Back to Journals » Drug Design, Development and Therapy » Volume 16
Intravenous Lidocaine Significantly Reduces the Propofol Dose in Elderly Patients Undergoing Gastroscopy: A Randomized Controlled Trial
Authors Hu S , Wang M, Li S, Zhou W, Zhang Y, Shi H, Ye P, Sun J, Liu F, Zhang W , Zheng L, Hou Q, Wang Y, Sun W, Chen Y, Lu Z, Ji Z , Liao L , Lv X , Wang Y, Wang X, Yang H
Received 3 June 2022
Accepted for publication 4 August 2022
Published 12 August 2022 Volume 2022:16 Pages 2695—2705
DOI https://doi.org/10.2147/DDDT.S377237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Song Hu,1– 3,* Mingxia Wang,2,3,* Siyu Li,2,* Wenyu Zhou,3 Yi Zhang,2 Haobing Shi,2 Pengcheng Ye,2 Jixiong Sun,2 Feng Liu,2 Wei Zhang,2 Li Zheng,3 Qianhao Hou,2 Yue Wang,2 Weixin Sun,2 Yuanli Chen,2 Zhenzhen Lu,4 Zhonghua Ji,2 Lijun Liao,2 Xin Lv,3 Yinglin Wang,2 Xiangrui Wang,2 Hao Yang2,3
1Graduate School, Wannan Medical College, Wuhu, 241002, People’s Republic of China; 2Department of Anesthesiology and Pain Management, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200120, People’s Republic of China; 3Department of Anesthesiology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, People’s Republic of China; 4Department of Biostatistics, Zhongshan Hospital, Fudan University, Shanghai, 200032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Yang; Xiangrui Wang, Department of Anesthesiology and Pain Management, Shanghai East Hospital, Tongji University School of Medicine, 150 Jimo Road, Shanghai, 200120, People’s Republic of China, Email [email protected]; [email protected]
Objective: Propofol-based sedation has been widely used for gastroscopy, but the risk of respiratory suppression in elderly patients should not be overlooked. Intravenous (IV) lidocaine during surgery can reduce the demand for propofol and the incidence of cardiopulmonary complications. We examined whether IV lidocaine reduces the dose of propofol and the occurrence of adverse events during gastroscopy in elderly patients.
Methods: We conducted a prospective, single-center, double-blind randomized controlled trial in elderly patients aged ≥ 65 years with ASA I-II. Subjects were randomly assigned to the lidocaine group (Group L, n=70), who received IV 1.5 mg kg− 1 lidocaine followed by a continuous infusion of 4 mg kg− 1 h− 1 lidocaine, or the normal saline group (Group N, n=70), who received an equal volume of saline in the same way.
Results: IV lidocaine reduced the total and maintenance propofol dose in Group L (p< 0.001), with no significant effect on the induction dose. The incidence of intraoperative hypoxia (p=0.035), emergency airway management events (p=0.005), duration of gastroscopy (p< 0.05), consciousness recovery time (p< 0.001), and postoperative pain (p=0.009) were all reduced in Group L. Patient (p=0.025) and gastroscopist (p=0.031) satisfaction was higher in Group L. Intraoperative hemodynamic parameters, the respiratory rate, the incidence of sedation-related events and anesthesiologist satisfaction were similar between the two groups.
Conclusion: IV lidocaine can significantly reduce the amount of propofol, the incidence of hypoxia and postoperative pain during gastroscopy in elderly patients, with a higher patient and gastroscopist satisfaction.
