Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Intracerebral Hemorrhage Induced Brain Injury Is Mediated by the Interleukin-12 Receptor in Rats
Authors Yue X, Liu L, Yan H, Gui Y, Zhao J, Zhang P
Received 26 August 2019
Accepted for publication 11 January 2020
Published 1 April 2020 Volume 2020:16 Pages 891—900
DOI https://doi.org/10.2147/NDT.S228773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Xuejing Yue,1 Lixia Liu,1 Haiqing Yan,2 Yongkun Gui,2 Jun Zhao,2 Ping Zhang2
1School of Basic Medical Sciences, Xinxiang Medical University, Xinxiang 453003, Henan, People’s Republic of China; 2Department of Neurology, The First Affiliated Hospital of Xinxiang Medical University, Xinxiang 453100, People’s Republic of China
Correspondence: Ping Zhang
Department of Neurology, The First Affiliated Hospital of Xinxiang Medical University, Xinxiang 453100, People’s Republic of China
Tel +86-373-3029917
Email [email protected]
Background: IL-12 inhibition of the endothelial cell functions and angiogenesis is mediated by the cross-talk between the lymphocyte and the endothelial cells, which plays a key role in inhibiting the process of angiogenesis in the eyeballs and in malignant tumors.
Methods: We established the intracerebral hemorrhage (ICH) rat model, and IL-12 receptor beta monoclonal antibody was injected into the ICH rats. Western blot, immunofluorescence and RT-qPCR were used to detect the gene expression. Brain water content, EB staining, Garcia test, Beam walking test and wire hanging test were used to assess the injury of brain in ICH rats.
Results: IL-12 gene was significantly increase in hematoma border tissue of ICH rats, and IL-12 protein mainly localized in monocytes. Anti-IL-12 treatment with IL-12 monoclonal antibodies could not only significantly decrease the brain water content and EB content in brain tissues of ICH rats, but also significantly increase the score of the Garcia, Beam balance and the Wire hanging test in ICH rats. Moreover, anti-IL-12 treatment significantly decrease the expression of pro-inflammatory gene, inflammatory gene, p-JAK2/JAK2 and p-STAT4/STAT4 protein, but significantly increase the expression anti-inflammatory gene and CD31 protein, and M2 macrophage ratio in hematoma border tissues of ICH rats. In vitro, rmIL-12 inhibited the tube formation of brain microvascular endothelial cells (BMVES) in BMVES and bone marrow-derived monocytes (BMDM) co-culture systems, but not work in a separately cultured BMVES system. In addition, Fedratinib not only reduced p-JAK2/JAK2 and p-STAT4/STAT4 protein expression in BMDM after treating with b-FGF and rmIL-12, but also significantly increased the tube formation of BMVES in BMVES and BMDM co-culture systems after treating with b-FGF and rmIL-12.
Conclusion: Blockade of IL-12 receptor attenuated brain injury after ICH in rat by promoting angiogenesis, and the mechanism might be related to blocking IL-12 could inhibit M2 cell activation via the JAK2/STAT4 pathway.
