Back to Journals » International Journal of Nanomedicine » Volume 18
Intravenous Injection of Na Ions Aggravates Ang II-Induced Hypertension-Related Vascular Endothelial Injury by Increasing Transmembrane Osmotic Pressure
Authors Song X, Li D, Gan L, Xiong X, Nie A, Zhao H, Hu Y, Li G, Guo J
Received 12 August 2023
Accepted for publication 3 December 2023
Published 11 December 2023 Volume 2023:18 Pages 7505—7521
DOI https://doi.org/10.2147/IJN.S435144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Xianrui Song,1,* Danyang Li,1,* Lingling Gan,2,* Xiyu Xiong,1 Aobo Nie,1 Huanhuan Zhao,3 Yunfeng Hu,1 Guangming Li,4 Jun Guo1
1Department of Biochemistry and Molecular Biology, School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210023, People’s Republic of China; 2Experiment Center for Science and Technology, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210023, People’s Republic of China; 3Basic Medical Experiment Center, School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210023, People’s Republic of China; 4Department of Anesthesiology, Huaian First People’s Hospital, Nanjing Medical University, Huaian, Jiangsu, 223001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Guo, School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210023, People’s Republic of China, Tel +86 13813909055, Email [email protected] Guangming Li, Department of Anesthesiology, Huaian First People’s Hospital, Nanjing Medical University, Huaian, Jiangsu, 223001, People’s Republic of China, Email [email protected]
Introduction: Extracellular protein nanoparticles (PNs) and ions perform synergistical functions in the control of transmembrane osmotic pressure (OP) under isotonic conditions. Intravenous injection may disrupt the ion balance and alter PN levels in blood plasma, changing transmembrane OP and damaging vascular endothelial cells.
Methods: Na ions were injected into AngII–induced HUVECs to simulate cell injury in vitro, and tail vein infusion of Na ions into hypertensive rats was performed to assess vascular damage. Optical measurements using an intermediate filament (IF) tension probe were conducted to detect indicators related to transmembrane OP. Immunofluorescence, Western blotting and small interfering RNA (siRNA) transfection were employed to investigate inflammasomes and the relationship between Abl2 and inflammation.
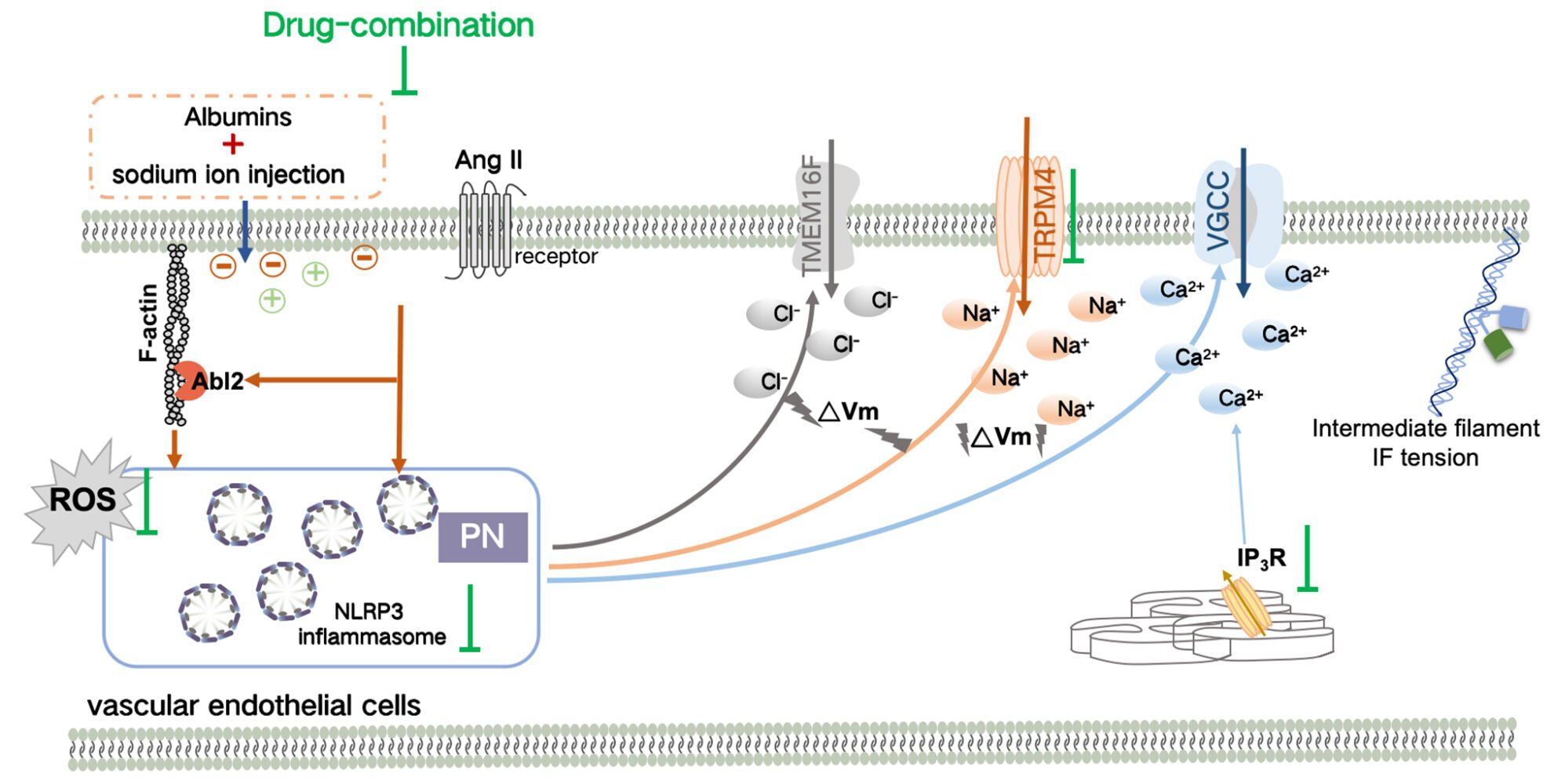
Results: Electrolyte injections with sodium ions (but not glucose and hydroxyethyl starch) induced the production of ASC and NLRP3 inflammasomes in Ang II–induced HUVECs; this in turn resulted in the disorder of calcium signals, and changes in transmembrane OP and cell permeability. Moreover, injection of Na ions into Ang II–induced HUVECs activated the mechanosensitive protein Abl2, involved in inflammation-induced transmembrane OP changes. A drug combination was identified that could induce OP recovery and block hyperpermeability induced by cytoplasmic inflammatory corpuscles in vivo and in vitro.
