Back to Journals » International Journal of General Medicine » Volume 17
Intramyocardial Hemorrhage Leads to Higher MACE Rate by Increasing Myocardial Infarction Volume in Patients with STEMI
Authors Wu Z, Jin X , Tudahun I, Wu S, Chen M, Tang J
Received 25 October 2023
Accepted for publication 18 January 2024
Published 24 January 2024 Volume 2024:17 Pages 275—285
DOI https://doi.org/10.2147/IJGM.S444360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Zhijian Wu,1– 3 Xiaotian Jin,1 Ilyas Tudahun,1 Shangjie Wu,2,3 Mingxian Chen,1 Jianjun Tang1
1Department of Cardiovascular Medicine, The Second Xiangya Hospital of Central South University, Changsha, 410011, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, People’s Republic of China; 3Hunan Centre for Evidence-Based Medicine, Changsha, 410011, People’s Republic of China
Correspondence: Jianjun Tang; Mingxian Chen, Department of Cardiovascular Medicine, The Second Xiangya Hospital of Central South University, No. 139 Middle Renmin Road, Furong District, Changsha, Hunan, 410011, People’s Republic of China, Fax + 86 731 85533525, Email [email protected]; [email protected]
Background and Aims: Whether IMH can directly cause persistent myocardial necrosis after reperfusion therapy in STEMI patients is still unclear. We conducted a prospective study to compare the cardiovascular parameters in patients with STEMI with and without IMH to explore the potential correlations between IMH and poor outcomes.
Methods and Results: We prospectively enrolled 65 consecutive patients with newly diagnosed STEMI admitted to the CCU of the Second Xiangya Hospital of Central South University between April 2019 and November 2021, all of whom underwent primary PCI. Of these, 38 (58.5%) and 27 (41.5%) patients were in the IMH-absent and IMH-present groups, respectively. At a mean time of 5– 7 days after reperfusion therapy, the volume of MI measured using LGE sequence was larger in STEMI patients with IMH than in patients without IMH (34.2 ± 12.7 cm3 vs 21.1 ± 13.1 cm3, P< 0.001). HsTNT levels were significantly higher in the IMH-present group than in the IMH-absent [2500.0 (1681.5– 4307.0) pg/mL vs 1710.0 (203.0– 3363.5) pg/mL, P=0.021] group during hospitalization. The LVEF measured using CMR in the IMH-present group was lower than that in the IMH-absent group (30.7 ± 9.8% vs 42.3 ± 11.0%, P < 0.001). The rate of MACE at 12 months in IMH-present group was significantly higher than in the IMH-absent group (9/27 VS 2/38, P = 0.012).
Conclusion: IMH can lead to further expansion of MI volumes in patients with STEMI, resulting in lower LVEF and higher MACE rate in the post-discharge follow-up.
Keywords: myocardial infarction, cardiac magnetic resonance, intramyocardial hemorrhage, infarction volume, outcome
Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is an emergency, severe cardiovascular disease. After the 1970s and the 1980s, with the introduction of reperfusion therapies such as thrombolysis, percutaneous coronary intervention (PCI), and coronary artery bypass graft surgery (CABG) in the treatment of acute myocardial infarction (AMI), the survival rate of patients with STEMI has greatly improved.1,2 However, cardiac function does not fully recover in 30–50% of patients, even with vascularization therapy, owing to irreversible myocardial necrosis, ischemia-reperfusion injury (IRI), and other factors.3,4 IRI often results in coronary microvascular injury, which includes two manifestations: intramyocardial hemorrhage (IMH) and microvascular obstruction (MVO), which can be visualized by cardiac magnetic resonance (CMR).5,6 Although epicardial vascular patency recovered because of microvascular injury, the vascular endothelial gap and erythrocyte exosmosis increased IMH.7 Previous studies8–10 have shown that patients with IMH have a worse prognosis. Some cardiologists have proposed that IMH can cause adverse left ventricular remodeling, and myocardial injury is further aggravated due to blood cell extravasation, iron deposition, and local inflammatory reaction.11 Despite various speculations, the specific mechanism by which IMH contributes to an adverse prognosis remains unclear.
