Back to Journals » Infection and Drug Resistance » Volume 17
Intrathecal Injection of Polymyxin B in a Child with Meningitis Caused by Carbapenem-Resistant Pseudomonas aeruginosa: A Case Report and Literature Review
Authors Wu M , Zhao J, Liu Z, Zhang H
Received 29 October 2023
Accepted for publication 20 January 2024
Published 24 January 2024 Volume 2024:17 Pages 249—258
DOI https://doi.org/10.2147/IDR.S445416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mei Wu,1,2,* Jingui Zhao,1,2,* Zhongqiang Liu,1,2 Haiyang Zhang1,2
1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haiyang Zhang, Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China, Tel + 86 15756273633, Fax + 86 15756273633, Email [email protected]
Background: Clinically, Carbapenem-resistant Pseudomonas aeruginosa (CRPA) meningitis is extremely difficult to cure and has a high mortality rate. Intrathecal injection of polymyxins B is suggested to be an effective anti-infective means to treat intracranial infection with CRPA. However, due to the potential drug toxicity of polymyxin B in children, this regimen has rarely been reported in pediatrics.
Case Description: A 5-year-old male patient diagnosed with Epstein-Barr virus-induced hemophagocytic syndrome (HPS) exhibited persistent fever for over a month despite antibacterial and chemotherapy regimens. During hospitalization, the patient presented with unconsciousness, nystagmus, and myasthenia. Cerebrospinal fluid (CSF) analysis indicated elevated leukocyte counts and protein levels. Sputum and blood cultures, as well as metagenomic next-generation sequencing (mNGS) of CSF, identified CRPA. Intravenous and intrathecal polymyxin B administration resulted in temperature normalization and amelioration of consciousness disturbances and nystagmus. Subsequent CSF analysis yielded normal results, while polymyxin B treatment exhibited no nephrotoxicity or neurotoxicity.
Conclusion: Intrathecal injection of polymyxin B in children with meningitis caused by CRPA is an effective treatment without remarkable adverse events.
Keywords: polymyxin B, intrathecal injection, carbapenem resistant, Pseudomonas aeruginosa, meningitis
Introduction
The incidence of Carbapenem-resistant gram-negative bacteria (CRGNB) has been on the rise. An international cohort study showed the rate of Pseudomonas spp was 14.3% in hospital-acquired bloodstream infections, and the rate of carbapenem resistance was up to 33.2%.1 Another investigation reported a rate of 4.9% for P. aeruginosa among 1939 isolated gram-negative bacteria from 21 hospitals in China between April 2019 and December 2021.2 Notably, most of the bacteriological susceptibility tests from various tissue samples indicated that the strain was sensitive to polymyxin B, indicating that the minimum inhibitory concentration (MIC) of polymyxin B against this strain was less than 2 mg/L.2 Polymyxins B was initially employed in the 1940s but was swiftly discontinued due to concerns of neurotoxicity and nephrotoxicity. However, it has been re-recommended, particularly for carbapenem-resistant gram-negative organisms that retain high antimicrobial susceptibility to polymyxins B, in recent years.3 Intrathecal administration of polymyxins B stands as an effective treatment for meningitis induced by CRGNB. Nonetheless, the utilization of intrathecal or intraventricular polymyxin B remains infrequent among pediatric patients.
Here, we report a child with meningitis attributed to carbapenem-resistant Pseudomonas aeruginosa (CRPA). The patient underwent both intravenous and intrathecal injection of polymyxin B, resulting in recovery without drug toxicity of side effect.
Case Presentation
A 5-year-old male patient was admitted to the pediatric intensive care unit (PICU) of West China Second University Hospital on November, 2022. The primary complaint was persistent fever for over a month accompanied by right neck lymphadenopathy. He had shaking chill and febrile peak of 41°C. Subsequently, the patient displayed altered consciousness, nystagmus, myasthenia, dyspnea, and gastrointestinal hemorrhage. The patient was previously healthy and scheduled vaccinated. He had not accepted immunosuppressive drugs or suffered from recurrent infections. He had no history of previous illnesses or family illnesses.