Conclusion: Changes in extracellular PNs and ions following chemical stimuli (Ang II) participate in the regulation of transmembrane OP. Furthermore, injection of Na ions causes vascular endothelial injury in Ang II-induced cells in vitro and hypertension rats in vivo, suggesting it is not safe for hypertensive patients, and we propose a new drug combination as a solution.
Keywords: Na ion injection, vimentin tension probe, protein-nanoparticles, transmembrane OP, Ang II-induced vascular endothelial injury, Abl2
Graphical Abstract:
Introduction
Intravenous infusion is a common treatment method in which drugs are intravenously injected into the bloodstream through blood vessels, resulting in a direct impact on the plasma environment.1 At present, the commonly used intravenous injections can be divided into three categories according to the drug composition: electrolyte injections (eg, sodium chloride injection and sodium bicarbonate injection), nutrition injections (eg, glucose injection, compound amino acid injection, and fat emulsion injection), and colloid injections (eg, hydroxyethyl starch 40 sodium chloride injection and dextran injection).2 According to the Van’t Hoff theory, commonly used intravenous injections have the same osmotic potential as vascular cells and are considered isotonic solutions, so intravenous injections are considered to be usually safe.3,4
However, in clinical treatment, colloidal osmotic pressure (OP) formed by plasma protein is generally believed to be extremely important and cannot be equated with ionic OP.5,6 Recent studies have found that protein nanoparticles (PNs) generated by the depolymerization of the cytoskeleton or the activation of inflammasome can induce the enhancement of intracellular ion OP via a change in the membrane potential and can contribute to cytopathic effects, indicating that PNs have a crucial effect on transmembrane OP of live cells.7,8 Furthermore, changes in extracellular PNs and ion components can synergistically induce cytoplasmic OP alteration.9 Studies have also shown that changes in physiological albumin, Ca2+, and K+ contents can result in intracellular PN production, driving further increases in cytoplasmic PN and ion content.5 In addition, extracellular albumins and intracellular PNs collaboratively contribute to electrochemical changes and the resultant ion rearrangement across membranes, further affecting transmembrane OP.10 Therefore, the transmembrane OP in live cells may be regulated during intravenous infusion by controlling extracellular PN content and ion composition, potentially resulting in endothelial cell damage, but the underlying mechanisms are not completely clear.
Some other factors were also found to be involved in the regulation of transmembrane OP in live cells.11,12 Previous studies reported that inhibition of the SUR1-TRPM4 channel can significantly downregulate the intracellular OP increase induced by angiotensin II (Ang II), associated closely with activation of the voltage-gated calcium channel (VGCC) and elevated calcium ions.13,14 Furthermore, Ang II is a typical stimulator of endothelial cell damage, indicating that chemical stimuli could alter transmembrane OP in the case of hypertension.15,16 Moreover, Abelson tyrosine-protein kinase 2 protein (Abl2) is involved in NF-κB signaling and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activation, contributing to oxidative stress, inflammation, and vascular leakage in vascular endothelial cells.17,18 Moreover, Abl2, as a mechanosensitive protein, can directly bind to F-actin via its ABD domain and then pull on cell membranes and microfilaments after the application of extracellular mechanical stimuli.19,20 Therefore, we speculate that Abl2 might also participate in the regulation of transmembrane OP.
The purpose of this study is to investigate the effect of intravenous injections on transmembrane OP changes and vascular endothelial cell lesion. In order to propose new research topics and potential methods for the safety evaluation of intravenous injections, we attempted to explore whether factors such as angiotensin II stimuli (chemical signals), tension receptor activation, and opening of ion channels can influence transmembrane OP and the structure and function of vascular endothelial cells.
Materials and Methods
Reagents
Ang II and N-acetylcysteine (NAC), which is a reactive oxygen species (ROS) inhibitor, were purchased from Yuanye Biotechnology (Shanghai, China). Further, 0.9% NaCl injection was purchased from Otsuka Pharmaceutical (Guangdong, China). The compound sodium chloride injection was purchased from Huiyingbi Group East Asia Pharmaceutical (Jiangxi, China). In addition, the 5% sodium bicarbonate injection was purchased from Kangyuan Pharmaceutical (Hunan, China), and the 5% glucose injection was purchased from Otsuka Pharmaceutical (Dalian, China). The hydroxyethyl starch 40 sodium chloride (HS40SC) injection was purchased from Fresenius Kabi Pharmaceutical (Beijing, China), and Beyotime Biotechnology (Shanghai, China) provided 4′,6-diamidino-2-phenylindole (DAPI). MCC950 and niclosamide were purchased from Selleck (Texas, USA). Heparin, Z-VAD-FMK, and 2-APB were purchased from MedChemExpress (New Jersey, USA). Jasplakinolide (JK) and Taxol (TAX) were purchased from TargetMol Chemicals Inc. (Massachusetts, USA), and nifedipine, glyburide, and tranilast were purchased from Macklin (Shanghai, China).
Endo-Free Plasmid Mini Kits were from Omega Bio-Tek (Norcross, USA). The Nitric Oxide Assay Kit was purchased from Beyotime Biotechnology (Shanghai, China). The Cell counting KIT-8 was purchased from Dojindo (Kyushu Island, Japan). The Super ECL Detection Reagent Kit was purchased from Yeasen Biotech Co, Ltd. (Shanghai, China).
Rabbit anti-NLRP3 antibodies were obtained from Aff-biotech (Liyang, China). Mouse anti-apoptosis-associated speck-like (ASC) antibodies and Rabbit anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). β-actin and α-tubulin antibodies were from Boster (Wuhan, China). Abl2 antibodies were from Proteintech (Chicago, USA). Fluorescein isothiocyanate (FITC)-Phalloidin was purchased from Solarbio (Beijing, China). Further, horseradish peroxidase (HRP)-labeled anti-rabbit, fluorescein isothiocyanate (FITC)-goat anti-mouse IgG (H+L), and fluorescein isothiocyanate (FITC)-goat anti-rabbit IgG (H+L) antibodies were purchased from Zsgb-Bio (Beijing, China).
