Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Intraarterial Lidocaine Administration for Pain Control by Water-in-Oil Technique in Transarterial Chemoembolization: in vivo and Randomized Clinical Trial
Authors Wang LZ, Hu XX, Shen XC, Wang TC , Zhou S
Received 5 August 2021
Accepted for publication 23 September 2021
Published 5 October 2021 Volume 2021:8 Pages 1221—1232
DOI https://doi.org/10.2147/JHC.S331779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Ahmed Kaseb
Li-Zhou Wang,1,* Xiao-Xia Hu,2,* Xiang-Chun Shen,2,3 Tian-Cheng Wang,4 Shi Zhou1
1Department of Interventional Radiology, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 2The State Laboratory of Functions and Application of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 3School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 4Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tian-Cheng Wang
Department of Radiology, The Second Xiangya Hospital of Central South University, Changsha, Hunan, 410011, People’s Republic of China
Tel +86 15073190153
Email [email protected]
Shi Zhou
Department of Interventional Radiology, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, 550004, People’s Republic of China
Tel +86 13809477708
Email [email protected]
Objective: To investigate the sustained release of lidocaine from a lidocaine–epirubicin–lipiodol emulsion created by water-in-oil (W/O) technique in vivo and evaluate the efficacy and safety of intraarterial lidocaine administration for intra- and postoperative pain control in transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC).
Methods: The in vivo concentrations of lidocaine were determined in tumor tissues after VX2 rabbit models for hepatic tumor were administered with intra-arterial lidocaine–epirubicin–lipiodol emulsion. A prospective randomized controlled clinical trial was performed, enrolling 70 consecutive patients who underwent TACE. Patients were randomized into two groups: Group A received an immediate bolus intraarterial lidocaine injection before TACE, and Group B received a lidocaine–epirubicin–lipiodol emulsion during TACE. Pain intensity was compared between the two groups using a visual analog scale (VAS) score before (Tbefore) and at 0 h (T0), 4 h (T4), 8 h (T8), 24 h (T24), 48 h (T48), and 72 h (T72) after the procedure. Adverse events and intake of analgesics were evaluated and compared between the two groups.
Results: The concentrations of lidocaine in tumor tissues were higher in experimental group than in control group at T0.5 (P=0.004), T1 (P=0.038), T4 (P=0.036), and T8 (P=0.029). In the clinical trial, VAS scores in Group B were significantly lower than in Group A at T0 (P=0.006), T4 (P=0.001), T8 (P=0.002), and T24 (P=0.005). The tramadol intake in Group B was significantly lower than in Group A (P=0.021). No significant difference was observed regarding the incidence of adverse events between the two groups.
Conclusion: This study demonstrated the effectiveness and safety of intraarterial lidocaine administration using the W/O technique in controlling intra- and post-TACE pain.
Keywords: carcinoma, hepatocellular, chemoembolization, therapeutic, lidocaine hydrochloride, pain management
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer with high cancer-related mortality.1–3 The standard therapy for intermediate-stage HCC is transarterial chemoembolization (TACE), and it has been proven to improve patient survival compared to the best supportive care method.4–9 However, TACE is associated with a high rate of side effects,10 among which pain is the most common clinical symptom and is frequently associated with prolonged hospitalization and poor compliance for repeat TACE.11,12 To date, there are various treatment modalities for controlling intra- and post-TACE pain, including nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids.13 However, both NSAIDs and opioids are limited by their shortcomings, such as common drug-related side effects and addiction in clinical practice.14
Lidocaine is a commonly used local anesthetic and is highly safe.15 Interestingly, intraarterial bolus injection of 100 mg lidocaine before chemoembolization has been documented to efficiently and safely alleviate intra-TACE pain.16 However, the effect of an intraarterial bolus injection of lidocaine is transient because it can rapidly enter circulation. Lipiodol plays a crucial role in TACE and has always been used as a carrier of chemotherapy drugs to achieve efficient delivery and sustained release of drugs.17 Based on this theory, we hypothesized that efficient transportation and sustained release of lidocaine could be achieved by using a water-in-oil (W/O) method for intraarterial lidocaine administration. Achieving sustained release of lidocaine may prolong the clinical effectiveness of lidocaine and decrease the intake of opioid analgesics.
In the present study, we investigated the sustained-release of lidocaine by the W/O technique in vivo and evaluated the efficacy and safety of the intraarterial lidocaine administration using a water-in-oil (W/O) method for the management of intra- and post-TACE pain.