Additionally, clinical questions have plagued us. STEMI patients with IMH usually present with more severe conditions and higher laboratory test results such as troponin and NT-ProBNP levels. These conditions indicate that patients with STEMI and IMH are likely to have worse clinical outcomes. This may be related to infarct volume or infarct area. Whether IMH is a concomitant phenomenon of severe MI or might IMH be involved in the worsening progression of MI remains unclear.
Therefore, we conducted a prospective study to compare the cardiovascular parameters in patients with STEMI with and without IMH to explore the potential pathophysiological correlations between IMH and poor cardiovascular outcomes.
Methods
Patients
We prospectively enrolled consecutive patients with newly diagnosed STEMI admitted to the coronary care units (CCU) of the Second Xiangya Hospital of Central South University between April 2019 and November 2021, all of whom underwent primary PCI. The diagnostic criteria were based on the fourth edition of the Global Definition of Myocardial Infarction (2018). Patients with clear prior reperfusion therapy (thrombolysis, PCI, or CABG) for ischemia were excluded (for specific inclusion and exclusion criteria, see Supplementary Material). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki12 and was reflected by prior approval from the Human Research Committee of the Second Xiangya Hospital of the Central South University (ethics approval number: Y2021453). Written informed consent was obtained from patients while the patient was in a clinically stable, non-congested condition or from their family members who could provide informed consent on behalf of the patients after they were informed about the objectives and procedures of the study. Their right to refuse participation at any time was assured. For this purpose, a one-page consent letter was attached as a cover page for each questionnaire stating the general objective of the study and issues of confidentiality that were discussed by the data collectors before proceeding to data collection.
Data Collection
Medical records were obtained from inpatient and emergency medical systems. Data including demographic characteristics, comorbidities, laboratory testing results, electrocardiography (ECG), PCI records, echocardiographic findings, and treatment were obtained. Follow-up was started at the time of STEMI diagnosis. The primary clinical endpoint [major adverse cardiac events (MACE)] was defined as a composite of cardiac death, reinfarction, and the occurrence of new heart failure (HF) after hospital discharge for the index event. To avoid double counting of patients with more than one event, each patient contributed only once to the MACE endpoint (death > reinfarction > HF). Data were obtained from medical records or telephone interviews with patients or relatives by two trained doctors. The final follow-up date was September 30, 2023. Survival time (months) was measured as the duration between the first day of hospitalization when the patient was diagnosed with STEMI and the date of MACE.
CMR Protocol
After admission, patients underwent CMR on 3.0 T scanners (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with an 18-channel body coil combined with a spine coil to determine whether IMH occurred. All CMR image analyses were performed using CVI42 (Circle Cardiovascular Imaging Inc.) by two radiologists with more than 3 years of CMR experience in consensus. IMH was assessed by T2 or T2 mapping quantification using a breath-hold, cardiac gated gradient echo sequence with eight echoes obtained in three matching short-axis slices before administration of the contrast agent, which was defined as a region of hypointense core within the infarcted area with a reduction in T2 signal intensities ≤ 20 ms. The infarct volume and MVO were measured with left ventricular short-axis delayed gadolinium-enhanced (LGE) images.
Statistical Analysis
Normally distributed parameters are expressed as mean ± standard deviation (SD), whereas non-normally distributed parameters are expressed as median (Q1-Q3) with interquartile interval (IQR). The classification values are expressed in numbers (percentages). Categorical data were reported as frequencies and percentages and compared using the chi-square or Fisher’s exact test. The unpaired Student’s t-test (if normal distribution) or Mann–Whitney U-test (non-normal distribution variable) was used to compare continuous variables between the two independent groups. If more than two groups were compared, the ANOVA or the Kruskal–Wallis test was used for analysis. The Kaplan–Meier curve was used to evaluate the difference in the incidence of endpoint events at 12 months post-discharge (log-rank method was used to calculate P-value). All tests were 2-tailed tests, and the statistical significance was set at P < 0.05. In this study, SPSS 26.0 (IBM Software Inc.), EmpowerStats3.0, and R (version 3.3.2) were used for statistical analysis, and R (version 3.3.2), GraphPad Prism V8.0 (GraphPad Software Inc.), and PowerPoint 2019 (Microsoft Inc.) were used for mapping.