Cell Culture Analysis
Human umbilical vein endothelial cells (HUVECs) were purchased from the ATCC Cell Bank. HUVECs were cultured in Dulbecco’s Modified Eagle’s Medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), 100 μg/mL penicillin, and 100 μg/mL streptomycin. Cells were grown in 5% CO2 in a humidified atmosphere at 37 °C. After growing to 80% confluence in cell culture bottles, the cells were used for experiments. For the experiment, HUVECs were starved for 6 h and then cocultured with Ang II (10 μmol/L, model) and drugs (inhibitor or activator) for 12 h for the detection of various indexes. Specifically, 0.9% NaCl, compound NaCl, 5% NaHCO3, 5% Glucose, and HS40SC injections were each mixed with the plasma-mimicking medium in a ratio of 1:9 (v/v) to simulate intravenous infusion into the human body, and the effect on Ang II–induced hypertensive HUVECs was monitored.
Establishment of Animal Models
Male hypertension Spragu-Dawley rats (170–190 g, 7–8 weeks old) were purchased from Shanghai Sippe-Bk Lab Animal Co Ltd. (Shanghai, China; SCXK(Hu) 2018–0006). The rats were placed in colony cages and had free access to food and water. The light/dark cycle was for 12 h, and the temperature was maintained at 21 ± 2 °C. Animal welfare and experimental procedures complied with the Provisions and General Recommendations of the Chinese Experimental Animals Administration Legislation. The animal experiment protocol was approved by the Experimental Animal Ethical Committee of Nanjing University of Chinese Medicine, China (permit No. SCXK (Hu) 2018-0006). The animals were acclimated to the laboratory conditions for at least 7 days before use in the experiments.
The heart rate and diastolic and systolic blood pressure of the hypertensive rats were determined before conducting the first experiment. Next, 2 mL of 0.9% NaCl, compound NaCl, and 5% NaHCO3 were separately injected into the tail vein four times a day, with an injection rate of 2 mL/min. The drug combination was combined with the following related inhibitor and activator to treat vascular injury: (1) heparin (0.5 mg/mL), a competitive antagonist of inositol triphosphate (IP3) receptors, similar to 2-APB; (2) nifedipine (30 μmol/L), an inhibitor of L-type VGCC; (3) glyburide (10 μmol/L), a SUR1-TRPM4 channel inhibitor; (4) niclosamide (1 μmol/L), an inhibitor of the calcium ion-sensitive chloride ion channel TMEM16F; (5) tranilast (50 μmol/L), an effective inhibitor of inflammasomes; and (6) NAC (50 μmol/L), a commonly used antioxidant. The effective drug concentrations used were as per previous reports.13,21–23 Furthermore, heart rate and diastolic and systolic blood pressure were detected after 2 weeks of treatment, and tissues were eventually collected for analysis of various indices.
Measurement of Transendothelial Electrical Resistance in HUVECs
The transendothelial electrical resistance (TEER) of HUVECs was determined by using the EVOM method.24 HUVECs were treated with 10 μmol/L Ang II and an inhibitor or activator, and the TEER value (Ω·cm2) of HUVECs was determined over 24 h. Further, the ratio of the TEER value at 12 h to that at 0 h was calculated.
Measurement of Cytoplasmic OP and Count Rate of Protein Particles
The cell culture medium, osmotic solution HEPES, and trypsin solution were all calibrated to 300 ± 10 Osm/kg. HUVECs were cultivated in 100 mm dishes. When the cell density reached >95%, the cells were stimulated with drugs for a certain duration. The cells were then digested and suspended in the HEPES isosmotic solution and transferred to 1.5 mL microcentrifuge tubes. Centrifugation (13,000×g, 5 min, 4 °C), ultrasonication (75% amplitude, 5 times, 5 s; Sonics and Materials, Connecticut, CT, USA), and another round of centrifugation (13,000×g, 10 min, 4 °C) were performed. Then, 50 µL of the supernatant solution (cytoplasm) was placed in 0.5 mL test tubes. Before use, the Osmomat 3000 freezing-point osmometer and 050 Membrane Osmometer (Gonotec, Berlin, Germany) were calibrated three times. The cytoplasmic OP was then measured. The concentration of cytoplasmic nanoparticles in terms of kilocycles per second (Kcps) was detected using a Nanosight NS300 (Malvern Instruments, Malvern, UK).
Measurement of Intracellular Calcium, Sodium, and Chloride Ions
Enhanced N-[ethoxycarbonylmethyl]-6-methoxy-quinolinium bromide (MQAE; Beyotime, China), Fura-2 AM (Molecular Probes, Abcam, Cambridge, MA, USA), and NaTrium Green-2 AM (ENG; Shanxi, China) were used to detect intracellular chloride, calcium, and sodium ion concentrations, respectively. Cells were grown adhesively in confocal dishes, and experiments were conducted when the cell density was 50%–70%. HUVECs were incubated with 5 μM MQAE for 30 min at 37 °C and washed 5 times with Krebs-HEPES buffer. The cells were incubated with 2 μM Fura-2 AM for 30 min at 37 °C and then treated with Hanks’ balanced salt solution (HBSS), before being incubated for another 30 min. HUVECs were incubated with 4 μM ENG-AM for 30 min at 37 °C, then treated with DMEM containing 1% FBS, and incubated for another 30 min. The fluorescence intensity was detected using a THUNDER Imaging Systems fluorescence microscope (Leica), and images were obtained every 60s. The normalized values of fluorescence intensity for MQAE (F0/Ft), Fura-2 AM (Ft/F0), and ENG (Ft/F0) were calculated based on the ion fluorescence just after (Ft) or before (F0) stimulation for 15 min, respectively.
Immunofluorescence Assay (IF)
Cells were seeded in 90 mm dishes. When the cells had grown to 70–80% confluence, the medium was removed and cells were treated with different drugs for a certain duration. Cells were then fixed using 4% paraformaldehyde for 30 min. Then, 2% Triton-100 was applied for permeabilization, and the cells were incubated with primary antibodies for 12 h at 4 °C. Washing with phosphate-buffered saline (PBS) was followed by the addition of goat anti-mouse-FITC secondary antibody (1:200 dilution), goat anti-mouse IgG (whole molecule)-tetramethylrhodamine isothiocyanate (TRITC) secondary antibody (1:200 dilution), and FITC-phalloidin. This was then followed by incubation for 2 h at 25 °C in the dark. Finally, 4′,6-diamidino-2-phenylindole (DAPI) was added to stain the cell nuclei. Fluorescent cells were observed under a confocal laser scanning microscope (SP5; Leica, Wetzlar, Germany).
