphPad Prism 7.0 statistical software. After one-way ANOVA of data from ELISA, immunohistochemistry and WB assays, all data obeyed normal distribution. p<0.05 and p<0.01 were considered to be statistically significant.
Results and Analysis
Results of the Drug Target Acquisition and Disease Target Acquisition
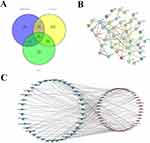
Information on the 43 chemical components of the active ingredients of S. chinensis and coenzyme Q10 is shown in Table 1. A total of 1027 predicted targets were obtained after re-integration of the data. Using the Gene Cards and OMIM databases, 7039 disease targets were searched and 710 differentially expressed genes were obtained between healthy patients and those with HF using the GEO database. The volcano plot (Figure 2A) and heat map (Figure 2B) show significant differences between normal body tissues and patient body tissues. Moreover, by combining the drug and disease targets, 45 core targets were obtained from online Venny diagram (Figure 3A).
 |
Figure 2 Results of GEO data mining. (A) Volcano map of differential gene expression in heart failure. (B) Heat map of differential gene expression in heart failure. |
Results of Network Construction
In the PPI network diagram, the 45 intersection targets have a relatively tight intersection relationship (Figure 3C). The network of “key component core targets” shows that Schisandra and Coenzyme 10 can exert anti-cardiac failure effects through synergy (Figure 3B).
Biofunctional and Pathway Enrichment Analysis Results
GO enrichment and KEGG pathway analyses were performed on 45 core targets using the DAVID database. A total of 104 enrichment results were obtained from GO enrichment analysis, including 73 items of biological process BP (biological process) (Figure 4A), 15 items of cellular component CC (Figure 4B), and Molecular function (MF) 16 items (Figure 4C). The top 20 entries were selected in BP according to the sorting of count values from largest to smallest, and CC and MF did not need to be selected. Among the top 20 items, BPs mainly involve response to xenobiotic stimulus, protein phosphorylation, and response to hypoxia; CCs mainly involve membrane, cell surface, and extracellular exosome; and MFs mainly involve identical protein binding, protein kinase binding, and macromolecular complex binding.
The results of KEGG pathway analysis involve 24 signaling pathways, and the results are shown in Figure 4D. The color after the node from red to green indicates the P-value from large to small, respectively; thus, the larger the green node, the higher the importance of the signaling pathway. The main pathways were those in cancer, lipid and atherosclerosis, and the PI3K-AKT signaling pathway.
Molecular Docking Results
The molecular docking results are shown in Table 2 and the interaction diagrams are shown in Figure 5. To further illustrate the binding activity between the target proteins and their corresponding compounds, we selected the key targets AKT1, PIK3CG, RELA, CHRM1, CHRM2 and NFKB1 using Discovery Studio 4.0 software. Positive drug validation experiments and molecular docking were performed, in which a higher score indicated higher binding activity. Each group has a corresponding positive group, and the docking scores of each group were compared with those of its corresponding positive group. The results showed that the that the target AKT1 had good binding activity with Schisandrin C, CQ10 and Citral. PIK3CG had good binding activity with Schisandrone, Chicanin and Schisanhenol B, and NFKB1 had good binding activity with Citral. RELA had good binding activity with Chicanin.CHRM1 had good binding activity with Gomisin O and Citral. CHRM2 had good binding activity with Citral. In addition, the conformation of the combination was stable, indicating that these may be the key components and targets of S.chinensis combined with CQ10 in the treatment of HF.
 |
Table 2 Docking Scores of Target Protein and Its Corresponding Compound |
Comparison of Cardiac Indices in Different Groups of Rats
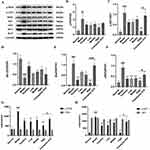
The effect of S.chisandra combined with coenzyme Q10 on HF was initially assessed by the cardiac coefficients (Figure 6A). The graphs showed that the cardiac coefficients of the model group was significantly higher than that of the normal group (P < 0.001, n=6), while the cardiac indices of the positive drug group and different dose groups were lower than that of the model group with significant differences (p < 0.05, n=6).