On physical examination, the patient had a blood pressure of 132/96 mmHg, temperature of 39 °C, heart rate of 144/min, respiratory rate of 34/min, oxygen saturation of 90%. The Glasgow Coma Scale (GCS) score was 9. Other positive signs included jaundice, pedal edema, ecchymosis, lymphadenopathy, pallor, and dyspnea. Moist rales appeared in both lungs without decreased breath sounds. The abdomen was grossly distended but without tenderness. The liver margin was palpable 6 cm below the costal margin. His neck stiffness and bilateral Babinski’s reflex were positive.
Before admission, the routine blood examination in emergency clinic revealed neutropenia at 0.06 × 109/L, anemia at 47 g/L, thrombocytopenia at 35 × 109/L and increased C-reactive protein at 149.83 mg/L. Liver function test results were deranged, with alanine aminotransferase (ALT) of 770 U/L, aspartate aminotransferase (AST) of 4466 U/L, a total bilirubin (TB) of 192.9 umol/L and lactate dehydrogenase (LDH) of 8108 U/L. Reduced fibrinogen levels (118 mg/dl), elevated ferritin levels (>2000 ng/mL) and positive polymerase chain reaction (PCR) of Epstein-Barr virus (EBV) were also observed. There was an increased presence of atypical lymphocytes and macrophages in the bone marrow. On admission, the routine blood examination also revealed neutropenia at 0.1 × 109/L, anemia at 80 g/L, thrombocytopenia at 45 × 109/L and increased C-reactive protein at 116.2 mg/L. The analysis showed liver dysfunction (ALT 104 U/L, AST 176 U/L, TB 144.5 umol/L, LDH 927 U/L). Other analyses showed markedly increased ferritin (11,448.5, normal range: 22–322 ng/mL), interleukin (IL)-2R (4942, normal range: 223–710 U/mL), IL-6 (626, normal range: < 5.9 pg/mL), IL-8 (1789, normal range: <62 pg/mL), IL-10 (618, normal range: < 9.1 pg/mL) and tumor necrosis factor-α (62, normal range: < 8.1 pg/mL). The fungi examination was positive, including galactomannan and 1,3-beta-D-glucan assays. The PCR of EBV was 4.37×104copies/mL. CSF analysis demonstrated a normal white blood cell count (3 × 106/L). CSF-protein levels (2270 mg/L) and lactate dehydrogenase (134 U/L) were elevated. Chest, abdominal and brain computerized tomography (CT) scans revealed pulmonary inflammation, pleural effusion, pelvic effusion and ventriculomegaly (Figure 1a and b). The initial diagnosis comprised sepsis, hemophagocytic syndrome (HPS) induced by EBV, which met the diagnostic criteria of hemophagocytic lymphohistiocytosis (HLH) guidelines,4 severe pneumonia, severe encephalitis, and aspergillosis.
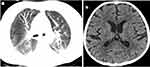 |
Figure 1 (a) Chest CT showed pulmonary inflammation; (b) Brain CT showed ventriculomegaly on admission. |
 |
Figure 2 (a) The variation of the patient’s temperature; (b) The variation of CRP; (c) The variation of leukocytes; (d) The variation of thrombocytes. |
Initially, the patient accepted chemotherapy based on the HLH-1994 protocol5 to treat HPS, linezolid and meropenem combined with micafungin for empirical anti-infection at a local medical facility before admission, but the clinical response was poor. Consequently, he was transferred to our PICU. Given an escalated inflammatory cascade and substantial elevation of inflammatory mediators, the patient underwent plasma exchange (PE) for 5 days as well as continuous renal replacement therapy (CRRT) for 3 days. Treatment encompassed acyclovir (30 mg/kg/d, q8h for 35 days), voriconazole (12 mg/kg/d, q12h for 30 days), and intravenous immunoglobulin (500 mg/kg/d for 4 days). Empirical antibiotic therapy included meropenem (120 mg/kg/d, q8h), vancomycin (40 mg/kg/d, q6h for 34 days). Dexamethasone and etoposide were implemented to address HPS according to the HLH-2004 protocol.4 Additional adjunctive measures included mannitol and glycerol fructose to reduce intracranial pressure, etamsylate, somatostatin, human fibrinogen, thrombocytes, plasma, hemocoagulase and hemorrhage transfusion to manage hemorrhaging and anemia, and a noninvasive ventilator to improve dyspnea. On the 3rd day of admission, the patient still suffered from a high fever, moderate unconsciousness, and dyspnea. Both the blood culture and the sputum culture indicated the presence of CRPA (Table 1 and Table 2). We used the VITEK 2 COMPACT microbial automatic identification and drug sensitivity analyzer (BioMerieux VITEK 2 Compact, Merieux biotech, France) for CRPA identification and susceptibility analysis. The evaluation criteria for antibiogram and drug susceptibility referred to the proposed standard by the Clinical and Laboratory Standards Institute (CLSI) M17-S32.6 The CSF-mNGS confirmed the involvement of P. aeruginosa (specific DNA sequence: 1187 reads, high confidence). The antibiotic regimen was then changed to combine polymyxin B with meropenem. The patient initially received a loading dose of polymyxin B (2.5 mg/kg) followed by standard dosing (1.5 mg/kg/d, q12h). Intrathecal injection of polymyxin B was also conducted, totaling 11 doses (5 mg/d for 4 days, followed by alternate-day administration for 2 weeks).