ROS Production Analysis
HUVECs were grown in the 24-well plates (2–4 × 105 cells/well), then HUVECs were treated with 10 μmol/L Ang II and the related inhibitor or activator for 12 h. The intracellular ROS generation was determined by measuring the oxidative conversion of cell-permeable DCFH-DA (10 μmol/L) to fluorescent dichlorofluorescein. The fluorescence was detected by a microplate reader (Thermo Varioskan LUX, MA, USA) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Western Blot
Cells were lysed using the radioimmunoprecipitation assay (RIPA) lysis buffer. Total proteins were extracted, and the protein concentrations were analyzed using a bicinchoninic acid (BCA) detection kit (Beyotime, Shanghai, China). Protein samples were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked using 5% non-fat milk for 2 h and then incubated with specific primary antibodies overnight at 4 °C. After incubation with specific secondary antibodies for 2 h, the immunoreactive protein bands were visualized using the enhanced chemiluminescent (ECL) chromogenic substrate and quantified by densitometry (Quantity One; Bio-Rad, Hercules, CA, USA). Actin or Tubulin was used as a negative control.
H&E Staining and Immunohistochemistry (IHC) Assay
The removed vascular tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-µm-thick sections. For histopathological assay, the sections were stained with hematoxylin and eosin (H&E) stain to observe pathological changes. The immunohistochemistry assay was performed as described previously.25 The vascular tissue sections were deparaffinized, and 3% H2O2 was used to block endogenous peroxidases. The sections were incubated in 10% normal goat serum for 60 min at room temperature. Goat anti-rabbit ASC or NLRP3 polyclonal antibody (1:100 dilution) was then added to the sections, and incubation was performed overnight at 4 °C. Subsequently, HRP-conjugated secondary antibodies were applied at room temperature for 1 h. Following incubation with DAPI for 10 min, images were obtained under a microscope (DMi8; Leica).
Evans Blue Staining
The hypertensive rats were treated with different injections in the tail vein for 2 weeks, and then 0.5% Evans blue (EB) dye was injected into the tail vein. When the eyes and skin of the rats were blue, the kidney tissue and heart tissue of the rats were taken out for homogenization. The supernatant was centrifuged at 4 °C, added to an equal amount of trichloroacetic acid, incubated, and centrifuged at 4 °C. The OD value was measured by the supernatant at 620 nm, and the content of EB in the tissue was calculated according to the standard curve.
Nitric Oxide (NO) Content
The vascular tissues in hypertensive rats were dissected. The tissue proteins were extracted using the RIPA lysis buffer. Lysates were centrifuged and supernatants were collected for subsequent analysis. The nitric-oxide-detection assay kit (Shanghai Biyuntian Biological Co., Ltd.) was then used to determine the NO content, according to the kit instructions.
Measurement of H2O2 Content in Vascular Tissue
Vascular tissues were isolated, and the proteins were extracted using the RIPA lysis buffer. Lysates were centrifuged for the collection of supernatants. The content of H2O2 in the supernatants was measured using the H2O2 assay kit (Jiancheng Bioengineering Institute, Nanjing, China) and normalized to the protein level.
Statistical Analysis
The CFP/FRET ratio was calculated using Image J (NIH, Bethesda, MD, USA). The FRET value in each subcellular region was measured for each cell, and the average value was calculated for several cells. Images were pseudo-colored using the 16-color map in Image J. Data are presented as mean ± SEM. Statistical analysis was performed by using GraphPad Prism 8.0. One-way analysis of variance (ANOVA) with the least significant difference test was used to determine the statistical significance, and p < 0.05 was considered to be significant. Each experiment was repeated at least three times, and more than 10 cells were imaged, with each condition being analyzed.
Construction of vimentin tension probes and selection of Ang II concentrations are explained in Supplementary Materials.
Results
Effect of Common Intravenous Injection on Ang II-Induced HUVECs
In order to identify the effect of intravenous injection on the transmembrane OP, which is closely associated with the cytoskeletal tension, we designed a vimentin tension probe based on FRET, then effectively evaluated the intermediate filament (IF) tension, which indicates a change in transmembrane OP (Figure S1). In addition, we selected the appropriate concentration (0.5 µmol/L; refer to Supplementary Materials) of Ang II to stimulate vascular endothelial cells for establishing a hypertensive model in vitro (Figure S2) and explored the effect of the intravenous injection on the transmembrane OP and endothelial cell damage. A plasma-mimicking medium was mixed with 0.9% NaCl, Compound NaCl, 5% NaHCO3, 5% glucose, and HS40SC in a ratio of 9:1 to simulate the intravenous infusion in a human body, and the effect on normal or Ang II (0.5 μmol/L)-induced HUVECs was evaluated. The results showed that all intravenous injections have little effect on the cell tension of normal endothelial cells (Figure 1A and C), but the electrolyte injection of 0.9% NaCl, Compound NaCl, and 5% NaHCO3, instead of 5% Glucose or HS40SC injection, could induce the upregulation of cell tension in Ang II–induced HUVECs (Figure 1B and D). Furthermore, the CFP/FRET ratio of Ang II–induced HUVECs was obviously higher (p < 0.001) than that of normal HUVECs (Figure 1E) in the case of the electrolyte injection with sodium ions (0.9% NaCl, Compound NaCl, and 5% NaHCO3). The data suggested that the electrolyte injection with sodium ions could significantly increase the transmembrane OP of the HUVECs. Further, the TEER value, cytoplasmic OP, and number of intracellular PNs were measured. The results showed that in the case of the injection of the electrolyte with sodium ions, instead of 5% Glucose or HS40SC injection, the TEER value, cytoplasmic OP, and number of intracellular PNs of Ang II–induced HUVECs were higher than those of normal HUVECs (p < 0.001, Figure 1F–H). Therefore, we speculate that electrolyte injection of sodium ions, instead of 5% Glucose and HS40SC injection, may affect cytoplasmic PNs and regulate transmembrane OP in Ang II–induced HUVECs, eventually resulting in vascular endothelial cell damage.
The Relationship Between Transmembrane OP and the Increased Number of Inflammation-Related PNs in Ang II-Induced HUVECs in Response to Sodium Ion Injection
The results of a previous study showed that both generation of inflammatory corpuscles and depolymerization of cytoskeleton could lead to the generation of cytoplasmic PNs.26 In the present study, the immunofluorescence assay results suggested that the ASC and NLRP3 protein expression increases and that the formation of ASC spot-like aggregates and intracellular ROS was also significantly upregulated in Ang II–induced HUVECs as a result of treatment with sodium ion injection; this indicates that sodium ion injection may lead to the production of inflammasomes in Ang II–induced HUVECs (Figure 2A and D). The immunofluorescence staining of the microfilament and microtubule showed slight cytoskeletal depolymerization in Ang II–induced HUVECs treated with sodium ion injection (Figure 2B and C). The above data indicate that sodium ion injection, instead of 5% Glucose or HS40SC injection, resulted in inflammasome activation, and the increase in the number of PNs resulted from inflammasome production, instead of cytoskeletal depolymerization in Ang II–induced HUVECs.