Results
Clinical Features of Patients According IMH
A total of 65 STEMI patients were enrolled, of whom 53 (81.5%) were male and 12 (18.5%) were female, with a mean age of 57.8 ± 10.5 years. Of these, 38 patients were in the IMH-absent group, and 27 (41.5%) were in the IMH-present group. The average age of the patients in the IMH-absent group was 58.3 ± 11.4 years, and 33 (86.8%) were male. Patients were examined with CMR 5.5 ± 2.3 days after presenting with AMI symptoms. Twenty-five (65.8%) patients had a history of smoking. Patients in the IMH-present group had a mean age of 57.1 ± 9.3 years, and 20 (74.1%) were male. Patients underwent CMR 5.4 ± 3.1 days after AMI symptoms, and 74.1% (20/27) of patients had a history of previous smoking. Demographic characteristics mentioned above, no significant difference was observed between the two groups.
The average systolic blood pressure (SBP) was 118.3 ± 19.1 mmHg and the average diastolic blood pressure (DBP) was 71.6 ± 11.5 mmHg in the IMH-absent group; The mean pulse was 81.1 ± 15.3 beats/min, and the patients in the IMH-present group had a mean SBP of 116.5 ± 21.2 mmHg, a mean DBP of 74.4 ± 15.6 mmHg, and a mean pulse of 79.1 ± 14.2 beats/min. In the IMH-absent group, 16 (42.1%) patients had comorbid hypertension, 10 (26.3%) had comorbid diabetes, and 7 (18.4%) had hyperlipidemia. In the IMH-present group, 14 (51.9%) patients had comorbid hypertension, 7 (25.9%) had comorbid diabetes, and 4 (14.8%) had hyperlipidemia. The remaining comorbidities are presented in Table 1. There were no statistically significant differences in patient comorbidities between the two groups.
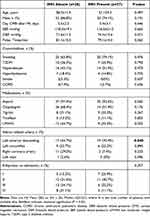 |
Table 1 Clinical Features of Patients According to IMH |
After admission, 37 (97.4%) patients in the IMH-absent group and 25 (92.6%) in the IMH-present group were prescribed aspirin antiplatelet aggregates, with no statistical difference between the two groups. See Table 1 for the other medications used. There was no statistical difference between the two groups in the use of drugs after admission or in the Killip class.
Cardiac Parameter Differences According to IMH
In the IMH-absent group, the culprit vessel in 17 (44.7%) patients was the anterior descending artery, the culprit vessel in nine (23.7%) patients was the circumflex artery, the culprit vessel in 11 (29.0%) patients was the right coronary artery, and the culprit vessel in one (2.6%) patient was the left main artery. In 19 (70.4%) patients in the IMH-present group, the culprit vessel was the circumflex artery, in 6 (22.2%) patients, the culprit vessel was the circumflex artery; and in 2 (7.4%) patients, the culprit vessel was the right coronary artery. The culprit vessel of the IMH-present was more frequently located in the anterior descending artery when the two groups were compared (P = 0.040). The routine blood examination results of the two groups are listed in Table 2, and there were no statistical differences in hemoglobin, white blood cell count, and platelet count between the two groups. The lipid profiles of the two groups of patients are shown in Table 2. Low-density lipoprotein cholesterol (LDL-C) was 2.4± 0.7 mmol/L in patients in the IMH-absent group and 2.6±0.8 mmol/l in patients in the IMH-present group, both of which were not statistically different (P = 0.273). Serum levels of C-reactive protein (CRP) were significantly greater in the IMH-present group than in the IMH-absent group (29.4 ± 32.9 mg/L vs 81.9 ± 68.5 mg/L, P = 0.010). Serum levels of CK-MB, a marker of myocardial injury, were also significantly higher in the IMH-present group than in the IMH-absent [161.6 (55.5–540.5) U/L vs 52.7 (25.8–303.0) U/L, P = 0.011] group. HsTNT levels were significantly higher in the IMH-present group than in the IMH-absent [2500.0 (1681.5–4307.0) pg/mL vs 1710.0 (203.0–3363.5) pg/mL, P = 0.021] group. NT-proBNP levels were not significantly different between the two groups [1901.0 (750.0–3233.5) pg vs 1618.0 (863.5–3066.8) pg/mL, P = 0.821]. Regarding the ECG of both groups of patients, all but 2 patients in the IMH-present group had sinus rhythm. There were no statistically significant differences in PR interval, QRS duration, or QTc duration between the two groups.