 |
Table 1 Susceptibility Results for P. Aeruginosa in Blood Samples |
 |
Table 2 Susceptibility Results for P. Aeruginosa in Sputum Sample |
On the 5th to 7th day of admission, a notable reduction in temperature was observed (Figure 2a). The patient’s level of consciousness improved compared to the preceding state (GCS score increased from 9 to 12). The previously positive Babinski’s reflex became negative, and the noninvasive ventilator changed to O2 0.5 L/min on the nasal cannula. Blood analyses indicated a gradual decrease in C-reactive protein levels (Figure 2b). On the 13th to 17th day, the blood routine examination showed gradual increases in leukocyte and thrombocyte counts (Figure 2c, 2d). Leukocytes in CSF were higher than before (333 × 106/L vs 3 × 106/L), but protein in CSF decreased (864 mg/L vs 2270 mg/L). Chest CT scan revealed pulmonary inflammation was relieved, but cavitary lesions formed, in contrast to the previous condition (Figure 3a). Brain MRI of the brain revealed ventriculomegaly, along with abnormal signal intensities observed in the bilateral precornu, the left frontal lobe, and the triangular region of white matter (Figure 3b). As the patient kept afebrile with improved dyspnea, fatigue, and drowsiness, previous treatment continued. During therapy, the patient exhibited purulent ear tract secretions, with corresponding culture results identifying CRPA. Levofloxacin ear drops were introduced. On the 28th day, the patient was still apyretic. His leukocyte, thrombocyte and CSF routine analyses were all within normal ranges. Blood culture and ear tract secretion culture did not indicate CRPA. Although sputum culture remained indicative of CRPA, we considered that colonizing bacteria had been formed. We downgraded the anti-infection regimen to ceftazidime and retained the voriconazole antifungal. The polymyxin B was discontinued after a course of 25 days. No adverse events, including nephrotoxicity, neurotoxicity, or skin hyperpigmentation, occurred during intrathecal and intravenous injection of polymyxin B.
On the 46th day, sputum culture turned negative, and P. aeruginosa was not detected in reexamination of CSF-mNGS. Eventually, the patient achieved successful discharge on day 60 with normal ferritin levels and continued to receive follow-up care in the Department of Pediatric Hematology and Oncology. No recurrence of intracranial infection was observed. The blood culture and the sputum culture remained negative for 3 months during the follow-up period. The timeline of the treatment and follow-up is presented in Figure 4.