To further explore whether the inflammation stress has an effect on the transmembrane OP in Ang II–induced HUVECs treated with the sodium ion injection, 50 μmol/L NAC (ROS inhibitor), 50 μmol/L MCC950 (NLRP3 inhibitor), or 20 μmol/L Z-VAD-FMK (Caspase-1 inhibitor) were administrated to HUVECs. The transmembrane OP recovered in Ang II–induced HUVECs treated with sodium ion injection (p < 0.01 or 0.001, Figure 2E–J), and the TEER value reduced (Figure 2K–M). However, with the addition of 10 μmol/L JK (microfilament stabilizer) and 10 μmol/L TAX (microtubule stabilizer), the transmembrane OP and TEER value showed a slight change (Figure 2E–M). These results suggest that the sodium ion injection is involved in the change in the transmembrane OP and cell permeability Ang II–induced HUVECs via cytoplasmic PN generation, which mainly resulted from the production of inflammasomes instead of the depolymerization of the cytoskeleton.
Role of Calcium Signals in Altering Transmembrane OP in Ang II-Induced HUVECs Following Sodium Ion Injection
Recent studies reported that the production of a large number of PNs resulted in increased intracellular ions due to the adsorption of intracellular cations, and this induced a change in the transmembrane OP and intracellular hyperosmolality.27 Therefore, we determined the Ca2+, Na+, and Cl− levels using Fura-2AM, ENG-2AM, and MQAE fluorescence probes, respectively, and found that intracellular Ca2+, Na+, and Cl− levels significantly increased after treatment of Ang II–induced HUVECs with sodium ion injection (p < 0.001, Figure 3A–C). IP3 can be combined with its receptor to release Ca2+ stored in the endoplasmic reticulum into the cytoplasm.24 We found that 100 μmol/L 2-APB (IP3 receptor inhibitor) could decrease the transmembrane OP and cell permeability in Ang II–induced HUVECs in response to sodium ion injection (p < 0.01, Figure 3D–L). Previous studies suggested that a change in the membrane potential could induce the activation of the VGCC and calcium ion inflow,28 and SUR1-TRPM4 as a non-selective univalent cation channel and TMEM16F as a chloride ion channel could be activated by calcium signals.11,29 We found that 30 μmol/L nifedipine (VGCC inhibitor), 10 μmol/L glyburide (SUR1-TRPM4 inhibitor), or 1 μmol/L niclosamide (TMEM16F inhibitor) could reverse the changes in transmembrane OP and TEER value, thereby reducing cell permeability in Ang II–induced HUVECs treated with the sodium ion injection (p < 0.01 or 0.001, Figure 3D–L). The results suggest that the change in the transmembrane OP due to the sodium ion injection was related to calcium signals and the activation of non-selective Na+ and Cl− channels in Ang II–induced HUVECs. Therefore, the downregulation of calcium signals and its related ion channels could inhibit transmembrane OP and cell permeability.
Role of Mechanosensitive Protein Abl2 in Inflammation and Regulation of Transmembrane OP in Ang II-Induced HUVECs Treated with Sodium Ion Injection
The results of previous studies indicated that the Abl2 protein could be involved in vascular endothelial lesion via the NF-κB signaling pathway,30 and Abl2 could bind directly to F-actin, thereby sensing mechanical stimuli and transmit the force exerted by microfilaments.31 The results of immunofluorescence staining showed the co-localization of Abl2 and actin in Ang II–induced HUVECs, but co-localization of Abl2 and actin was not observed in Ang II–induced HUVECs stimulated with sodium ion injection (Figure 4A and B), indicating that the Abl2 protein may be activated by sodium ion injection in Ang II–induced HUVECs. In addition, previous studies reported that the Abl family participated in inflammation through the oxidative stress pathway.32 To investigate the role of Abl2, HUVECs were transfected with Abl2-siRNA to downregulate endogenous Abl2 (p < 0.001, Figure 4C). In Ang II–induced HUVECs treated with sodium ion injection, Abl2-transfection could significantly decrease the protein level of ASC and NLRP3, and downregulate ASC spot-like aggregation and ROS generation (p < 0.001 or 0.0001, Figure 4D–G). The Abl2 protein might be related to the formation of inflammasomes. Further, Figure 4H–J show that transmembrane OP and TEER value decreased in sodium-ion-injection-treated HUVECs induced with Ang II after transfection of Abl2 (p < 0.001). The results suggest that the co-treatment with the sodium ion injection and Ang II could significantly increase the inflammasome production in HUVECs, and this is related closely to Abl2 activation, transmembrane OP, and endothelial cell permeability.