 |
Table 2 Laboratory Examination and Cardiac Parameters According to IMH |
The mean IMH volume in the IMH group was 5.6 ± 4.1 cm3. The IMH group had a significantly higher MI volume than the IMH-absent group (34.2 ± 12.7 cm3 vs 21.1 ± 13.1 cm3, P < 0.001), and LVEF was also significantly lower in the IMH group than the IMH-absent group (30.7 ± 9.8% vs 42.3 ± 11.0%, P < 0.001). Twelve (41.4%) patients in the IMH-absent group present with ventricular aneurysms, whereas 11 (44.0%) patients in the IMH-present group present with ventricular aneurysms, and there was no statistical difference between the two groups (P = 0.846). Undescribed narrative results are listed in Table 2.
IMH and Clinical Outcome
These patients were followed up for a median of 16 (11–17) months, and 16 patients experienced a MACE (death, n = 1; new congestive heart failure, n = 11); reinfarction, n = 4). The Kaplan–Meier curve was used to compare MACE-free survival in the IMH-present and IMH-absent groups. The rate of MACE at 12 months in the IMH-present group was significantly higher than in the IMH-absent group (9/27 VS 2/38, P = 0.012), as shown in Figure 1.
 |
Figure 1 Kaplan–Meier curve showing the risk of MACE according to the presence or absence of IMH. |
Compared with the MACE-absent group, the MACE-present group have fewer men (62.5% VS 87.8%, P = 0.05), with a higher CK-MB [342 (1112-624) u/L VS 56.0 (24–216) u/L, P = 0.005] and HsTNT levels [3600 (2489–6551) pg/mL VS 1700.0 (253.3–3076.0) pg/mL, P < 0.001]. As for LVEF, LVEF measured by echocardiographic (39.1 ± 10.7% VS 46.3 ± 11.9%, P < 0.001) and CMR (28.7 ± 8.8% VS 40.4 ± 11.4%, P = 0.002), and the MACE-present group was smaller than the MACE-absent group. The incidences of IMH (75% VS 30.6%, P = 0.02) and MVO (87.5% VS 49%, P = 0.07) were much higher in the MACE group than in the MACE-absent group. The remaining differences in clinical characteristics are presented in Table 3.
 |
Table 3 Patient Characteristics According to Major Adverse Cardiac Events |
We investigated the prognostic factors for MACE using univariate and multivariate Cox hazard analyses in our study, as presented in Table 4. This study reveals that not only MVO (HR 3.940, 95% CI 1.000–10.250, P = 0.049), but also IMH (HR 3.151, 95% CI 1.000–9.931, P = 0.039) are independent risk factors in patients with STEMI.