Serum Levels of TNF-α and IL-1β
The serum concentrations of TNF-α and IL-1β were significantly higher in the HF model group compared to the normal group (P < 0.05, n=6). TNF-α and IL-1β were reduced in the positive drug group and the co-administration group as well as the single CQ10 group compared to the model group, but were higher in the single CQ10 group compared to the co-administration group. The treatment effect was significant in the co-administration group compared to the single coenzyme Q10 group. The results are shown in Figure 6B and C. (During testing of the kit, each sample was tested twice, with intra-batch CV values less than 10%)
HE Results
The results of HE staining showed that ISO induced disorderly arrangement of cardiomyocytes. Moreover, compared to the model group, the disorderly arrangement of cardiomyocytes was reduced in the positive drug group, low, medium, and high dose groups of S. chinensis combined with CQ10 and CQ10 groups after treatment, as shown in Figure 7A.
The overall structure of myocardial tissue in the normal group was normal, with no sign of degeneration such as tissue myocardial fiber-sparing edema necrosis. Moreover, the tissue interstitium was not significantly dilated and congested with blood vessels, and the tissue showed low levels of inflammatory cell infiltration.
In the model group, the overall structure of myocardial tissue was abnormal, the arrangement of myocardial fibers was disordered, and many myocardial fibers were loose, edematous, and necrotic, with high levels of inflammatory cell infiltration in the necrotic area.
Compared to the model group, the overall structure of myocardial tissue in the low and medium dose groups was mildly abnormal, the interstitial tissue was slightly vasodilated and congested, and the tissue showed low levels of inflammatory cell infiltration. The overall structure of the myocardial tissue in the high-dose group and positive drug group was largely normal; the tissue myocardial fibers were arranged neatly; no myocardial fiber sparing edema necrosis or other degeneration were observed in the tissue; no obvious vascular dilatation or congestion were found in the tissue interstitium; and no obvious inflammatory cell infiltration was found.
In the CQ10 group, the overall structure of the myocardial tissue was mildly abnormal, with slight edema of myocardial fibers, slight enlargement of myocardial fiber gaps, congested and dilated blood vessels in the tissue interstitium, and a small amount of inflammatory cell infiltration in the tissue (the yellow arrows show myocardial fibers, the red arrows show interstitial blood vessels, and the black arrows show inflammatory cells, as shown in Figure 5A).
These results suggest that CQ10 and S. chinensis combined with CQ10 have a protective effect on cardiac function in vivo, but the therapeutic effect of the CQ10 group was lower than that of the S. chinensis combined with CQ10 group.
TUNEL Results
The apoptosis rate of cardiomyocytes in the model group was significantly higher than that in the normal group (P < 0.001, n=3), and treatment with positive drugs, S. chinensis combined with CQ10 and single CQ10 significantly reduced the apoptosis rate. The treatment effect was significant in the combined administration group compared to the single CQ10 group (P < 0.001, n=3) (Figure 7B and C).
Immunohistochemistry Results
Bcl-2 expression was down-regulated and Bax expression was up-regulated in the model group. In the positive drug group, treatment with S. chinensis combined with CQ10 and the single CQ10 decreased Bax expression (Figure 7D) and increased Bcl-2 expression (Figure 7E). Using Image-Pro Plus 6.0 software, the same brown color was selected as a uniform criterion for judging the positivity of all photos, and each photo was analyzed to derive the integrated optical density (IOD) value of each photo (Figure 7F).
Western Blotting Results
Compared to the normal group, the Bcl-2 protein expression level in the rat HF model group was lower than that in the normal group, and the protein expression of p-PI3K/PI3K, p-AKT/AKT, P65, and Bax was higher than those in the normal group. Compared to the rat HF model group, the treatment and positive drug groups showed elevated Bcl-2 protein expression and decreased p-PI3K/PI3K, p-AKT/AKT, P65, and Bax protein expression. The effect of S. chinensis combined with CQ10 on elevating Bcl-2 protein expression and decreasing p-PI3K/PI3K, p-AKT/AKT, P65, and Bax protein expression was more significant compared with the effect of CQ10 alone (Figure 8). This result indicates that S. chinensis combined with CQ10 and single CQ10 can inhibit the PI3K-AKT pathway, and that the inhibitory power of S. chinensis combined with CQ10 is more significant.