 |
Figure 4 The timeline of the treatment and follow-up. |
Discussion
P.aeruginosa, a highly virulent and aggressive gram-negative bacterium, is infrequently implicated in meningitis. However, when clinically relevant risk factors are present, P. aeruginosa can easily transform into opportunistic infections, with a potential risk of infection in the cerebrospinal fluid. The risk factors encompass CSF leakage or shunt, neurosurgery, neurosurgical devices, head trauma, immune deficiency, chemotherapy, invasive tubes, and nosocomial infections in children. The development of resistance to P. aeruginosa depends on multiple resistance mechanisms, including natural resistance mechanisms, acquired resistance mechanisms and adaptive resistance mechanisms. Under the combined intervention of integrated resistance mechanisms, P. aeruginosa is easily transformed into CRPA, which is extremely difficult to remove. The European Society for Clinical Microbiology and Infectious Diseases (ESCMID) recommended that severe infections caused by CRPA can be treated with polymyxin, aminoglycoside, or fosfomycin, using two drugs that are active in vitro.7 The utilization of intrathecal injection of polymyxin B in pediatrics, particularly in China, is extremely lacking in clinical experience. Some patients died of aggressive deterioration before a definitive diagnosis of CRPA meningitis was made. Polymyxin B is not absorbed from the gastrointestinal tract and achieves generally poor penetration in the CSF, even by intravenous admission. Intrathecal or intraventricular administration of antibiotics can be an optimal choice in meningitis caused by gram-negative pathogens to obtain an appropriate CSF concentration.7,8 In this case, the patient’s recovery was achieved through intravenous and intrathecal administration of polymyxin B.
Polymyxins, including polymyxin E and polymyxin B, combine with lipopolysaccharide (LPS) locating on gram-negative microbes surface, which reduces outer membrane integrity and induces self-promoted uptake to disrupt the overall membrane. Polymyxins E also inhibit the type 2 nicotinamide adenine dinucleotide dehydrogenase (NDH-2) to suppress bacterial respiration and accumulate reactive oxygen species, and polymyxin B is a better inhibitor compared to polymyxin E.9 In CRPA meningitis, it is difficult for polymyxins (molecular mass: 1163 Da) to permeate the blood-brain barrier, which allows low molecular mass (< 450 Da) to get through. To obtain therapeutic concentrations of CSF (MIC > 2 μg/mL), intrathecal or intraventricular injection of polymyxins is conducted. Polymyxins induce neurotoxicity by increasing oxidative stress injury and mitochondrial pathology, activating the apoptotic pathway and autophagy.10 Polymyxin B is given as polymyxin B sulfate, the active form, most of which is cleared independently of the kidney. Polymyxin E is given as polymyxin methanesulphonate (CMS). CMS is an inactive prodrug that needs to transfer to several derivatives and excreted in the urine. Exposure of renal tubular cells to CMS can induce cell cycle arrest, apoptosis and oxidative stress.11 As a result, CMS has a higher risk of nephrotoxicity than polymyxin B. Clinical studies also showed polymyxin B had fewer adverse events than polymyxin E with the same efficacy.12,13 Besides, polymyxins have a narrow antibacterial spectrum limited to a subset of gram-negative bacilli. To ameliorate these side effects of polymyxins, It needs to monitor renal function and electrolyte balance, evade concurrent nephrotoxic or neurotoxic agents, and optimize polymyxins. Unfortunately, there were only pharmacokinetic assays of polymyxin B, especially in CSF, by intrathecal or intraventricular injection of children.14,15 Here, we reviewed intrathecal or intraventricular administration of polymyxin B in children.
To date, only four articles have reported pediatric patients who underwent intrathecal or intraventricular administration of polymyxin B (Table 3).16–19 Among them, all had received neurosurgical interventions. The preterm neonate accepted ventriculoperitoneal shunting after anti-infection therapy due to persistent hydrocephalus. Our patient exhibited ventriculomegaly on the brain CT scan and MRI, yet external ventricular drainage was not implemented. Isolated microorganisms encompassed Acinetobacter baumannii, P. aeruginosa, and Klebsiella pneumoniae. The dosages of Polymyxin B varied across these reports. The neonate received intraventricular polymyxin B at 40,000 units per dose for 14 doses.16 Xing H18 selected 50,000 U once per day for the first 4 days, then 50,000 U once every other day. Another boy in early childhood received 5mg once per day.19 International consensus guidelines recommend a once-daily intraventricular or intrathecal administration of 5 mg for adults.7 The recommended polymyxin B dosing regimen specifies 5 mg for adults and older children. The recommended dose for newborns and infants is 2mg once daily for 3 to 4 days followed by every other day dosing. This regimen should span at least two weeks to achieve microbial eradication. Therefore, we opted for a dosage of 5 mg once daily for 4 days, followed by every other day administration for 2 weeks. A study revealed that the concentration of polymyxin B in CSF was notably higher in individuals who received polymyxin through intrathecal and intravenous routes compared to those administered solely intravenously.20 Among the reported patients, two experienced skin hyperpigmentations, while one encountered neurotoxicity during treatment. However, both of these adverse effects resolved following the completion of polymyxin B therapy.18,19 No adverse events were observed during our treatment. The patient afflicted by Klebsiella pneumoniae infection succumbed to septic shock, with a persistent positive CSF culture at the time of death. The other patients survived and achieved CSF sterilization. Furthermore, to enhance clinical outcomes, it was imperative to reposition, remove drainage, or discontinue lumbar drainage after each injection.