Keywords: lidocaine, propofol, gastroscopy, elderly patient, sedation
Introduction
Gastroscopy is one of the most important tools for diagnosing upper gastrointestinal diseases, but nausea, vomiting, and other adverse reactions during the examination make it difficult for many patients to cooperate effectively, especially for the elderly who are more prone to dangerous events.1 With rapidly developing anesthesia techniques and the spread of the concept of comfort care, painless gastroscopy has gradually replaced traditional gastroscopy.2
Propofol has gradually become the most common sedative-hypnotic drug used in painless gastroscopy due to its rapid effect, short half-life, and good sedative effect, but it has disadvantages such as a weak analgesic effect and suppression of respiratory and circulatory systems. In particular, the degeneration of heart, brain, lung, and other organ functions, poor tolerance to anesthetic drugs, and many concomitant diseases in elderly patients make the degree of anesthesia difficult to control and the risk of anesthesia higher, which to a certain extent limits the application of propofol in gastroscopy in elderly patients.3 In addition, hypoxemia caused by propofol sedation remains the main cause of procedural sedation and analgesia (PSA) complications.4,5 The use of propofol combined with other drugs to reduce the risk of PSA complications has been widely studied. For instance, the combination of dexmedetomidine and propofol has been used to reduce propofol doses during endoscopy.6 However, IV dexmedetomidine may cause bradycardia and hypertension, endangering the safety of the patient.7 Currently, opioids such as sufentanil combined with propofol IV anesthesia are more commonly used in gastrointestinal endoscopy.8 However, opioids are not suitable for elderly patients due to their susceptibility to respiratory depression, hypotension, and other cardiovascular adverse events. Therefore, there is still a need to find a more appropriate sedative to combine with propofol for performing clinical gastroscopy in elderly patients.
Lidocaine, an amide local anesthetic, is a potential complement to propofol sedation. During surgery on the viscera, IV lidocaine is beneficial.9 IV lidocaine alleviates visceral pain in experimental animals as well as abdominal pain in humans.10,11 In addition, Labaille et al showed that IV lidocaine enhanced the ventilatory response to carbon dioxide in healthy subjects.12 Forster et al showed that IV lidocaine with ketamine during colonoscopy reduced the demand for propofol by 50%.13 Therefore, lidocaine in combination with propofol has been applied as a strategy to reduce the total propofol demand in sedation. However, there have been no studies reported in the literature on the effect of IV lidocaine on intraoperative propofol dose and the incidence of perioperative adverse events in elderly patients undergoing painless gastroscopy. In elderly patients undergoing painless gastroscopy, we hypothesized that IV lidocaine would reduce the propofol dose and the incidence of perioperative adverse events. Therefore, we conducted a prospective, double-blind, randomized controlled trial to investigate whether IV lidocaine infusion would reduce the overall need for propofol on top of propofol sedation and to assess the safety of IV lidocaine applied to painless gastroscopy in the elderly.
Method
Study Design
This was a single-center, patient-and-investigator blinded, randomized trial conducted at Shanghai East Hospital, Tongji University, enrolling elderly patients undergoing painless gastroscopy. The study performed conducted between July 2021 and April 2022. The study protocol received ethical approval from the Ethics Committee of Shanghai East Hospital [No. 2021 (047)] and was registered with the Chinese Clinical Trials Registry (ChiCTR2100048878) on July 19, 2021. Participants were asked to sign an informed consent before enrolling in the study. This study complied with the Declaration of Helsinki and the guidelines for reporting parallel group randomized trials, the Consolidated Standards for Reporting Trials 2010 (CONSORT).14
Participants
From July 2021 to April 2022, eligible elderly patients who underwent painless gastroscopy were evaluated for inclusion. Inclusion criteria: (i) age ≥65 years; (ii) patients undergoing painless gastroscopy; (iii) ASA I-II. Exclusion criteria: (i) Arrhythmia with II- or III-degree atrioventricular block. (ii) severe hepatic, or renal dysfunction; (iii) chronic obstructive pulmonary disease and recent asthma attacks, or hypoxemia; (iv) central nervous system disease or neuropsychiatric disorders such as epilepsy; (v) gastrectomy history, or full stomach and upper gastrointestinal bleeding; (vi) use of sedatives, sleeping pills or analgesics for>3 months; (vii) allergy to lidocaine; (viii) refusal to sign the informed consent form.
Study Protocol
The patients were randomly divided into Group L or Group N by the random number table method. The patient grouping was known only to the nurse anesthetist and research assistant in charge of the draw; neither the patient, the anesthesiologist, nor the gastroscopist were aware of the patient grouping. Study medications were dispensed by the anesthesiologist, who was not involved in patient care or the collection of study variables. Unmarked medications were given to the anesthesiologist who performs the sedation by the nurse anesthetist according to the patient grouping.
The subject patients routinely fasted for 8 hours before painless gastroscopy, the peripheral veins of the right upper limb were opened at the gastrointestinal endoscopy center, and 50 mL of sodium lactate Ringer’s solution was slowly dripped, and oxygen was administered via nasal cannula (2 L min−1) while lying on the examination bed in the left lateral position. The heart rate, blood pressure, electrocardiogram, and pulse oximetry were detected and recorded after the patient rested for 15 minutes.