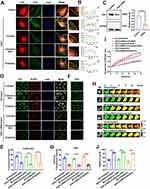 |
Figure 4 Role of mechanosensitive protein Abl2 in inflammation to regulate transmembrane OP in Ang II–induced HUVECs treated with sodium ion injection. HUVECs were incubated with Ang II and 0.9% NaCl, Compound NaCl, or 5% NaHCO3 injection for 24 h (mixed with plasma-mimicking medium at 1:9). (A) Representative images of cells immunostained with Abl2 and actin (scale bar: 20 μm). (B) Pearson curve was used to show colocalization of Abl2 protein and actin between points A and B in each group in Figure 4A. (C) Expression of Abl2 in HUVECs transfected with Abl2 siRNA. Hypertensive HUVECs transfected with or without Abl2 siRNA were incubated with 0.9% NaCl, Compound NaCl, or 5% NaHCO3 injection. (D) Representative images of cells immunostained with ASC (FITC) and NLRP3 (TRITC) (scale bar: 20 μm). (E) Quantitative statistics of fluorescence intensity of ASC and NLRP3. (F) Immunofluorescence staining of ROS in HUVECs (scale bar = 40 μm). (G) Quantitative statistics for fluorescence intensity of ROS. (H) Images obtained at 15-min time lapse in FRET analysis (calibration bar: 0.1–1.5; scale bar: 10 µm). (I) Standardized CFP/FRET ratio of vimentin within 15 min. (J) Ratio of transendothelial electrical resistance (TEER) values of HUVECs at 12 h and 0 h. NC, normal; Ang II, Angiotensin II. Data are expressed as mean ± SD (n = 3). *p < 0.05, ***p < 0.001, ****p < 0.0001. |
Reversal of Sodium Ion-Injection-Stimulated Injury of Ang II-Induced HUVECs by Drug‑combination Treatment
In order to reverse the damage to Ang II–induced vascular endothelial cells caused by sodium ion injection, we selected drug combinations to act on Ang II–induced HUVECs stimulated with 0.9% NaCl, Compound NaCl, 5% NaHCO3 injection. Specifically, we analyzed whether the drug combinations could improve the transmembrane OP and reverse the cell damage caused by sodium ion injections in Ang II–induced HUVECs. Different drug combinations of heparin, nifedipine, glyburide, niclosamide, tranilast, and NAC were used to treat Ang II–induced HUVECs stimulated with sodium ion injection. All combinations of different drugs could reverse transmembrane OP and TEER changes (p < 0.001, Figure 5A–C). Among them, the drug combination of heparin, glyburide, tranilast, and NAC was more effective in Ang II–induced HUVECs injected with 5% NaHCO3 (p < 0.0001, Figure 5A–C). Similarly, this drug combination could also result in the recovery of the transmembrane OP and enhance the TEER value in Ang II–induced HUVECs injected with 0.9% NaCl or compound NaCl (p < 0.001, Figure 5D–F); this suggests that by inhibiting calcium signals, inflammation, and oxidative stress, this drug combination could effectively reverse the change in the transmembrane OP and cell permeability caused by sodium ion injection. In order to further investigate the effect of the drug combinations on the inflammation-induced transmembrane OP, the ASC and NLRP3 expression levels, cytoplasmic OP, and PN content are measured. Immunofluorescence staining and Western blotting showed that the drug combination could significantly reduce NLRP3 (Figure 5G and H), and immunofluorescence staining also showed that the drug combination could decrease ASC and Caspase-1 expression, and ASC spot-like aggregation in Ang II–induced HUVECs injected with sodium ions (Figure 5H and I). The drug combination also had a significant inhibitory effect on cytoplasmic OP and the number of PNs (p < 0.0001, Figure 5J and K). In summary, the drug combination could downregulate the expression of NLRP3 and ASC inflammasomes, decrease the number of PNs, and improve transmembrane OP and the cell permeability, indicating that it reverses Ang II–induced hypertension-related vascular endothelial injury because of sodium ion injection.
Reversal of Sodium Ion Injection-Induced Vascular Endothelial Damage of Hypertensive Rats by Drug‑combination Treatment
To verify the mechanisms described above in vitro and analyze the effect of the drug combination of heparin, glyburide, tranilast, and NAC, we employed rat hypertensive models and then injected sodium ion injection intravenously into the rats. The results showed substantial EB staining of heart and kidney tissues in hypertensive rats, but this drug combination could obviously inhibit the accumulation of EB and improve vascular permeability (p < 0.01, Figure 6A and B, Figure S3A). In hypertensive rats stimulated with sodium ion injection, the drug combination did not change heart rate or systolic and diastolic blood pressure (Figure 6C–E; the blood pressure in normal rats ranged from 85 to 115 mmHg, while the blood pressure in hypertensive rats was >115 mmHg; these results showed that both systolic and diastolic blood pressure were higher than 115 mmHg in each group). H&E staining and immunofluorescence assay results showed that sodium ion injection induced vascular endothelial inflammation in hypertensive rats, characterized by inflammatory cells densely surrounding and infiltrating surrounding tissues, and an increase in ASC and NLRP3 proteins, and these two proteins overlapped with staining by the endothelial cell marker CD31 (Figure 6F–I). Immunohistochemistry also revealed ASC and NLRP3 protein accumulation (Figure S3B), indicating the accumulation of inflammatory factors in vascular endothelial cells. Additionally, the H2O2 content also increased, and the drug combination could significantly reduce inflammation-related proteins and H2O2 levels (p < 0.01 or 0.001, Figure 6F–J). In addition, the drug combination could also significantly increase the amount of NO released, thus effectively reverse the damage to hypertensive vascular endothelial cells (p < 0.01, Figure 6K). These results suggest that sodium ion injection could result in vascular endothelial cell inflammation followed by cell damage in hypertensive rats, and that the above mentioned drug combination could safely and effectively mitigate vascular endothelial damage.
Discussion
In the present study, changes in transmembrane OP were found to be correlated with the damage caused by intravenous injection of sodium ions in hypertensive patients. In addition to PNs and ion composition, chemical signals also play crucial roles in affecting the transmembrane OP in live cells. In particular, the Ca2+ signal and mechanosensitive protein Abl2 are involved in transmembrane OP regulation. Sodium ion injections can cause the accumulation of NLRP3 inflammasomes, opening of Ca2+ channels, and activation of Abl2 in response to Ang II–induced HUVECs; consequently, the transmembrane OP balance is disturbed, cell permeability increases, and endothelial cell damage occurs. The safety of intravenous injections was analyzed by considering the transmembrane OP, and a new drug combination is further established as a treatment measure.
The increase in the transmembrane OP resulting from the opening of ion channels, was found to associate closely with PN-induced membrane potential, Ca2+ signals, and chemical stimuli. A previous study reported that extracellular and intracellular PN-ion adsorption could disrupt the membrane potential equilibrium and induce activation of voltage-gated ion channels. Nifedipine, a VGCC blocker, was found to attenuate vimentin tension and protein nanoparticle-induced osmotic pressure (PN-OP), suggesting that a change in the membrane potential caused a change in the OP and was associated with intracellular PN-ion adsorption.31,32 The results of the present study suggest that calcium signals are necessary for the influx of large quantities of ions and the enhancement of intracellular OP (hyperosmolarity). Heparin, a competitive inhibitor of the IP3 receptor, inhibits the calcium-induced increase in OP elicited by Ang II stimulus in vascular endothelial cells. Moreover, calcium signals were reported to be involved in the activation of nonselective voltage-gated ion channels, like SUR1-TRPM4 and TMEM16A/F.33,34 SUR1-TRPM4 and TMEM16A/F were found to be associated closely with intracellular hyperosmolarity via mass Na+ and Cl− influx in response to Ang II or bradykinin stimuli.35,36 Chemical signals are also very important in regulating transmembrane OP. Ion channels can also be activated and sensitized by the kinase signal in response to chemical stimuli.37 Calcium ion/calmodulin-dependent protein kinase IIγ (CaMKIIγ) is involved in the increase in Ca2+-activated chloride current (ICl·Ca) induced by TMEM16A,38 and the activity of protein kinase IIδ (CaMKIIδ) is closely associated with the openness of the TRPM4 channel and Na+ influx.39 Furthermore, calmodulin can be activated effectively by Ang II treatment. Therefore, the sensitivity of TMEM16A and SUR1-TRPM4 channels can be upregulated in hypertensive patients and involved in influx of large quantities of cations and anions in response to electrolyte injection. Furthermore, the Abl2 protein was also found to be involved in endothelial cell inflammation and in the changes in the transmembrane OP and permeability. To summarize, we propose a method that combines the use of multiple sources of PNs, calcium signals, and chemical factors to control the transmembrane OP and thereby reduce endothelial-cell damage.