 |
Table 3 Neonatal and Pediatric Cases Receiving Intrathecally or Intraventricularly of Polymyxin B |
In this case, the combination of intravenous and intrathecal polymyxin B administration led to the successful resolution of meningitis caused by CRPA in a pediatric patient. It was vital that the therapy did not result in nephrotoxicity, neurotoxicity, skin hyperpigmentation, or chemical meningitis. Nevertheless, it was regrettable that therapeutic drug monitoring (TDM) or pharmacokinetic assays were not conducted. In addition, there was a lack of identification of drug-resistant enzyme types in this case. P. aeruginosa produces a variety of β-lactamases, including hyperspectral β-lactamases (ESBLs), induced cephalosporinase (AmpC), carbapenase and penicillase. Among them, carbapenenase mainly consists of the metallic enzymes IMP and VIM. But some studies found that P. aeruginosa carrying NDM-1 enzyme was resistant to polymyxin.18,19 Given the limited and sporadic reports on intraventricular or intrathecal polymyxin B usage in pediatric patients, coupled with small sample sizes, further investigations are warranted to comprehensively assess the efficacy and potential adverse events associated with polymyxin B administration through intraventricular or intrathecal routes in children.
Conclusion
Polymyxin B, employed as salvage therapy against CRGNB, effectively resolved meningitis induced by CRPA through combined intravenous and intrathecal administration in children. Notably, the efficacy and safety of this drug were worthy of recognition in our study. Further research and clinical practice are required to be conducted through well-designed studies with an ample clinical cohort, aiming to optimize the rational use of Polymyxin B in children with CRPA meningitis.
Abbreviations
CRPA, Carbapenem-resistant Pseudomonas aeruginosa; mNGS, metagenomic next-generation sequencing; CRGNB, Carbapenem-resistant gram-negative bacteria; MIC, minimum inhibitory concentration; PICU, pediatric intensive care unit; GCS, Glasgow Coma Scale; EBV, Epstein-Barr virus; CSF, cerebrospinal fluid; IL, interleukin; HPS, hemophagocytic syndrome; HLH, hemophagocytic lymphohistiocytosis; PE, plasma exchange; CRRT, continuous renal replacement therapy; ESCMID, European Society for Clinical Microbiology and Infectious Diseases; LPS, lipopolysaccharide; NDH-2, type 2 nicotinamide adenine dinucleotide dehydrogenase; Da, Dalton; CMS, polymyxin methanesulphonate; TDM, therapeutic drug monitoring; ESBLs, hyperspectral β-lactamases; AmpC, cephalosporinase.
Data Sharing Statement
Data will be provided by the corresponding author upon reasonable request.
Ethical Approval
The study adhered to the guidelines outlined in the 1964 Helsinki Declaration and its subsequent amendments, or equivalent ethical standards. The studies involving human participants were reviewed and approved by the ethics committee of West China Second University Hospital. Written informed consent was obtained from the patient and his immediate family members for the publication of any potentially identifiable images or data included in this case report prior to inclusion.
Acknowledgments
The authors thank all the clinical staff contributed to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Sichuan Medical Youth Innovative Research Project (No. Q22028).
Disclosure
All authors declare that the research is conducted in the absence of any commercial relations or financial relationships of interest that might be a constant source of interest.