Patients in Group L were administered intravenously a bolus dose of 1.5 mg kg−1 lidocaine followed by a continuous infusion of 4 mg kg−1 h−1, then 1.5 mg kg−1 propofol was injected slowly until consciousness was lost. In Group N, patients were administered an equal volume of saline by IV injection followed by a continuous infusion of an equal volume of saline and then 1.5 mg kg−1 propofol was injected slowly until loss of consciousness. Depth of sedation was assessed with the Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S).15 The score scale is described as 5: Respond readily to name spoken in normal tone; 4: Lethargic response to name spoken in normal tone; 3: Responds only after the name is called loudly and/or repeatedly; 2: Responds only after mild prodding or shaking; 1: Response only after painful trapezius squeeze; 0: No response after painful trapezius squeeze. The patient’s state of consciousness was continuously observed after induction of anesthesia, and gastroscopy was started when the patient lost consciousness and the MOAA/S score was ≤2.
During gastroscopy, additional propofol 20–30 mg was administered intravenously to maintain the depth of sedation when the patient had a MOAA/S score of ≥3 or when painful facial features, limb movement, and hemodynamics were present. During sedation, all patients breathed spontaneously with 2 L min−1 of oxygen via nasal cannula. If the patient develops decreased oxygen saturation and the anesthesiologist rules out interference from the volumetric pulse waveform, the nasal cannula oxygen flow is increased from 2 to 6 L min−1 while the mandible is lifted bilaterally until pulse oxygen saturation (SpO2) is ≥95%, using mask ventilation to assist with breathing if necessary, and tracheal intubation if severe hypoxia cannot be relieved. Hypotension (systolic blood pressure (SBP) <90 mmHg or 20% decrease in base value) persisting for ≥1 minute was treated with phenylephrine (40 μg, IV). Bradycardia (heart rate (HR) <50 bpm) was treated with atropine (0.5 mg, IV). Repeated the procedure if necessary. In the post-anesthesia care unit (PACU), the patients were observed further following the procedure.
Measurements
The primary endpoints measured were propofol consumption (induction dose, maintenance dose, and total dose). The secondary endpoints were: the incidence of hypoxia (75%≤ SpO2 < 90% for < 60 seconds), increase the flow of oxygen (increase the flow of oxygen to 6L min−1), severe hypoxia (SpO2 < 75% or 75% ≤ SpO2 < 90% for > 60 seconds), emergency airway management situations (hypoxemia cannot be corrected by increasing oxygen flow but instead requires jaw-thrust maneuvers or interruption of endoscopic procedures for mask-assisted ventilation or emergency tracheal intubation), intraoperative hemodynamic parameters (SBP, diastolic blood pressure (DBP), mean arterial pressure (MAP), HR), respiratory rate (RR), duration of gastroscopy (time between the entry of the gastroscope into the mouth and the end of the examination when the gastroscope exits the mouth), consciousness recovery time (time between the end of the examination and the return of consciousness), and sedation-related adverse events (hypotension, bradycardia (<50 beats min−1), cough, hiccups, nausea, vomiting). Sedation-related adverse events were recorded and managed using the Sedation Adverse Event Reporting International (SIVA) tool, which has been published in the British Journal of Anaesthesia 108(1):13–20 (2012).16 The Visual Analogue Scale (VAS) was used to evaluate postoperative pain of patients as well as patient, anesthesiologist, and gastroscopist satisfaction. The staff involved in the evaluation of these variables were not aware of the assigned group of patients.
Sample Size
The sample size was calculated using Pass software version 15.0 (NCSS, Kaysville, UT, USA). Based on the results of a pilot study, the propofol requirements during gastroscopy were 125.18±28.56 mg in Group N and 112.21±23.38 mg in Group L. Assuming a two-sided α=0.05 and statistical power of 0.8, the sample size was calculated to 63 patients in each group. Considering a loss to follow-up of 10%, 70 patients were required for each group.