Mechanism Description
In this study, 42 active ingredients of S. chinensis as well as CQ10 were selected for a network pharmacological study, and 1027 drug genes, 7039 disease genes, 710 differentially expressed genes, 104 GO functions, and 24 KEGG pathways were obtained. Molecular docking results showed the mechanism of action of Schisandra chinensis combined with CQ10 for treating heart failure may involve components of CQ10, Citral, Schisandrone, Schisanhenol B, Gomisin O, Schisandrin C. It was hypothesized that the anti-HF effect might be related to the regulation of PI3K-Akt signaling pathway. The rat HF model was established using the IOS method, and the rats were treated with the positive drug; low, medium, and high doses of S. chinensis combined with CQ10; and CQ10 alone. During the experiment, the serum levels of TNF-α and IL-1β in the rats in each group were detected using ELISA, and the myocardial tissue, myocardial fibers, and inflammatory cells in the skin lesions of rats were observed using HE staining. Immunohistochemistry was used to detect the expression of Bcl-2 and Bax in cardiac tissues, TUNEL staining was used to detect the apoptosis rate of cardiomyocytes, and Western blotting was used to show the protein expression levels of p-AKT/AKT, p-PI3K/PI3K, p65, BAX, and BCL-2 in cardiac injury. Taken together, our results suggest that the mechanism of action of S. chinensis combined with CQ10 may occur via a reduction in the inflammatory response by inhibiting the PI3k-AKT pathway and reducing the secretion of inflammatory factors IL-1β and TNF-α, thereby indicating that S. chinensis combined with CQ10 has a synergistic effect on the treatment of HF (Figure 9).
 |
Figure 9 The mechanism of Schisandra chinensis combined with coenzyme Q10 inhibiting heart failure through PI3K/AKT signal pathway. |
Discussion
Due to high readmission rates, morbidity and mortality, HF occupies a large amount of clinical healthcare resources and has been a hot and difficult research topic in the field of cardiovascular research.4 Given the multiple pathological processes involved in HF, drugs that interfere with cardiac energy metabolism serve as potential therapeutic agents for HF. In this study, a target network was constructed and analyzed based on 42 active ingredients of S.chinensis and CQ10 obtained from the literature, and multiple drug targets were screened to determine the upstream and downstream target proteins of the signal transduction pathway to predict the relevant targets and corresponding mechanisms of action, indicating that the signal transduction pathway of HF is PI3K/AKT. After that, an animal model of isoproterenol-induced heart failure was successfully established. Numerous studies in the literature have shown that9–15,18 mono-flavored S.chinensis as well as mono-flavored CQ10 can inhibit cardiomyocyte apoptosis and have a protective effect on cardiomyocytes. Based on this, this paper used S.chinensis in combination with CQ10 confirmed the ameliorative effect of S.chinensis in combination with CQ10 on isoproterenol-induced heart failure. It further revealed that CQ10, Citral, Schisandrone, Schisanhenol B, Gomisin O, Schisandrin C et al are important compounds in the treatment of heart failure with S.chinensis combined with CQ10.
Studies suggest that the mechanism of action in heart failure may be related to local and systemic activation of inflammatory signaling cascades and apoptosis, the inflammatory response in HF is characterized by the induction and activation of multiple pleiotropic cytokines and chemokines, leading to cardiac remodeling and functional deterioration. PI3K/Akt is a classical signal transduction pathway that directly regulates the expression of Bcl-2 and Bax and plays an important role in regulating cardiomyocyte survival, myocardial remodeling and inflammation. The phosphorylation level of Akt, PI3K, is one of the most important markers of the PI3K/Akt signaling pathway. Phosphatidylinositol (PI3K) is a cytoplasmic lipid kinase and AKT is a key kinase (serine/threonine kinase) in the PI3K pathway. AKT activation requires membrane interactions and phosphorylation of serine 473 (AKT-Ser473) and threonine 308Akt (AKT-Thr308). After multiple stimulations, the pH structural domain interacts with phosphatidylinositol 3,4,5-trisphosphate (PIP3) produced by PI3K, allowing cytoplasmic AKT to enter the plasma membrane. Then, AKT-PIP3 interaction, induced by conformational changes between the structural domains is open conformation of AKT, exposing Thr308 and Ser473 subsequently phosphorylated to phosphatidylinositol-dependent protein kinase, and phosphorylation of Thr308 and Ser473 fully activates AKT, which is involved in a variety of regulatory cell proliferation and growth through phosphorylation of downstream kinases.26 Studies have shown that over-activated P-Akt can lead to cardiac hypertrophy, induce Bax gene expression, promote cardiomyocyte apoptosis, and accelerate the deterioration of cardiac function. Therefore, inhibition of the inflammatory response of the organism as well as apoptosis may be effective in the treatment of HF.