Sodium ions, glucose, and HS40SC injections are isotonic injections, but only sodium ions can lead to the production of intracellular PNs, change in the ion contents, activation of ion channels, and changes in the transmembrane OP, finally resulting in damage to the hypertensive endothelial cells. The reason for 5% glucose and HS40SC not damaging the endothelial cells may be that these do not rely on ion channels for transport and absorption after entering the blood plasma.40,41 The results of previous studies showed that the permeability of ion channels could be regulated by controlling the membrane potential and the ligand and ion concentrations; however, glucose is not an electrolyte and cannot not ionize to form free ions, hence it has little impact on ion channel opening or transmembrane OP under isotonic conditions.42 A change in albumin content (or other PNs) in blood plasma could result in alteration of the transmembrane OP via absorption of K+ and Ca2+ cations.4 However, HS40SC injection as a plasma substitute, similar to albumins, did not affect the transmembrane OP by altering the membrane potential and activating ion channels.43 HS40SC was speculated to keep the colloid particles and membrane potential in the blood plasma stable. Therefore, the changes in the membrane potential and opening of voltage-gated ion channels substantially affect the transmembrane OP and are therefore important factors to assess the sodium ion-injection-induced damage to the hypertensive endothelial cells.
Clinical studies found that in hypertensive patients, a decline in the kidney function to remove excess water and sodium leads to “water retention”.44 If hypertensive patients received a large amount of sodium ion injection intravenously, the renal burden is increased, resulting in increased water retention.45 Hyperosmosis of kidney cells can inhibit water efflux from kidneys.46 This in turn leads to an increase in extracellular fluid volume, which can cause an increase in cardiac output, leading to an increase in blood pressure. This is consistent with the results of the present study showing that sodium ion injection could cause hyperosmosis in Ang II–induced vascular endothelial cells and alter vascular permeability in hypertensive rats. Therefore, we speculate that sodium ion injections may not prove effective in the treatment of hypertensive patients, especially patients with water retention. Hypertensive patients may suffer from sodium deficiency due to factors such as improper diet or renal dysfunction, and supplementing large quantities of substances present in sodium injections may further increase blood pressure. Based on this knowledge, we identified a new drug combination that could inhibit the damage caused by sodium ion injection in hypertensive rats and vascular endothelial cells from the perspective of transmembrane OP. Therefore, this drug combination could be combined with sodium ion injection to improve water retention, but the clinical value of this approach requires clinical trial validation.
Conclusion
In summary, the transmembrane OP is an important factor to quantify the sodium-ion-injection-induced damage to Ang II–induced vascular endothelial cells. In addition to PNs, ions, and ion channels that were considered in a previous study that focused on the transmembrane OP, in this study, chemical stimuli such as Ang II and the activation of mechanosensitive proteins have also been identified as key factors regulating the transmembrane OP. Furthermore, from the perspective of transmembrane OP, sodium ion injections are not safe for patients with hypertension-related vascular injury, and drug combination adjuvant therapy could provide a method for clinically safe treatment.
Acknowledgments
Xianrui Song, Danyang Li and Lingling Gan are co-first authors for this study. This work was supported by grants from the National Natural Science Foundation of China [grant numbers 82273908, 82073826] and the Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine [grant number 2020YLXK005].
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Baek SH, Jo YH, Ahn S, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 2021;181:81–92. doi:10.1001/jamainternmed.2020.5519
2. Liang F. Pharmacy. Beijing: People’s Health Publishing House; 2016.
3. Fleer G. Polymers at interfaces and in colloidal dispersions. Adv Colloid Interface Sci. 2010;159(2):99–116. doi:10.1016/j.cis.2010.04.004
4. Fleer GJ, Tuinier R. Analytical phase diagrams for colloids and non-adsorbing polymer. Adv Colloid Interface Sci. 2008;143(1–2):1–47. doi:10.1016/j.cis.2008.07.001
5. Zheng Z, Wang Y, Li M, et al. Albumins as extracellular protein nanoparticles collaborate with plasma ions to control biological osmotic pressure. Int J Nanomedicine. 2022;17:4743–4756. doi:10.2147/IJN.S383530
6. Belinskaia DA, Voronina PA, Shmurak VI, et al. Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. 2021;22(19):10318. doi:10.3390/ijms221910318
7. Fletcher DA, Mullins D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi:10.1038/nature08908
8. Caudron N, Arnal I, Buhler E, et al. Microtubule nucleation from stable tubulin oligomers. J Biol Chem. 2002;277:50973–50979. doi:10.1074/jbc.M209753200
9. Higaki A, Caillon A, Paradis P, et al. Innate and innate-like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21(1):4. doi:10.1007/s11906-019-0907-1
10. Li C, Chen L, Wang Y, et al. Protein nanoparticle-related osmotic pressure modifies nonselective permeability of the blood-brain barrier by increasing membrane fluidity. Int J Nanomedicine. 2021;16:1663–1680. doi:10.2147/IJN.S291286
11. Woo SK, Kwon MS, Ivanov A, et al. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013;288(5):3655–3667. doi:10.1074/jbc.M112.428219
12. Diszházi G, Magyar Z, Lisztes E, et al. TRPM4 links calcium signaling to membrane potential in pancreatic acinar cells. J Biol Chem. 2021;297(3):101015. doi:10.1016/j.jbc.2021.101015