Statistics
Statistical analyses were performed after excluding patients who were considered ineligible after enrollment. All data were checked for normal distribution using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation and nonnormally distributed data were expressed as median with interquartile range. Continuous variables were analyzed using the Student’s t-test or Mann–Whitney U-test. Categorical variables were compared using the Pearson’s χ2 test or Fisher’s exact test, as appropriate. p-value less than 0.05 was considered statistically significant. Data analyses were conducted using SPSS 24.0 (SPSS Inc., Chicago, IL, USA).
Results
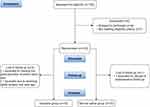
A total of 159 patients were evaluated for participation in the study. Among them, 3 had severe hypertension, 2 had renal failure, 1 was on antidepressants for more than 3 months, 1 had a recent acute bronchial asthma attack, and 9 patients were excluded because they refused to provide written informed consent. Finally, 143 patients were recruited for our study. Three patients were excluded after randomization; one patient was excluded for leaving the postoperative recovery room early, another for receiving nasal surgery a year earlier, and another for refusing postoperative follow-up. A total of 140 patients (70 in each group) completed gastroscopy (Figure 1). There were no significant differences in demographic characteristics between the two groups (Table 1).
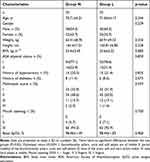 |
Table 1 The Demographic and Clinical Characteristics of Patients |
Primary Outcome
The total propofol dose was reduced by 19.71 mg (95% CI, 11.33 to 28.09, p<0.001, Table 2) in Group L (107.32±21.39 mg) when compared with Group N (127.03±28.24 mg), and the maintenance dose was reduced by 20.70 mg (95% CI, 15.15 to 26.25, p<0.001, Table 2) in Group L (23.96±14.34 mg) when compared with Group N (44.66±18.59 mg); meanwhile, no significant difference was found between the two groups in terms of induction dose (Table 2).
 |
Table 2 Propofol Consumption at Different Phases |
Secondary Outcomes
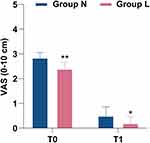
Compared with Group N, the number of hypoxia cases in Group L decreased by 14.2% (27.1% vs 12.9%, p=0.035, Table 3), and there was no significant difference between the two groups in severe hypoxia. Compared with Group N, the number of emergency airway management events in Group L decreased by 14.2% (17.1% vs 2.9%, p=0.005, Table 3). The main difference was that the number of patients required to increase the oxygen flow (17.1%, 25.7% vs 8.6%, p=0.007, Table 3) and the jaw thrust (14.2%, 17.1% vs 2.9%, p=0.005, Table 3) in Group L decreased, but there was no significant difference in mask-assisted ventilation and intubation (Table 3). No significant differences in SBP, DBP, MAP, HR or RR were found between the N and L groups. Interestingly, we found that Group L was more stable than Group N in terms of hemodynamic parameters during the period from T1 to T5 (Figure 2). Compared with Group N (5.22±1.04 mins), the duration of gastroscopy decreased by 0.707 mins (95% CI, 0.382 to 1.033, p<0.001, Table 4) in Group L (4.51±0.90 mins); compared with Group N (2.76±1.65 mins), the consciousness recovery time decreased by 0.904 mins (95% CI, 0.464 to 1.345, p<0.001, Table 4) in Group L (1.86±0.86 mins). There were no significant differences between Group N and Group L in terms of sedation-related adverse events, such as hypotension, bradycardia, cough, hiccups, nausea, and vomiting (Table 5). The pain score measured by VAS at the recovery of consciousness after gastroscopy in Group L (2.4 (1.2, 3.2)) was significantly lower than that in Group N (2.85 (2.00, 3.70)) (95% CI, 0.2 to 1.1, p=0.009). Similarly, the pain score measured 10 minutes after recovery of consciousness was the same as before (L: 0.20 (0.00, 0.80), N: 0.50 (0.00, 1.00)) (95% CI, 0 to 0.3, p=0.027) (Figure 3). The satisfaction of patients and gastroscopists in Group L was higher than that in Group N, but there was no significant difference in the satisfaction of anesthesiologists (the satisfaction of the patient, p=0.025; the satisfaction of gastroscopists, p=0.031, Table 6). No cardiac arrhythmias were observed during gastroscopy, and the patient had no signs of toxicity (eg, dizziness, drowsiness, blurred vision, oral paralysis, metallic odor) after awakening.