Keywords: interleukin-12, intracerebral hemorrhage, vascular regeneration, macrophages
Introduction
Intracerebral hemorrhage (ICH) is a type of stroke with a high incidence of mortality and disability.1 Van Asch et al2 reported that between 1980 and 2008, the ICH incidence rate globally was 51.8/100,000/year in Asia, 24.2/51.8/100,000/year in caucasians, 22.9/51.8/100,000/year in blacks, 19.6/51.8/100,000/year in Spain; and it increases with age. The mortality rate after a month of detection of ICH was 40.4% and the lowest mortality rate is observed in the Japanese population who have a rate of 16.7%.2,3
ICH causes the blood to enter the brain parenchyma via the ruptured blood vessels, that in turn causes ischemia, inflammation, edema, cytotoxicity, etc., to result in a series of pathological changes in the neurons, such as hematoma formation, enlargement and ischemia and hypoxia and edema of surrounding brain tissue.4,5 Recent studies have reported that one-third of the patients with ICH experience progressive deterioration of their central nervous system after a period of time following onset,6 suggesting that in addition to the acute neuronal damage caused by hematoma, there is secondary damage to the surrounding tissues of the hematoma.7 The area in the tissue lesion and in which edema forms around the hematoma following cerebral hemorrhage is called the penumbra or penumbra of the hematoma,8,9 and studies have reported that the formation of neovascularization in the penumbra is induced by the reperfusion and oxygen supply within time to reduce the process of neuronal apoptosis and necrosis, which are key factors affecting the prognosis of ICH.8,9
Interleukin-12 (IL-12) formed by the combination of two subunits, p35 and p40, via disulfide bonds10 is a key pro-inflammatory factor in the cellular immune response process, mainly secreted by the dendritic cells and macrophages in the antigen-presenting cells and B lymphoblasts, upon stimulation by antigen,11,12 that in turn activate the NK and T cells to regulate inflammation.13 Previous studies have shown that the IL-12 inhibition of the endothelial cell functions and angiogenesis is mediated by the cross-talk between the lymphocyte and the endothelial cells,14 which plays a key role in inhibiting the process of angiogenesis in the eyeballs15,16 and in malignant tumors.17,18 However, the expression of IL-12 in penumbra of the hematoma and its function are not yet known. In this study, we observed that blockade of the interleukin-12 receptor attenuated the brain injury induced by the intracerebral hemorrhage in rat and IL-12 inhibits the process of angiogenesis in the hematoma border zone via the JAK2/STAT4 pathway. Thus, blocking the interleukin-12 receptor in the ICH patients is a potential treatment strategy for ICH.
Materials and Methods
Animal and ICH Models
A total of 80 Normal Sprague-Dawley rats (300–350 g, 15- 16-weeks-old, male: female = 1:1) were purchased from the First Affiliated Hospital of Xinxiang Medical University and fed by the animal center (free-feeding, 12h each day and night, 22±2°C). The rats were first divided into 2 groups according to gender, and then grouped according to the random number table method, and each group of rats had the same sex ratio. This study was carried out in strict accordance with the recommendations in the Guide for the International Cooperation Committee of Animal Welfare (ICCAW), which is responsible for animal care and use in China. All animal experiments are approved and regulated by the Animal Ethics Committee in the First Affiliated Hospital of Xinxiang Medical University.
ICH model: 50 μL of whole blood was drawn from the femoral artery of the rat into the ipsilateral striatum, inserted into the striatum through a 26 G diameter needle, the depth of the skull was 5.8 mm or less, and the blood was manually injected for more than 10 min.
Sham group: 50 μL of whole blood was drawn from the femoral artery of the rat, but no more after that.
Anti-IL-12 treatment: ICH rats in anti-IL-12 group were injected with 1 mg/kg IL-12 R beta (MAB8650, R&D Systems, USA) monoclonal antibody by tail vein.19
Injection procedure: Inject the IL-12 R beta antibody immediately after the ICH model is established, and then wait 6 hrs and 12 hrs to re-inject the IL-12 R beta antibody. After 24 hrs of ICH, we performed an antibody injection once a day until the rats were sacrificed.
Western Blot
Western blot was used to detect the protein expression in tissues and cell lysates as previously described.20,21 The primary antibody were incubated in 4°C overnight and the primary antibody were anti-IL-12 p40 antibody (1:1000, ab77373, abcam, UK), anti-JAK2 antibody (1:500, ab39636, abcam, UK), anti-JAK2 (phospho Y1007) antibody (1:500, ab195055, abcam, UK), anti-STAT4 antibody (1:2000, ab235946, abcam, UK) and anti-STAT4 (phospho Y693) antibody (1:500, ab28815, abcam, UK). The second antibody was incubated for 1 hr at room temperature. BeyoECL Plus kit (P0018S, Beyotime, Shanghai, China) was used to chromogenic and densitometry protein bands with Beckman Coulter Immunoassay System (UniCel DxI 800, Beckman, CA, USA).
Real-Time Fluorescence Quantitative Polymerase Chain Reaction
mRNA expression levels were detected by Real-time fluorescence Quantitative Polymerase Chain Reaction (RT-qPCR) as previously described.20,21 Total RNA was extract with RNAiso Plus (D9108A, taraka, Japan) and cDNA was synthesized with cDNA reverse transcription kit (6210A, taraka, Japan) in PCR amplifier (S-1000, BIO-RAD). And GoTaq® qPCR Master Mix (A6002, Promega, USA) was used to detect the mRNA expression by fluorescence quantitative polymerase chain instrument (FQD-96A, Shanghai Bori Technology Co., Ltd., China). The primers are shown in Table 1.
 |
Table 1 Primers for RT-qPCR |
Flow Cytometry
At 7 days after ICH, we sacrificed the rats to separate the hematoma border tissue of brain, and preparation of single cell suspension and antibody incubation as previously described.22 Antibodies (Percp-Cy5.5-CD45, FITC-CD31, FITC-CD3 and PE-CD11b) were all purchase from BD biosciences and incubated on 4°C for 30 mins.