This study focuses on the effects of inflammatory factors and cardiomyocyte apoptosis in ISO-induced heart failure and the mechanisms of drug action. The histopathological changes of the heart were first observed by HE staining as well as preliminary detection of cardiac coefficients, and the results showed that S.chinensis combined with CQ10 reversed the pathological changes of the myocardial tissue and the cardiac coefficients were significantly decreased. At the same time, the inflammatory factors in serum and myocardial cell apoptosis were verified, The results showed that the expression of inflammatory factors IL-1β and TNF-α in serum was significantly higher in the model group than in the normal group, and there was a significant decrease in inflammatory factors after the drug administration intervention, TUNEL analysis showed that the apoptotic rate of cardiomyocytes was higher in the model group than in the normal group, and the apoptotic cells were significantly reduced after treatment with S.chinensis combined with CQ10 as well as single flavor CQ10. Next, the expression of Bax, a key apoptotic protein in the PI3K-Akt signaling pathway, and Bcl-2 was analyzed by immunohistochemistry, and the results showed that Bcl-2 expression was downregulated and Bax expression was upregulated in the model group, and Bcl-2 protein expression was upregulated and Bax protein expression was downregulated after the drug administration intervention. Finally, Western blotting was performed to verify, the experimental results which confirmed that p-AKT, p-PI3K, P65 and BAX levels were elevated in the myocardium of heart failure patients, while Bcl-2 levels were decreased, In addition, treatment with S.chinensis combined with CQ10 significantly reduced p-AKT, p-PI3K, P65 and BAX levels, increased Bcl-2 levels and improved cardiac function. Importantly, S.chinensis combined with CQ10 was significantly more effective than CQ10 alone in the treatment of heart failure.
Research showed24 that CQ10 could protect primary chicken cardiomyocytes during heat stress by upregulating autophagy and inhibiting the PI3K/Akt/mTOR pathway. Schisandrin B has been shown to inhibit the proliferation of cardiac fibroblasts and collagen deposition in neonatal Sprague–Dawley (SD) rats by inhibiting PI3K and p-AKT targets. In this study, we validated the PI3K/Akt signaling pathway related proteins by using S.chinensis combined with CQ10 and single flavor CQ10, and found that S.chinensis combined with CQ10 could downregulate PI3K-AKT inflammatory pathway, inhibit the inflammatory response of cardiomyocytes and reduce apoptosis with significantly stronger efficacy than the single flavor CQ10 group, indicating that S.chinensis combined with CQ10 has a synergistic effect on blocking PI3K/AKT pathway. Among them, compounds such as Coenzyme Q10, Citral, Schisantheri E, and Gomisin S inhibited p-AKT levels; compounds such as Schisantheri E, Schisantherin D, and Angeloylgomisin H inhibited p-PI3K; and compounds such as Gomisin D, Coenzyme Q10, and Gomisin E inhibited NF-KB. The results showed that S.chinensis combined with CQ10 had synergistic effects on improving cardiac function, inhibiting myocardial cell apoptosis, reducing the level of inflammatory cytokines and regulating PI3K/Akt signal pathway. At the same time, this study also has the following shortcomings: 1. only male rats were used to study the heart failure model 2. only one heart failure model was used, which would limit the direction of drug treatment 3. the treatment time period was too short 4. Echocardiography was not used to examine the heart function. The group will focus on these aspects in the follow-up study of heart failure and correct them to improve the study of drug mechanism of action.
Conclusion
This study confirms the potential of S.chinensis combined with CQ10 intervention for the treatment of heart failure. The results of the network-based pharmacology combined with mechanistic validation provide new insights to fully elucidate the effects of S.chinensis combined with CQ10 in the treatment of heart failure. Specifically, S.chinensis combined with CQ10 reduced the expression of inflammatory factors as well as the rate of apoptosis in cardiomyocytes; therefore, modulation of PI3K/Akt signaling pathway may be a potential mechanism for the synergistic treatment of heart failure with S.chinensis combined with CQ10.