 |
Table 3 Respiratory-Related Adverse Events and Interventions |
 |
Table 4 Duration of Gastroscopy and Consciousness Recovery Time |
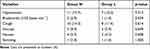 |
Table 5 Incidence of Sedation-Related Adverse Events |
 |
Table 6 Satisfaction of Patients, Anesthesiologists and Gastroscopists |
Discussion
This research demonstrated that IV lidocaine combined with propofol reduced propofol consumption, the incidence of hypoxia, emergency airway management events, duration of gastroscopy, and consciousness recovery time in elderly patients during gastroscopy in comparison to propofol sedation alone. IV lidocaine also reduced postoperative pain and improves patient and gastroscopist satisfaction scores. In addition, there were no adverse effects associated with lidocaine.
With the increasing global aging and human awareness of disease prevention, the number of patients in the digestive endoscopy center is increasing, most of whom are elderly. Elderly patients can detect gastrointestinal lesions early through gastroscopy, and can also receive timely treatment when lesions, such as resectable polyps, are found. However, elderly patients are more likely to experience serious complications, such as hypoxia, cardiac arrhythmias or more life-threatening conditions when undergoing gastroscopy. Therefore, the current focus on finding a safer and more appropriate propofol combination sedation drug for painless gastroscopy in elderly patients is particularly important. Low doses of ketamine have the effect of sparing propofol, and it has been shown that propofol–ketamine combination IV anesthesia causes fewer cardiopulmonary adverse reactions than propofol alone.17 Unfortunately, the risk that ketamine-assisted IV anesthesia may lead to postoperative nausea, vomiting, delirium, and other adverse effects cannot be ignored, especially in the elderly.
The propofol-sparing effect of lidocaine has been particularly evident during surgical stimulation. Altermatt et al18 found that perioperative IV lidocaine in patients undergoing elective laparoscopic cholecystectomy reduced the need for propofol. In addition, low-dose IV lidocaine is useful in decreasing the pain of propofol injection, shortening anesthesia induction time, and reducing propofol dose in young adult patients undergoing painless gastrointestinal examination.19 Forster et al13 investigated the sedative effects of IV lidocaine in combination with propofol and ketamine undergoing painless colonoscopy in adult patients. The results found that IV lidocaine resulted in a significant 50% reduction in propofol requirements and alleviated immediate post-examination pain and fatigue. In obese patients undergoing painless colonoscopy, IV lidocaine significantly reduces the occurrence of hypoxia and apnea episodes.20 In elderly patients during painless colonoscopy, adjunctive IV lidocaine during propofol sedation also leads to a significant reduction in propofol supplementation.21 Likewise, IV lidocaine significantly reduces the need for propofol during endoscopic retrograde cholangiopancreatography (ERCP) procedures, thereby improving sedation quality and endoscopist satisfaction.22 Recent studies have also shown that IV lidocaine significantly reduces the ED50 of the induction dose of propofol in adult patients during gastroscopy.23 Another meta-analysis suggests that IV or topical lidocaine may help improve sedation with propofol in patients undergoing gastrointestinal endoscopic procedures.24 In our study of elderly patients undergoing painless gastroscopy, IV lidocaine combined with propofol sedation was found to lead to a significant reduction in the total and maintenance dose of propofol required for the examination, which is in agreement with prior studies. However, we observed no significant difference in the dose of propofol used for induction in both groups. The possible reasons are as follows: (i) In general, the same level of sedation can be achieved in elderly patients with a smaller dose of anesthetics than in younger patients, thus we give only a below-average induction dose (1.5 mg kg−1) of propofol; (ii) induction sedation for gastroscopy is a fairly short procedure, and in many cases only one induction dose of propofol is required to bring the patient to the operating condition, thereby reducing the consumption of propofol required for induction. The statistical differences between the groups will be narrowed when these factors are taken into account.