Immunofluorescence
The brain tissue sections were fixed with 4% paraformaldehyde, 0.2% TritonX-100 was ruptured; Rabbit serum and rat serum were incubated for 1 hr at room temperature, and washed with PBS for 3 times; The primary antibody was incubated overnight at 4 degrees and the secondary antibody was incubated for 1 hr at room temperature. Primary antibodies: Anti-CD11b antibody (1:200, ab8878, abcam, UK) and anti-IL-12 p40 antibody (1:2000, ab77373, abcam, UK); Secondary antibodies: TRITC-conjugated goat-rabbit IgG (C5872, Jackson Immunoresearch, USA), Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (A11008, Invitrogen, USA). At last, 5 ug/mL DAPI (D8417, SIGMA, USA) at room temperature for 5 mins.
Brain Injury Assessment
Brain Water Content
The rats were sacrificed to obtain brain tissue, and the weight (A) was weighed and then dried in a 100°C oven for 24 h until the weight was no longer reduced and weighed (B). Brain water content = (A-B)/A.
EB Staining
The extent of blood-brain barrier damage was monitored by EB staining. One hour before being sacrificed, 2% of EB was injected through the tail vein. After 1 hr of circulation, when the heart was perfused with pre-cooled saline until the clear flow of the right atrium, the brain was quickly decapitated, the wet mass was weighed with an electronic balance, and the rat brain was placed in a 3 mL formamide solution. Centrifuge in a 45 °C water bath for 24 h, 10,000 r/min for 10 min, take the supernatant, and measure the A value (wavelength 620 nm) with a multi-function microplate reader. The sample EB content (μg·mL −1) was calculated from the EB solution standard curve. Brain tissue EB content (μg·g-1) = EB content of the sample to be tested (μg.mL-1) × Formamide capacity (mL)/brain wet weight (g).
Garcia Test
The Garcia neurofunctionality test method reference is Chen et al.23 The score of normal rats in the Garcia neurofunctionality test is 18.
Beam Walking Test
The Beam walking test method reference is Manaenko et al.24 The score of normal rats in the Beam walking test is 5.
Wire Hanging Test
The wire hanging test method reference is Manaenko et al.25 The score of normal rats in the Garcia neurofunctionality test is 5.
Tube Formation
We isolated and cultured rat BMVES26 and BMDM27 as previously described. The Matrigel (354230, BD biosciences, USA) was melted in advance and placed in the lower hole of the ibidi angiogenic slide (81506, IBIDI, German). After gelation, the cell suspension was added to the upper hole of the angiogenic slide.
The independent culture system: 20,000 BMVES/50 μL was added to the upper hole of the angiogenic slide. Co-culture system: 20,000 BMVES/30 μL and 10,000 BMDM/20 μL was added to the upper hole of the angiogenic slide. For cell culture medium, we selectively added 50 ng/mL b-FGF, 0.02 ng/mL rmIL-12 (SRP4163, MERCK, German) and 2 nM Fedratinib (HY-10409, MCE, USA).
The inoculated cells were incubated in an incubator (37°C+5% CO2) for 6 hrs and then washed twice with PBS. 5 μM of calcein (17783, sigma, USA) was added to the wells, and incubated at 37 °C for 30 mins, and then washed 3 times with PBS. Observed under a Fluorescence microscope (FCK-50C, CAIKON, China). We quantified the total length of the image tube using angioquant v1.33 software (Mathworks, USA).
Statistical Analysis
The data in this study was analyzed by Statistical Product and Service Solutions 20.0 (IBM, USA), and the data was presented as (Mean ± Standard Deviation). Data between the two groups were compared by student’s t test, and multiple groups were compared with one-way ANOVA that duncan test as post hoc test. And P<0.05 means significant difference.