Abbreviations
S.chinensis, Schisandra chinensis; CQ10, coenzyme Q10; HF, heart failure; CNKI, China’s National Knowledge Infrastructure; GEO, gene expression omnibus;PPI, protein-protein interaction BP, biological process; CC, cellular component; MF, molecular function; ISO, isoprenaline; HE, Hematoxylin-eosin;TUNEL, Terminal Deoxynucleotidyl Transferase-Mediated deoxy-UTP-fluorescein nick end labeling SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; PVDF, polyvinylidene fluoride; TBST, Tris buffered saline + Tween; ECL, electrochemiluminescence; ELISA, Enzyme-linked immunosorbent assay;SD, standard deviation; ANOVA, analysis of variance; IOD, integrated optical density.
Ethics Approval and Consent to Participate
The experimental animals were obtained with the informed consent of all participants. The institutional review board of the Shaanxi University of Chinese Medicine approved this experimental, code SCXK (Sichuan) 2020–030.
Ethics
The human data involved in this study from GeneCards public database and the study meets national and international guidelines for research on humans. Therefore, the ethical approval has been granted an exemption (IEC for Medical of The Second Affiliated Hospital of Shaanxi University of Chinese Medicine).
Acknowledgment
Thanks to all the peer reviewers for their opinions and suggestions. We would also like to thank Ms. Yu Shangshang for her great contribution to this study.
Funding
This work was supported by the Engineering Technology Research Center. Research and Application Demonstration of Key Technologies for the Industry of Chinese Herbs such as Tianma and Panax notoginseng in Southwest China (2021YFD1601000), Shaanxi Province, supported by the Engineering and Technology Research Center for the Application and Development of Chinese Herbs in Qinling. Research on comprehensive development and utilization of medicinal herbs of “Wu Wei Zi” of Qin Medicine (2021-02-22-014) Key scientific research on the inheritance and innovation of Chinese medicine and the development of “Qin Medicine” (2021-03-22-010).
Disclosure
Yunfeng Bai is affiliated with Shaanxi Dongtai Pharmaceutical Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Tao Y-G, Huang X-F, Wang J-Y, et al. Exploring molecular mechanism of huangqi in treating heart failure using network pharmacology. Evid Based Complement Alternat Med. 2020;2020:1–17. doi:10.1155/2020/6473745
2. Wu RM, Jiang B, Li H, et al. A network pharmacology approach to discover action mechanisms of Yangxinshi Tablet for improving energy metabolism in chronic ischemic heart failure. J Ethnopharmacol. 2020:246. doi:10.1016/j.jep.2019.112227
3. Shao M, Guo D, Lu W, et al. Identification of the active compounds and drug targets of Chinese medicine in heart failure based on the PPARs-RXRα pathway. J Ethnopharmacol. 2020:257. doi:10.1016/j.jep.2020.112859
4. Zhao -D-D, Zhang X-Q, Yang T, et al. Exploring the therapeutic mechanism of Tingli Dazao Xiefei decoction on heart failure based on network pharmacology and experimental study. Evid Based Complement Alternat Med. 2021;2021:1–15. doi:10.1155/2021/6645878
5. Yu YD, Xiu YP, Li YF, et al. To explore the mechanism and equivalent molecular group of radix astragali and semen lepidii in treating heart failure based on network pharmacology. Evid Based Complement Alternat Med. 2021. doi:10.1155/2021/5518192
6. Li X, Zhong J, Zeng Z, et al. MiR-181c protects cardiomyocyte injury by preventing cell apoptosis through PI3K/Akt signaling pathway. Cardiovasc Diagnosis Ther. 2020;10(4):849–858. doi:10.21037/cdt-20-490
7. Li L, Hao J, Jiang X, et al. Cardioprotective effects of ulinastatin against isoproterenol-induced chronic heart failure through the PI3K‑Akt, p38 MAPK and NF-κB pathways. Mol Med Rep. 2018;17(1):1354–1360. doi:10.3892/mmr.2017.7934
8. Xing NN, Qu HD, Ren WC, et al. Research progress on the main chemical components and modern pharmacological effects of Schisandra chinensis. Chin J Expl Trad Med Formulae. 2021;27(15):210–218. Chinese. doi:10.13422/j.cnki.syfjx.20211407
9. Yang SQ. Regulation of Nrf2/HO-1/GPX4 iron death pathway by Schisandra chinensis ethereal in reduces myocardial injury in diabetic mice. J Chin Med Mater. 2022; (07):1714–1722. in Chinese. doi:10.13863/j.issn1001-4454.2022.07.033
10. Yang M. Effects of pentosidine alcohol methyl on the regulation of cT-I, cT-T, and ET-1 in norepinephrine-induced cardiac hypertrophic injury. Chin J Vet Sci. 2020; (08):1553–1559. in Chinese. doi:10.16303/j.cnki.1005-4545.2020.08.20
11. Zhang WG. Protective effect of Schisandra chinensis ester A on hypoxia-reoxygenated cardiomyocytes. Chin J New Drugs. 2018;17:2052–2056. in Chinese.