In recent years, concerns about hypoxemia caused by propofol sedation have increased, but there has been no consensus among clinical practices. A high prevalence of hypoxemia may be related to the increased risk of brain injury, myocardial ischemia, and mechanical ventilation, resulting in increased mortality. Propofol alone often fails to obtain satisfactory anesthetic effects and is prone to respiratory depression and hypotension and other adverse reactions. Hypoxia and apnea are precisely the most common cardiopulmonary complications of gastrointestinal endoscopy.25 It has been shown that ketamine used with propofol results in fewer respiratory depressions and a lower number of adverse respiratory events.17,26 Lidocaine produces significant propofol sparing effect and increases the ventilatory response to carbon dioxide.27 This is consistent with our finding that fewer patients in Group L experienced decreased oxygen saturation and emergency airway management events. The procedures required in the event of an emergency airway management event include jaw thrust, mask-assisted ventilation and intubation. These interventions can generally effectively and quickly improve the patient’s oxygen saturation and restore the patient’s hemodynamic stability but can also interrupt gastroscopy, thereby affecting the endoscopist’s working conditions and satisfaction. It has been suggested that age over 65 years, higher body mass index (BMI), and ASA III may increase the incidence of hypoxemia during endoscopic.28–30 While in our study severe hypoxia was not significantly different among the two groups, whether this is relevant to our criterion of including only ASA I–II patients is not known.
After propofol induction, SBP, DBP, MAP, HR, and RR in both groups gradually decreased to the lowest point and then returned to baseline levels, and there were no significant differences between the overall blood pressure and heart rate parameters of the two groups at each time point from T1 to T5. This deviates from our initial hypothesis that the addition of lidocaine to propofol would minimize hemodynamic fluctuations and respiratory depression by reducing the need for propofol. It appears that the vasodilatory properties and sedative effects of lidocaine might counteract some of the benefits of lower doses of propofol. Nonetheless, we found that Group L was more stable than Group N in terms of hemodynamic parameters during the overall period from the initiation of sedation induction to the patient’s recovery of consciousness.
At the recovery of consciousness after gastroscopy and 10 minutes after recovery of consciousness, we used the VAS method to measure the pain scores of the patients, and the results showed that the pain scores of Group L were lower than those of Group N. This result is consistent with previous reports finding that IV lidocaine-assisted propofol induction significantly reduces the incidence of postoperative pain after endoscopic surgery.13 In addition, the duration of gastroscopy and the consciousness recovery time was lower in Group L than in Group N. Since gastroscopy procedures and post-operative care are often performed in the digestive endoscopy center, shortening the entire process will facilitate bed rotation and thus improve the efficiency of hospital admissions. Similarly, we found that patients and gastroscopists in Group L had a higher satisfaction level, which would reduce the fear and discomfort of patients during the examination. It also allows the gastroscopist to obtain a more comfortable feeling of the procedure, which is beneficial for the examination and maybe one of the reasons for the shorter gastroscopy time. There was no difference in the number of sedation-related adverse events between the two groups. These findings suggest that, despite the insufficient evidence of clinical significance, moderate lidocaine-assisted propofol sedation has potential clinical benefit. Importantly, these findings may form a basis for future research and interventions.
Lidocaine has a sparing effect on propofol that has not been fully elucidated. The sedative effect of propofol is mainly mediated by directly activating the GABAA- Cl−channel complex, thereby enhancing the influx of Cl− and promoting inhibitory transmission.31,32 It has been shown that lidocaine can exert its effect of reducing nerve cell excitability by inhibiting γ-aminobutyric acid reuptake and enhancing GABA receptor-mediated intracellular flow of Cl−.33 The mechanism of action by propofol and lidocaine on nerve cells allows speculation of a possible sedative synergistic effect when the two are combined. Furthermore, the Hans et al study showed that lidocaine reduced propofol consumption only in the context of noxious stimuli.34 Thus the sparing effect of lidocaine on propofol may also be achieved by blocking peripheral stimulation.
This study has the following limitations. First, this was a single-center study and the sample size was not large enough. Second, only relatively healthy patients (ASA I-II) were recruited in this study, and patients with ASA III and above and those with recent acute high-risk diseases were excluded. Therefore, further studies on the risks and benefits of lidocaine-assisted propofol IV sedation in gastroscopy in these high-risk patients are warranted. Third, we did not measure objective indicators of sedation level, such as the bispectral index (BIS) or electroencephalogram (EEG) monitoring, during the procedure; thus, when using MOAA/S scores and subjective observation techniques, there may be slight differences in the judgment of individual patients on the depth of sedation, which has been shown in another work to affect the incidence of hypoxemia.35 Finally, we did not design an experimental protocol based on a dose-dependent effect of lidocaine and measuring lidocaine concentrations in patient plasma, which would have been more helpful to discover the association between lidocaine concentrations and propofol dose. Therefore, this will be the primary question to be explored in our next research.