References
1. Tabah A, Buetti N, Staiquly Q, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. 2023;49(2):178–190. doi:10.1007/s00134-022-06944-2
2. Xi J, Jia P, Zhu Y, et al. Antimicrobial susceptibility to polymyxin B and other comparators against Gram-negative bacteria isolated from bloodstream infections in China: results from CARVIS-NET program. Front Microbiol. 2022;13:1017488. doi:10.3389/fmicb.2022.1017488
3. Nation RL, Li J, Cars O, et al. Framework for optimisation of the clinical use of polymyxin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis. 2015;15(2):225–234. doi:10.1016/S1473-3099(14)70850-3
4. Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi:10.1002/pbc.21039
5. Henter JI, Aricò M, Egeler RM, et al. HLH-94: a treatment protocol for hemophagocytic lymphohistiocytosis. HLH study Group of the Histiocyte Society. Med Pediatr Oncol. 1997;28(5):342–347. doi:10.1002/(sici)1096-911x(199705)28:5<342::aid-mpo3>3.0.co;2-h
6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. In: CLSI Supplement M100-S32. Wayne, PA: Clinical and Laboratory Standards Institute; 2022.
7. Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti‐infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi:10.1002/phar.2209
8. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861
9. Li Z, Velkov T. Polymyxins: mode of Action. Adv Exp Med Biol. 2019;1145:37–54. doi:10.1007/978-3-030-16373-0_4
10. Dai C, Xiao X, Li J, et al. Molecular Mechanisms of Neurotoxicity Induced by Polymyxins and Chemoprevention. ACS Chem Neurosci. 2019;10(1):120–131. doi:10.1021/acschemneuro.8b00300
11. Azad MAK, Nation RL, Velkov T, Li J. Mechanisms of Polymyxin-Induced Nephrotoxicity. Adv Exp Med Biol. 2019;1145:305–319. doi:10.1007/978-3-030-16373-0_18
12. Truong CB, Durham SH, Qian J. Comparisons of adverse event reporting for polymyxin versus polymyxin B using the US Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf. 2021;20(5):603–609. doi:10.1080/14740338.2021.1890024
13. Thomas R, Velaphi S, Ellis S, et al. The use of polymyxins to treat carbapenem resistant infections in neonates and children. Expert Opin Pharmacother. 2019;20(4):415–422. doi:10.1080/14656566.2018.1559817
14. Tran TB, Velkov T, Nation RL, et al. Pharmacokinetics/pharmacodynamics of polymyxin and polymyxin B: are we there yet? Int J Antimicrob Agents. 2016;48(6):592–597. doi:10.1016/j.ijantimicag.2016.09.010
15. Velkov T, Dai C, Ciccotosto GD, Cappai R, Hoyer D, Li J. Polymyxins for CNS infections: pharmacology and neurotoxicity. Pharmacol Ther. 2018;181:85–90. doi:10.1016/j.pharmthera.2017.07.012
16. Piparsania S, Rajput N, Bhatambare G. Intraventricular polymyxin B for the treatment of neonatal meningo-ventriculitis caused by multi-resistant Acinetobacter baumannii--case report and review of literature. Turk J Pediatr. 2012;54(5):548–554.
17. Ye J, Tan L-H, Shen Z-P, et al. Polymyxin for the treatment of intracranial infections of extensively drug-resistant bacteria in children after neurosurgical operation. World J Pediatr. 2020;16(5):528–532. doi:10.1007/s12519-020-00350-8
18. Xing H, Cheng C, Zhang Y, et al. Successful Treatment With Intrathecal and Intravenous Polymyxin B-Based Combination Against MDR Acinetobacter baumannii Meningitis in Pediatric Patient: a Case Report. Front Pediatr. 2021;9:564991. doi:10.3389/fped.2021.564991
19. Fitzrol DN, Ang SY, Suhaimi A, Yeap TB. Rare occurrence of polymyxin B-induced hyperpigmentation in a child with ventriculitis. BMJ Case Rep. 2023;16:4. doi:10.1136/bcr-2022-253959
20. Bhandari RK, Pandey AK, Shafiq N, et al. polymyxin disposition in the cerebrospinal fluid when administered either intravenously alone or with intraventricular/intrathecally in neonates/pediatric patients with culture-proven meningitis. Pediatr Neonatol. 2022;63(2):190–191. doi:10.1016/j.pedneo.2021.07.012
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.