Results
IL-12 Is Highly Expressed After ICH
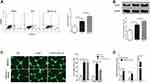
At different times after ICH, we sacrificed the rats to separate the hematoma border tissue and measured the IL-12 gene expression. As shown in Figure 1, The expression of IL-12 gene increased after ICH and peaked at 24 hrs after ICH, and then gradually decreased. The expression of IL-12 gene gradually stabilized after 7 days of ICH, but it was still significantly higher than normal. In addition, we also found that IL-12 p40 mRNA expression was highest in CD45+CD31+ cells in hematoma border zone at 7 days after ICH (Figure 2A), and immunofluorescence showed that CD31+ and IL-12 p40 protein expressed in the same cell in hematoma border zone at 7 days after ICH (Figure 2B).
IL-12 Blockade Attenuates Brain Damage After ICH
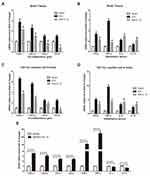
As shown in Figure 3A and B, at 7 days after ICH, although there was no significant difference in brain water content and EB content in contralateral brain tissue between rats in difference group, brain water content and EB content in ICH group was significantly higher than that in sham group, and anti-IL-12 treatment could significantly reduce brain water content and EB content in ICH rats. Moreover, the score of Garcia, Beam balance and the Wire hanging test in rats of ICH group was significantly lower than in the sham group, and anti-IL-12 treatment could significantly increase the score of Garcia, Beam balance and Wire hanging test in ICH rats (Figure 3C).
IL-12 Blockade Promotes Angiogenesis After ICH
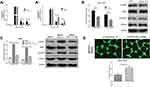
At 7 days after ICH, we analyzed the cell types in the hematoma border zone and found that (Figure 4A) the ratio of CD45-CD31+ cells in ICH group was significantly higher than that in sham group, but significantly lower than that in Anti-IL-12 group. And Western blot also showed that CD31 protein expression in ICH group was significantly higher than that in sham group, but also significantly lower than that in Anti-IL-12 group (Figure 4B). In vitro, we found there was no significant difference in the tube length between b-FGF group and b-FGF+rmIL-12 group in a separately cultured BMVES system, but the tube length in b-FGF+rmIL-12 group was significantly shorter than that in b-FGF group in the BMVES and BMDM co-culture systems (Figure 4C). Furthermore, rmIL-12 treatment could significantly increase Thbs1 and Arg1 mRNA expression but decrease Ang mRNA expression (Figure 4D).
IL-12 Blockade Activates M2 Macrophages and Inhibits JAK2/STAT4 Pathway After ICH
As shown in Figure 5A–D, we found out that whether in the hematoma border tissue or in CD11b+ cells derived from hematoma border tissue at 7 days after ICH, the expression levels of pro-inflammatory factor gene and inflammatory factor gene in ICH rats were significantly higher than those in Sham group, but anti-IL-12 treatment could significantly decrease them. We also found that rmIL-12 not only induced high expression of pro-inflammatory genes (CXCL1, CXCL2, CCL4 and CCL5) and inflammatory genes (IFN-γ and TNF-α) in BMDM cells in vitro, but also induced low expression of anti-inflammatory genes (IL-4 and IL-13) in BMDM cells in vitro (Figure 5E).
At 7 days and 14 days after ICH, compared with the ICH rats without treatment, the proportion of iNOS+ cells in CD45+Gr-1-CD11b+ macrophages in hematoma border zone was significantly decrease in ICH rats with anti-IL-12 treatment (Figure 6Aa), and the proportion of Arginase1+ cells in CD45+Gr-1-CD11b+ macrophages in hematoma border zone was significantly increase (Figure 6Ab). In addition, we also found that the expression of p-JAK2/JAK2 and p-STAT4/STAT4 protein in the hematoma border tissue of ICH rats with anti-IL-12 treatment was significantly lower than that in ICH rats (Figure 6B). Similarly, rmIL-12 induced p-JAK2/JAK2 and p-STAT4/STAT4 protein expression significantly increase, and Fed, a JAK2 inhibitor, could significantly decrease the p-JAK2/JAK2 and p-STAT4/STAT4 protein expression in BMDM after treatment with rmIL-12 (Figure 6C). More importantly was Fed also significantly increase the tube length in BMVES and BMDM co-culture systems after treating with b-FGF and rmIL-12 (Figure 6D).