Conclusion
Under our study conditions, IV lidocaine can significantly reduce propofol consumption, the incidence of hypoxia, and postoperative pain during gastroscopy in elderly patients, with a higher patient and gastroscopist satisfaction. This method is safe and worthy of clinical application.
Data Sharing Statement
The de-identified data for individual participants underlying our results can be accessed with approval from the corresponding author Hao Yang 1 year after publication. The study protocol, statistical analyses, and clinical study report will also be available.
Funding
This study was supported by the Program of Shanghai Academic Research Leader (No.21XD1402800); Shanghai “Rising Stars of Medical Talent” Youth Development Program: Outstanding Youth Medical Talents; the Young Elite Scientist Sponsorship Program by CAST (2018QNRC001); Shanghai Pujiang Program(2020PJD050); Academic medicine leader’s training Program in health systems of Pudong New Area(PWRd2020-06), the Basic Research Program for Young Elite Scientist by Shanghai Association for the Study of Pain(2018SASP01); the Research Program for Young Scientist by Shanghai Society of Anesthesiology (2019SSA).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shao LJ, Zou Y, Liu FK, et al. Comparison of two supplemental oxygen methods during gastroscopy with propofol mono-sedation in patients with a normal body mass index. World J Gastroenterol. 2020;26(43):6867–6879. doi:10.3748/wjg.v26.i43.6867
2. Cai G, Huang Z, Zou T, et al. Clinical application of a novel endoscopic mask: a randomized controlled trial in aged patients undergoing painless gastroscopy. Int J Med Sci. 2017;14(2):167–172. doi:10.7150/ijms.16919
3. Hao L, Hu X, Zhu B, Li W, Huang X, Kang F. Clinical observation of the combined use of propofol and etomidate in painless gastroscopy. Medicine. 2020;99(45):e23061. doi:10.1097/MD.0000000000023061
4. Abdelmalak BB, Cata JP, Bonilla A, et al. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: an observational study. Br J Anaesth. 2013;110(2):241–249. doi:10.1093/bja/aes378
5. Chiumello D, Brioni M. Severe hypoxemia: which strategy to choose. Crit Care. 2016;20(1):132. doi:10.1186/s13054-016-1304-7
6. Padiyara TV, Bansal S, Jain D, Arora S, Gandhi K. Dexmedetomidine versus propofol at different sedation depths during drug-induced sleep endoscopy: a randomized trial. Laryngoscope. 2020;130(1):257–262. doi:10.1002/lary.27903
7. Edokpolo LU, Mastriano DJ, Serafin J, Weedon JC, Siddiqui MT, Dimaculangan DP. Discharge readiness after propofol with or without dexmedetomidine for colonoscopy: a randomized controlled trial. Anesthesiology. 2019;131(2):279–286. doi:10.1097/ALN.0000000000002809
8. Yin S, Hong J, Sha T, et al. Efficacy and tolerability of sufentanil, dexmedetomidine, or ketamine added to propofol-based sedation for gastrointestinal endoscopy in elderly patients: a prospective, randomized, controlled trial. Clin Ther. 2019;41(9):1864–1877.e1860. doi:10.1016/j.clinthera.2019.06.011
9. Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–1246. doi:10.1007/s40265-018-0955-x
10. Ness TJ. Intravenous lidocaine inhibits visceral nociceptive reflexes and spinal neurons in the rat. Anesthesiology. 2000;92(6):1685–1691. doi:10.1097/00000542-200006000-00028
11. Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106(1):
12. Labaille T, Clergue F, Samii K, Ecoffey C, Berdeaux A. Ventilatory response to CO2 following intravenous and epidural lidocaine. Anesthesiology. 1985;63(2):179–183. doi:10.1097/00000542-198508000-00011
13. Forster C, Vanhaudenhuyse A, Gast P, et al. Intravenous infusion of lidocaine significantly reduces propofol dose for colonoscopy: a randomised placebo-controlled study. Br J Anaesth. 2018;121(5):1059–1064. doi:10.1016/j.bja.2018.06.019
14. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001
15. Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133(2):675–701. doi:10.1053/j.gastro.2007.06.002