12. Sun HX. Protective effect of Schisandra chinensis ethylene on myocardial ischemia-reperfusion injury in rats. Food Sci. 2016;37(01):203–207. in Chinese.
13. Zhang X, Zhao Y, Bai D, et al. Schizandrin protects H9c2 cells against lipopolysaccharide-induced injury by downregulating Smad3. J Biochem Mol Toxicol. 2019;33(5):e22301. doi:10.1002/jbt.22301
14. Gong S, Liu J, Wan S, et al. Schisandrol A attenuates myocardial ischemia/reperfusion-induced myocardial apoptosis through upregulation of 14-3-3θ. Oxid Med Cell Longev. 2021;2021:5541753. doi:10.1155/2021/5541753
15. Su Y. Effects of Schisandra chinensis on myocardial antioxidant capacity and morphological changes in aging rats. Chin J Gerontol. 2015;35(11):2944–2945. in Chinese.
16. lv C. 52 cases of chronic heart failure treated with sacubitril valsartan combined with coenzyme Q10. Herald Med. 2020;11:1511–1515. in Chinese.
17. Wu B, Xu X, Yang Y, et al. Clinical observation on the treatment of chronic heart failure with Astragalus membranaceus capsules combined with coenzyme Q10. J Guangdong Med Univ. 2019;6:662–665. inChinese.
18. Xu J, Huang B, Tang S, et al. Co-enzyme Q10 protects primary chicken myocardial cells from heat stress by upregulating autophagy and suppressing the PI3K/AKT/mTOR pathway. Cell Stress Chaperones. 2019;24(6):1067–1078. doi:10.1007/s12192-019-01029-4
19. Liu CX, Sun H, Hu B, et al. Inhibitory effect of northern schisandra berry etherein on proliferation of rat cardiac fibroblasts and its PI3K/Akt/P27 kip1 signaling pathway mechanism. J Jilin Univ. 2022;(01):52–58. in Chinese. doi:10.13481/j.1671-587X.20220107
20. Yu S, Guo Q, Jia T, et al. Mechanism of action of nicotiflorin from tricyrtis maculata in the treatment of acute myocardial infarction: from network pharmacology to experimental pharmacology. Drug Des Devel Ther. 2021;15:2179–2191. doi:10.2147/DDDT.S302617
21. Bai WY. Research progress on chemical composition and pharmacological effects of Schisandra chinensis. Chin Trad Patent Med. 2019;9:2177–2183. in Chinese.
22. Xia CX. Study on the chemical composition of Schisandra chinensis. Chin Trad Patent Med. 2017;39(03):547–550. in Chinese.
23. Huang Y. Advances in the chemical composition and antitypical activity of Schisandra chinensis against type 2 diabetes. Chin Tradit Herbl Drugs. 2019;50(07):1739–1744. in Chinese.
24. Huang S, Zhang D, Li Y, et al. Schisandra sphenanthera: a comprehensive review of its botany, phytochemistry, pharmacology, and clinical applications. Am J Chin Med. 2021;49(7):1577–1622. doi:10.1142/S0192415X21500749
25. Yang K, Qiu J, Huang Z, et al. A comprehensive review of ethnopharmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et Wils. J Ethnopharmacol. 2022;284:114759. doi:10.1016/j.jep.2021.114759
26. Zhou J, Duan X, Wang J, et al. Valsartan regulates PI3K/AKT pathways through lncRNA GASL1 to improve isoproterenol-induced heart failure. Forensic Clin Appli Biomarkers Cardiovasc Dis. 2022. doi:10.1155/2022/1447399
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.