Discussion
We observed that the IL-12 is highly expressed in the hematoma border region of the ICH model rats and blocking its receptor effectively attenuates the ICH induced brain damage in rats. The ICH damages the brain by the following two mechanisms:1 1) Primary brain damage, referred to as the physical damage of the brain tissues caused by the ICH induced clotting of blood in the brain. Following the onset of ICH, there is an enlargement of the hematoma which results in increased intracranial pressure, followed by compression of the brain tissues-related leading to the presentation of the cerebral blood flow disorder (cerebral ischemia), with the eventual incidence of cerebral palsy; and 2) Secondary brain damage is referred to the primary brain damage triggered cascade, associated with the physiological response of the hematoma and the release of the coagulation components. Thus, hematomas play a critical role in both primary and secondary brain injury in ICH as evident from these resultant effects:28,29 1) Hematoma occupying oppression caused microcirculatory disorders. Cerebral hemorrhage is followed immediately by a decrease of the regional cerebral blood flow (rCBF) due to the compression of the hematoma, which causes incidence of cerebral ischemic injury; and 2) Cerebral blood flow can automatically adjust the disorder but the incidence of brain edema can cause dysfunction of the cerebral vascular autoregulation. Therefore, recovery of rCBF in areas of cerebral ischemia is critical for the patients to recover from cerebral hemorrhage.
In the current study, we observed that blocking the IL-12 receptor attenuated the ICH associated brain damage and increased the expression of the CD31 protein in the hematoma region of rats with cerebral hemorrhage. CD31, also known as Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), is a biomarker of the endothelial cells and angiogenesis.30 It is known that neovascularization restores blood and oxygen supply to the tissues to attenuate the hypoxic-ischemic induced injury and promote repair of the tissues, which reverses the apoptosis events in the neurons, glial and endothelial cells.9 From the current study results, we also observed that blocking the IL-12 receptors promoted angiogenesis in the hematoma areas of rats with ICH, which can be used to attenuate the ICH associated brain damage in rats. Our data is supported by the previous work of Morini et al,16 who found that transfection of the gene IL-12 plasmid significantly prevented the growth and vascularization of the highly angiogenic KS-Imm Kaposi’s sarcoma and TS/A murine mammary carcinoma tumors in nude mice. Further, Wigginton et al31 reported that IL-12 effectively inhibited the process of angiogenesis in malignant tumor tissues. Recent years have also provided more information on the role of IL-12 as an anti-angiogenic factor in animal models, for example, Kan et al, reported that knocking out IL-12, which is highly expressed in the heart tissue from the heart-damaged rats, facilitated p35 to repair the damaged heart tissue by promoting the process of angiogenesis.32
Previously, it has been shown that the IL-12, which is mainly produced by the dendritic cells (DCs) and macrophages, promotes the immune function of the T helper1 (Th1) cells by inducing the expression of IFN-γ, and plays a role in promoting mitosis of the T cells, and its anti-angiogenic factor is associated with the T cells and macrophages.14,33 In the current study, we observed that IL-12 is mainly localized in the monocytes; further rmIL-12 did not affect angiogenesis of the BMVES cells in vitro, but significantly inhibited its angiogenesis in the BMVES and BMDM co-culture systems. This suggests that rmIL-12 inhibits the process of angiogenesis via the BMDM pathway and does not directly act on BMVES. In addition, we observed that rmIL-12 induces the in vitro expression of Thbs1 and Arg1 and inhibition of angiopoietin, which promotes angiogenesis.34 Thbs1, a cellular matrix protein, is known to negatively regulate the angiogenic function by regulating the survival and migration of the endothelial cells by modulating the vascular endothelial growth factor.35,36 Arg1 is an intracellular enzyme that inhibits the process of angiogenesis and endothelial cell function by modulating the activity of the nitric oxide synthase.37 Further studies have shown that blocking the IL-12 receptor not only promotes the expression of pro-inflammatory gene in the hematoma region and hematoma region CD11b+ cells, but also affects the expression of IFN-γ, TNF-α, IL-4 and IL-13. In addition, our study also found that blocking the IL-12 receptor significantly reduced the proportion of the M1 macrophages and increased the proportion of the M2 macrophages in the hematoma area of rats with ICH. Th cells are of two types, viz., the Th1 cells and Th2 cells. The Th1 cells are mainly driven by IL-12 of the macrophages, which induces signaling of the IFN-γ and TNF-α cytokines, while the IL-4 effects the Th2 cells function via the signaling associated with the IL-4, IL-5 and IL-13 cytokines. Previously, it has been shown that the cytokine-mediated Janus kinase/signaling and transcriptional activator (JAK/STAT) signaling pathways play a critical role in the differentiation of the Th1 and Th2 cells, and IL-12 is known to mediate the JAK2/STAT4 pathway. We observed in the current study that blocking the IL-12 receptor significantly reduced the phosphorylated levels of JAK2/JAK2 and STAT4/STAT4 proteins in the hematoma region of rats with cerebral hemorrhage, and inhibition of JAK2/STAT4 with rmIL-12promoted angiogenesis. The results safely suggests that blocking the IL-12 receptor reduces the IL-12-induced Th1 cell differentiation process.