16. Mason KP, Green SM, Piacevoli Q. Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: a consensus document from the world SIVA international sedation task force. Br J Anaesth. 2012;108(1):13–20. doi:10.1093/bja/aer407
17. Yan JW, McLeod SL, Iansavitchene A. Ketamine-propofol versus propofol alone for procedural sedation in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2015;22(9):1003–1013. doi:10.1111/acem.12737
18. Altermatt FR, Bugedo DA, Delfino AE, et al. Evaluation of the effect of intravenous lidocaine on propofol requirements during total intravenous anaesthesia as measured by bispectral index. Br J Anaesth. 2012;108(6):979–983. doi:10.1093/bja/aes097
19. Wang J, Duan J, Xie C, Yu Y, Lu Y. Comparison between intravenous nalbuphine and lidocaine in reducing propofol-induced injection pain during gastroscopy: a randomized controlled trial. Pain Ther. 2020;9(2):563–571. doi:10.1007/s40122-020-00188-y
20. Li X, Lv X, Jiang Z, et al. Application of intravenous lidocaine in obese patients undergoing painless colonoscopy: a prospective, randomized, double-blind, controlled study. Drug Des Devel Ther. 2020;14:3509–3518. doi:10.2147/DDDT.S266062
21. Chen M, Lu Y, Liu H, et al. The propofol-sparing effect of intravenous lidocaine in elderly patients undergoing colonoscopy: a randomized, double-blinded, controlled study. BMC Anesthesiol. 2020;20(1):132. doi:10.1186/s12871-020-01049-z
22. Liu J, Liu X, Peng LP, Ji R, Liu C, Li YQ. Efficacy and safety of intravenous lidocaine in propofol-based sedation for ERCP procedures: a prospective, randomized, double-blinded, controlled trial. Gastrointest Endosc. 2020;92(2):293–300. doi:10.1016/j.gie.2020.02.050
23. Liu H, Chen M, Lian C, Wu J, Shangguan W. Effect of intravenous administration of lidocaine on the ED50 of propofol induction dose during gastroscopy in adult patients: a randomized, controlled study. J Clin Pharm Ther. 2021;46(3):711–716. doi:10.1111/jcpt.13335
24. Hung KC, Yew M, Lin YT, et al. Impact of intravenous and topical lidocaine on clinical outcomes in patients receiving propofol for gastrointestinal endoscopic procedures: a meta-analysis of randomised controlled trials. Br J Anaesth. 2022;128(4):644–654. doi:10.1016/j.bja.2021.08.036
25. Qadeer MA, Lopez AR, Dumot JA, Vargo JJ. Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations. Digestion. 2011;84(1):37–45. doi:10.1159/000321621
26. Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg. 2001;92(6):1465–1469. doi:10.1097/00000539-200106000-00022
27. Gross JB, Caldwell CB, Shaw LM, Laucks SO. The effect of lidocaine on the ventilatory response to carbon dioxide. Anesthesiology. 1983;59(6):521–525. doi:10.1097/00000542-198312000-00006
28. Haines DJ, Bibbey D, Green JR. Does nasal oxygen reduce the cardiorespiratory problems experienced by elderly patients undergoing endoscopic retrograde cholangiopancreatography? Gut. 1992;33(7):973–975. doi:10.1136/gut.33.7.973
29. Wani S, Azar R, Hovis CE, et al. Obesity as a risk factor for sedation-related complications during propofol-mediated sedation for advanced endoscopic procedures. Gastrointest Endosc. 2011;74(6):1238–1247. doi:10.1016/j.gie.2011.09.006
30. Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi:10.1038/nrdp.2015.15
31. Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10(29):3639–3649. doi:10.2174/1381612043382846
32. Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–1558. doi:10.1007/s40262-018-0672-3
33. Nordmark J, Rydqvist B. Local anaesthetics potentiate GABA-mediated Cl- currents by inhibiting GABA uptake. Neuroreport. 1997;8(2):465–468. doi:10.1097/00001756-199701200-00018
34. Hans GA, Lauwick SM, Kaba A, et al. Intravenous lidocaine infusion reduces bispectral index-guided requirements of propofol only during surgical stimulation. Br J Anaesth. 2010;105(4):471–479. doi:10.1093/bja/aeq189
35. Zhou X, Li BX, Chen LM, et al. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc. 2016;30(11):5108–5116. doi:10.1007/s00464-016-4861-6
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.