13. Qian Z, Wang Q, Qiu Z, et al. Protein nanoparticle-induced osmotic pressure gradients modify pulmonary edema through hyperpermeability in acute respiratory distress syndrome. J Nanobiotechnology. 2022;20:314. doi:10.1186/s12951-022-01519-1
14. Luo ZW, Ovcjak A, Wong R, et al. Drug development in targeting ion channels for brain edema. Acta Pharmacol Sin. 2020;41(10):1272–1288. doi:10.1038/s41401-020-00503-5
15. Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10(6):1045–1059. doi:10.1089/ars.2007.1986
16. Hernanz R, Briones AM, Salaices M, et al. New roles for old pathways? A circuitous relationship between reactive oxygen species and cyclo-oxygenase in hypertension. Clin Sci (Lond). 2014;126(2):111–121. doi:10.1042/CS20120651
17. Negri S, Faris P, Moccia F. Reactive oxygen species and endothelial Ca2+ signaling: brothers in arms or partners in crime? Int J Mol Sci. 2021;22(18):9821. doi:10.3390/ijms22189821
18. Ushio-Fukai M. Vascular signaling through G protein-coupled receptors: new concepts. Curr Opin Nephrol Hypertens. 2009;18(2):153–159. doi:10.1097/MNH.0b013e3283252efe
19. Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol. 2008;294(3):H1103–1118. doi:10.1152/ajpheart.00903.2007
20. Castro MM, Rizzi E, Prado CM, et al. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29(3):194–201. doi:10.1016/j.matbio.2009.11.005
21. Pedre B, Barayeu U, Ezeriņa D, et al. The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol Ther. 2021;228:107916. doi:10.1016/j.pharmthera.2021.107916
22. Hamada K, Mikoshiba K. IP3 receptor plasticity underlying diverse functions. Annu Rev Physiol. 2020;82:151–176. doi:10.1146/annurev-physiol-021119-034433
23. Lawana V, Singh N, Sarkar S, et al. Involvement of c-Abl kinase in microglial activation of NLRP3 inflammasome and impairment in autolysosomal system. J Neuroimmune Pharmacol. 2017;12(4):624–660. doi:10.1007/s11481-017-9746-5
24. Rado M, Flepisi B, Fisher D. Differential effects of normoxic versus hypoxic derived breast cancer paracrine factors on brain endothelial cells. Biology (Basel). 2021;10(12):1238. doi:10.3390/biology10121238
25. Rana A, Lowe A, Lithgow M, et al. Use of deep learning to develop and analyze computational hematoxylin and eosin staining of prostate core biopsy images for tumor diagnosis. JAMA Netw Open. 2020;3(5):e205111. doi:10.1001/jamanetworkopen.2020.5111
26. Karch JM, Lee JS. Pulmonary fluid extraction and osmotic conductance, sigmaK, measured in vivo. J Appl Physiol (1985). 1998;84(3):769–781. doi:10.1152/jappl.1998.84.3.769
27. Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi:10.1073/pnas.0409594102
28. Hahn NE, Meischl C, Kawahara T, et al. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol. 2012;180:2222–2229. doi:10.1016/j.ajpath.2012.02.018
29. Cho CH, Lee YS, Kim E, et al. Physiological functions of the TRPM4 channels via protein interactions. BMB Rep. 2015;48(1):1–5. doi:10.5483/BMBRep.2015.48.1.252
30. Letsiou EA, Rizzo N, Sammani S, et al. Differential and opposing effects of imatinib on LPS- and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308(3):L259–269.
31. Woodring PJ, Hunter T, Wang JY. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J Biol Chem. 2001;276(29):27104–27110. doi:10.1074/jbc.M100559200
32. Stephens RS, Servinsky LE, Rentsendorj O, et al. Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase. Am J Physiol Cell Physiol. 2014;306(6):C559–569. doi:10.1152/ajpcell.00375.2012
33. Laryushkin DP, Maiorov SA, Zinchenko VP, et al. Role of L-type voltage-gated calcium channels in epileptiform activity of neurons. Int J Mol Sci. 2021;22(19):10342. doi:10.3390/ijms221910342
34. Walcott BP, Kahle KT, Simard JM. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9(1):65–72. doi:10.1007/s13311-011-0087-4
35. Schreiber R, Ousingsawat J, Wanitchakool P, et al. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca2+ and plasma membrane lipid. J Physiol. 2018;596(2):217–229. doi:10.1113/JP275175
36. Yu D, Thelin WR, Rogers TD, et al. Regional differences in rat conjunctival ion transport activities. Am J Physiol Cell Physiol. 2012;303(7):C767–780. doi:10.1152/ajpcell.00195.2012
37. Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. J Neurosurg. 2010;113(3):622–629. doi:10.3171/2009.11.JNS081052
38. Lin CX, Lv XF, Yuan F, et al. Ca2+/calmodulin-dependent protein kinase II γ-dependent serine 727 phosphorylation is required for TMEM16A Ca2+-activated Cl- channel regulation in cerebrovascular cells. Circ J. 2018;82(3):903–913. doi:10.1253/circj.CJ-17-0585
39. Hu Y, Kaschitza DR, Essers M, et al. Pathological activation of CaMKII induces arrhythmogenicity through TRPM4 overactivation. Pflugers Arch. 2021;473(3):507–519. doi:10.1007/s00424-020-02507-w
40. Berweck S, Lepple-Wienhues A, Stöss M, et al. Large conductance calcium-activated potassium channels in cultured retinal pericytes under normal and high-glucose conditions. Pflugers Arch. 1994;427(1–2):9–16. doi:10.1007/BF00585936
41. Innes A, Tingle S, Ibrahim I, et al. Use of dextran 40 after pancreas transplant may reduce early inflammation and significant bleeding compared to a heparin-based protocol. Transplant Proc. 2021;53(2):712–715. doi:10.1016/j.transproceed.2020.10.020
42. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751–1760. doi:10.2337/diabetes.49.11.1751
43. Ates T, Gezercan Y, Menekse G, et al. The effects of stereotactic cerebroventricular administration of albumin, mannitol, hypertonic sodium chloride, glycerin and dextran in rats with experimental brain edema. Turk Neurosurg. 2017;27(6):917–923. doi:10.5137/1019-5149.JTN.17231-16.1
44. Grillo A, Salvi L, Coruzzi P, et al. Sodium intake and hypertension. Nutrients. 2019;11(9):1970. doi:10.3390/nu11091970
45. Titze J. Water-free Na+ retention: interaction with hypertension and tissue hydration. Blood Purif. 2008;26(1):95–99. doi:10.1159/000110573
46. Danziger J, Zeidel ML. Osmotic homeostasis. Clin J Am Soc Nephrol. 2015;10(5):852–862. doi:10.2215/CJN.10741013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.