 |
Table 4 Univariate and Multivariate Cox Hazard Analyses of Predictors for MACE |
Discussion
AMI, a highly lethal acute and critical illness, has a significant reduction in mortality after the invention and promotion of reperfusion therapy, especially PCI, but still a proportion of patients, despite receiving timely and effective reperfusion therapy, the prognosis has not improved significantly, and cardiologists are considering that there may be other mechanisms involved.13 However, given the limitations of research methods, most are limited to animal and autopsy pathological studies, and some mechanistic questions regarding AMI still need to be explored. In recent decades, with the continuous development of noninvasive imaging devices, such as CMR and positron emission tomography (PET), let us understand more about AMI and reperfusion myocardial injury.14 Reperfusion myocardial injury often leads to coronary microcirculation disorders and the no-reflow phenomenon after PPCI in STEMI patients.15 IMH and MVO share microcirculatory damage as a common pathophysiological mechanism. Previous studies16 have been relatively clear on the adverse effects of MVO after reperfusion therapy in STEMI; however, the significance of IMH in STEMI patients is limited to poor prognostic factors, and the specific mechanism remains unclear. Previous studies17 suggested that IMH mainly causes chronic adverse remodelling of ventricular remodeling by iron-containing compounds deposited in the interstitium of cardiomyocytes, which further contributes to the worse prognosis of patients with STEMI.18 Bulluck et al11 demonstrated that iron deposition after IMH is closely related to persistent inflammation within the myocardium and adverse left ventricular remodeling. Behrouzi et al19 demonstrated that sustained deferiprone administration in a post-MI pig model reduced the inflammatory response and myocardial remodeling in pigs with MI combined with IMH. We speculate that IMH can directly cause persistent myocardial necrosis after reperfusion therapy in patients with STEMI; however, the available evidence is insufficient. Therefore, the present study aimed to answer this question by analyzing the clinical data of the patients, as well as the CMR records.
The present prospective study suggests that at a mean time of 5–7 days after reperfusion therapy, the volume of MI measured using the LGE sequence was larger in STEMI patients with IMH than in patients without IMH (Figure 2a). The difference in MI volume was more pronounced in the IMH group than in the MVO subgroup (Figure 2b). At this point, patients in the IMH-present group had a lower LVEF, whereas patients in the MVO-present group did not have a significantly lower LVEF than those in the MVO-absent group (Figure 3a–c). We believe that IMH caused further expansion of myocardial infarction. It is possible that the LGE-based measurement of the myocardial infarction volume and IMH volume was inaccurate in this study. Therefore, we consecutively collected daily hsTnT results during the patients’ hospitalization and depicted two sets of curves, as shown in Figure 2c. We found that the peak value and area under the curve of HsTnT in IMH-present group were larger than those in the IMH-absent group, and these two indexes can also suggest that the time and intensity of myocardial injury in IMH group were much larger than those in non IMH group. We also measured the volume of IMH and MI. Theoretically, IMH can expand the volume of myocardial infarction, and the volume of IMH should be related to the volume of MI, but we did not find a linear relationship between these two variables (Figure 2d). This may be because of the small sample size or the fact that the volume of IMH is not linearly related to the final volume of the patient’s MI, and we do not know how to interpret it.
The critical factors determining the volume of myocardial infarction are the size of the vascular bed or the amount of myocardium downstream from the culprit lesion, as well as the duration of ischemia in that territory.20 Therefore, the restoration of myocardial perfusion by opening the occluded vessel as soon as possible is key to reducing the volume of myocardial infarction in patients with STEMI. In a recent animal study, Liu et al21 demonstrated that IMH causes further expansion of the MI volume in a beagle dog model of MI. This landmark study, through CMR and PET examination of the heart in a beagle dog model, suggested that IMH could affect cardiomyocyte salvage in an animal model and let us refocus on this negligible phenomenon of IMH. Our study is complementary and confirmatory as it provides further evidence that IMH can result in larger infarcts in humans. In addition, we believe that this is the key factor for the worse prognosis of patients in the IMH group, rather than the chronic remodeling of the ventricles, as previously thought, due to iron deposition.
The present study has some limitations, the first of which is the small sample size. The sample size was small, mainly because enrollment in prospective studies is difficult, so even though we enrolled 65 patients over a 2-year period, although there were some significant differences, inferences to larger populations should be made with caution. Second, data on serial myocardial activity profiles of patients with STEMI were lacking in this study. If it is possible to perform a cardiac scan of a STEMI patient with IMH, it may be possible to fully confirm the conclusions of this study. Finally, it is ideal to use the LGE sequence to evaluate the MI volume. However, the time of CMR detection for each patient cannot be fixed after PCI, because the clinical patient status is different. This may have led to the difference between the myocardial infarction volume in this study and the actual myocardial infarction volume in the patient.
Conclusions
Using data on CMR and serum troponin levels, this prospective study demonstrated that IMH can lead to further expansion of MI volumes in patients with STEMI, resulting in a lower LVEF in patients who also had a higher MACE rate in the following post-discharge follow-up.