Conclusion
IL-12 highly expressed in the hematoma area of ICH rats, induces the differentiation of Th1 through the JAK2/STAT4 pathway to inhibit the process of angiogenesis, while blockade of the IL-12 receptor attenuate the ICH associated brain injury in rat by promoting angiogenesis.
funding
This research was supported by the funds of Xinxiang Medical University doctoral research start-up funding project (XYBSKYZZ201725) and the National Natural Science Foundation of China (81471349).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi:10.1016/S1474-4422(12)70104-7
2. van Asch CJ, Luitse MJ, Rinkel GJ, Van D, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi:10.1016/S1474-4422(09)70340-0
3. Inagawa T. Risk factors for primary intracerebral hemorrhage in patients in Izumo City, Japan. Neurosurg Rev. 2007;30(3):225–234. doi:10.1007/s10143-007-0082-8
4. Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke. 2001;32(12):2932–2938. doi:10.1161/hs1201.099820
5. Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2017;S0028390817304501.
6. Xue M, Del Bigio MR, Muizelaar JP. Intracortical hemorrhage injury in rats: relationship between blood fractions and brain cell death editorial comment: relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721. doi:10.1161/01.STR.31.7.1721
7. Orito K, Hirohata M, Nakamura Y, et al. Leakage sign for primary intracerebral hemorrhage: a novel predictor of hematoma growth. Stroke. 2016;47(4):958–963. doi:10.1161/STROKEAHA.115.011578
8. Mayer SA, Lignelli A, Fink ME, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage. Stroke. 1998;29(9):1791. doi:10.1161/01.STR.29.9.1791
9. Emanueli C, Madeddu P. Angiogenesis gene therapy to rescue ischaemic tissues: achievements and future directions. Br J Pharmacol. 2001;133(7):951–958. doi:10.1038/sj.bjp.0704155
10. Richard C, Lorraine ON, MG M, et al. Interleukin 12 and interleukin 23 play key pathogenic roles in inflammatory and proliferative pathways in giant cell arteritis. Ann Rheum Dis. 2018;77(12):1815–1824.
11. Zheng H, Ban Y, Wei F, Ma X. Regulation of interleukin-12 production in antigen-presenting cells. Oxygen Transport to Tissue XXXIII. 2001;79(1):117–138.
12. O’Shea JJ, Paul WE. Regulation of THI differentiation |[ndash]| controlling the controllers. Nat Immunol. 2002;3(6):506. doi:10.1038/ni0602-506
13. Zhu H, Li J, Wang S, Liu K, Wang L, Huang L. Hmgb1-TLR4-IL-23-IL-17A axis promote ischemia-reperfusion injury in a cardiac transplantation model. Transplantation. 2013;95(12):1448–1454. doi:10.1097/TP.0b013e318293b7e1
14. Strasly M, Cavallo F, Geuna M, et al. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. 2001;166(6):3890. doi:10.4049/jimmunol.166.6.3890
15. Lee S, Zheng M, Deshpande S, Eo SK, Hamilton TA, Rouse BT. IL-12 suppresses the expression of ocular immunoinflammatory lesions by effects on angiogenesis. J Leukoc Biol. 2002;71(3):469.
16. Morini M, Albini A, Lorusso G, et al. Prevention of angiogenesis by naked DNA IL-12 gene transfer: angioprevention by immunogene therapy. Gene Ther. 2004;11(3):284–291. doi:10.1038/sj.gt.3302175
17. Angiolillo AL, Sgadari C, Tosato G. A role for the interferon-inducible protein 10 in inhibition of angiogenesis by interleukin-12. Ann N Y Acad Sci. 2010;795(1):158–167. doi:10.1111/j.1749-6632.1996.tb52664.x
18. Passer BJ, Cheema T, Wu S, Wu C-L, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20(1):17–24. doi:10.1038/cgt.2012.75
19. !!! INVALID CITATION !!!.
20. Bai J, Yao X, Jiang L, et al. Taurine protects against As2O3-induced autophagy in livers of rat offsprings through PPARγ pathway. Sci Rep. 2016;6:27733. doi:10.1038/srep27733
21. Wang S, Yang Z, Bo Y, et al. C1q/tumor necrosis factor-related protein-3 attenuates brain injury after intracerebral hemorrhage via AMPK-dependent pathway in rat. Front Cell Neurosci. 2016;10. doi:10.3389/fncel.2016.00237
22. Bao C, Wang B, Yang F, Pang J, Jiang Y, Chen L. Blockade of interleukin-7 receptor shapes macrophage alternative activation and promotes functional recovery after spinal cord injury. Neuroscience. 2017;371:518–527.
23. Chen S, Ma Q, Krafft PR, et al. P2X7 receptor antagonism inhibits p38 mitogen-activated protein kinase activation and ameliorates neuronal apoptosis after subarachnoid hemorrhage in rats. Crit Care Med. 2013;41(12):E466–E474. doi:10.1097/CCM.0b013e31829a8246
24. Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J. Hydrogen inhalation ameliorated mast cell–mediated brain injury after intracerebral hemorrhage in mice. Crit Care Med. 2013;41(5):1266–1275. doi:10.1097/CCM.0b013e31827711c9
25. Manaenko A, Fathali N, Chen H, et al. Heat shock protein 70 upregulation by geldanamycin reduces brain injury in a mouse model of intracerebral hemorrhage. Neurochem Int. 2010;57(7):844–850. doi:10.1016/j.neuint.2010.09.001
26. Parkinson FE, Hacking C. Pericyte abundance affects sucrose permeability in cultures of rat brain microvascular endothelial cells. Brain Res. 2005;1049(1):8–14. doi:10.1016/j.brainres.2005.04.054
27. Su S, Jiang L. Isolation of mouse bone marrow-derived monocytes; 2015.
28. Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86(2):272. doi:10.3171/jns.1997.86.2.0272
29. Peeling J, Yan H-J, Corbett D, Xue M, Bigio MRD. Effect of FK-506 on inflammation and behavioral outcome following intracerebral hemorrhage in rat. Exp Neurol. 2001;167(2):341–347. doi:10.1006/exnr.2000.7564
30. Maharani A, Aoshima K, Onishi S, Gulay KCM, Kobayashi A, Kimura T. Cellular atypia is negatively correlated with immunohistochemical reactivity of CD31 and vWF expression levels in canine hemangiosarcoma. J Vet Med Sci. 2018;80.
31. Wigginton JM, Park JW, Taub D, Brunda MJ, Strieter R, Wiltrout RH. The role of t cells, apoptosis and inhibition of angiogenesis in the antitumor activity of IL-12/IL-2. J Immunother. 1997;20(5):406.
32. Kan X, Wu Y, Ma Y, Zhang C, Du J. Deficiency of IL-12p35 improves cardiac repair after myocardial infarction by promoting angiogenesis. Cardiovasc Res. 2015;109(2):249. doi:10.1093/cvr/cvv255
33. Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87(9):3877. doi:10.1182/blood.V87.9.3877.bloodjournal8793877
34. Bilimoria J, Singh H. The effect of interleukin 1 beta on vascular angiopoietin 1 signalling. Heart. 2017;103(Suppl5):
35. Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285(50):38923–38932. doi:10.1074/jbc.M110.172304
36. Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122(10):1822–1832. doi:10.1182/blood-2013-01-482315
37. Chen M, Zhao J, Ali IH, et al. SOCS3 deficiency in myeloid cells promotes retinal degeneration and angiogenesis through arginase-1 up-regulation in experimental autoimmune uveoretinitis. Am J Pathol. 2018;188(4):1007–1020. doi:10.1016/j.ajpath.2017.12.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.