Data Sharing Statement
Data available on reasonable request from the authors.
Ethics Approval and Consent to Participate
The study protocol was performed in accordance with the ethical guidelines of the Declaration of Helsinki 27, and was approved by the Human Research Committee of the Second Xiangya Hospital of Central South University. Written informed consent was obtained from patients while the patient was in a clinically stable, non-congested condition or from their family members who could provide informed consent on behalf of the patients after they were informed about the objectives and procedures of the study. Their right to refuse participation at any time was assured.
Consent for Publication
All authors declare that the submitted work is original and has not been published before (either in English or any other language) and that the work is not under consideration for publication elsewhere.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Hunan Provincial Health Commission (grant number 202203013651).
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
1. Haeck JD, Koch KT, Bilodeau L, et al. Randomized comparison of primary percutaneous coronary intervention with combined proximal embolic protection and thrombus aspiration versus primary percutaneous coronary intervention alone in ST-segment elevation myocardial infarction: the PREPARE (PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation) study. JACC Cardiovasc Interv. 2009;2:934–943. doi:10.1016/j.jcin.2009.07.013
2. Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. doi:10.1136/heart.88.2.119
3. Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi:10.1016/j.jacc.2015.02.032
4. Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi:10.1152/physrev.00024.2007
5. Robbers LF, Eerenberg ES, Teunissen PF, et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–2353. doi:10.1093/eurheartj/eht100
6. Beijnink C, van der Hoeven NW, Konijnenberg L, et al. Cardiac MRI to visualize myocardial damage after ST-segment elevation myocardial infarction: a review of its histologic validation. Radiology. 2021;301:4–18. doi:10.1148/radiol.2021204265
7. Betgem RP, de Waard GA, Nijveldt R, et al. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol. 2015;12:156–167. doi:10.1038/nrcardio.2014.188
8. Amabile N, Jacquier A, Shuhab A, et al. Incidence, predictors, and prognostic value of intramyocardial hemorrhage lesions in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2012;79(7):1101–1108. doi:10.1002/ccd.23278
9. Reinstadler SJ, Stiermaier T, Reindl M, et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2019;20(2):138–146. doi:10.1093/ehjci/jey101
10. Husser O, Monmeneu JV, Sanchis J, et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol. 2013;167:2047–2054. doi:10.1016/j.ijcard.2012.05.055
11. Bulluck H, Rosmini S, Abdel-Gadir A, et al. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging. 2016;9. doi:10.1161/CIRCIMAGING.116.004940
12. World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. doi:10.1001/jama.1997.03540350075038
13. Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9:407–415. doi:10.4330/wjc.v9.i5.407
14. Garcia MJ, Kwong RY, Scherrer-Crosbie M, et al. State of the art: imaging for myocardial viability: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging. 2020;13:e53. doi:10.1161/HCI.0000000000000053
15. Reffelmann T, Kloner RA. The ”no-reflow” phenomenon: basic science and clinical correlates. Heart. 2002;87:162–168. doi:10.1136/heart.87.2.162
16. Ganame J, Messalli G, Dymarkowski S, et al. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–1449. doi:10.1093/eurheartj/ehp093
17. Cokic I, Kali A, Wang X, et al. Iron deposition following chronic myocardial infarction as a substrate for cardiac electrical anomalies: initial findings in a canine model. PLoS One. 2013;8:e73193.
18. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137:1949–1964. doi:10.1161/CIRCULATIONAHA.117.030693
19. Behrouzi B, Weyers JJ, Qi X, et al. Action of iron chelator on intramyocardial hemorrhage and cardiac remodeling following acute myocardial infarction. Basic Res Cardiol. 2020;115:24. doi:10.1007/s00395-020-0782-6
20. Reimer KA, Jennings RB. The ”wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Investigat. 1979;40:633–644.
21. Liu T, Howarth AG, Chen Y, et al. Intramyocardial hemorrhage and the ”wave front” of reperfusion injury compromising myocardial salvage. J Am Coll Cardiol. 2022;79:35–48. doi:10.1016/j.jacc.2021.10.034
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