Methods
TACE Procedure in vivo
All experimental procedures involving animals were approved by the Institutional Animal Ethics Committee of Guizhou Medical University and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The rabbit VX2 hepatocellular carcinoma model was established as follows:
(1) VX2 tumor cells (GuangZhou Jennio Biotech Co., Ltd, China) were implanted into the femoribus internus subcutaneous of the rabbits for proliferation. After 2 weeks, an auxetic VX2 tumor (almost 2 cm in diameter) was separated in sterile conditions and made into 1×1×1 mm3 pieces of tumor tissue. The pieces were stored in physiological saline.
(2) The rabbits underwent surgery under the guidance of ultrasound, and the left hepatic lobe was selected as an area for the microinjection of VX2 tissue pieces. Subsequently, three pieces of VX2 tissue were implanted in the candidate hepatic area, and the puncture site was covered with a gelatin sponge (Figure 1).
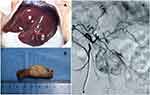 |
Figure 1 VX2 tumor tissue is implanted in the candidate hepatic area (A). The size of the tumor is approximately 2 cm (B). The hepatic arteriography of the tumor (C). |
(3) All the rabbits were administered intramuscular injections of penicillin for 3 days after the implantation of VX2 tumors. The success of VX2 tumor implantation was confirmed by ultrasound after 2 weeks.
Two weeks after successive VX2 tumor implantation, the rabbit VX2 HCC models were treated under general anesthesia, and the arteria cruralis of each rabbit was bluntly dissected. Subsequently, a 2.1-Fr microcatheter was placed into the hepatic tumor-feeding artery from the arteria cruralis under digital subtraction angiography (DSA) (Figure 1). After catheterization, the TACE-control group received intraarterial lidocaine (Hebei Tiancheng Pharmaceutical Co., Ltd, China) followed by an injection of the mixture of epirubicin (Shandong New Time Pharmaceutical Co., LTD, China) and Lipiodol (approximately 3 mL). The dosage of lidocaine and epirubicin was 20 mg and 10 mg, respectively. The TACE-experimental group received the lidocaine–epirubicin–lipiodol emulsion created using the W/O technique (approximately 3 mL) with 20 mg and 10 mg lidocaine and epirubicin, respectively.
Measurement of Lidocaine Concentrations in Tumor Tissues and Peripheral Blood
Tumor samples and peripheral blood samples were collected at 0.5 (T0.5), 1 (T1), 4 (T4), 8 (T8) and 24 (T24) hours after TACE in three rabbits in both the TACE-control group and TACE-experimental group. The VX2 tumor tissues were harvested, weighed, and then homogenized by adding 0.9% saline solution. The collected samples were centrifugated at 12,000 rpm for 10 min, and after centrifugation, the supernatant samples were frozen at 4°C. The supernatant samples and peripheral blood samples were measured by HPLC (High-Performance Liquid Chromatography).
Randomized Clinical Trial
A randomized controlled trial was performed (registered at www.chictr.org.cn with the registry Number of ChiCTR1800019271, registered at 02-November-2018). This prospective study was approved by the institutional review board of the Second Xiangya Hospital of Central South University and was conducted according to the Declaration of Helsinki. Patients who underwent TACE from November 3, 2018, to May 1, 2020, were enrolled. All patients signed informed consent. The details of the clinical trial process, including the pros and cons of the interventions, were explained by physicians. Eligible patients were randomly assigned to Group A and Group B in a 1:1 ratio. Patients in Group A received an intraarterial bolus injection of lidocaine immediately before the injection of epirubicin-Lipiodol emulsion (the ratio of epirubicin solution to Lipiodol was 1:2). Patients in Group B received intraarterial lidocaine-epirubicin mixture mixed with Lipiodol using a three-way stopcock (the ratio of lidocaine-epirubicin mixture to Lipiodol was 1:2). The formation of the lidocaine–epirubicin–lipiodol emulsion is shown in Figure 2. The dosage of lidocaine administered in both groups was 100 mg. A statistician generated a random assignment via a computer-generated random number. Interventional radiologists, nurses, and patients were blinded to the study design. Only the pharmacist who prepared the medication for patients was aware of the study design; however, he was not allowed to communicate with other personnel involved in the study.
Patient Eligibility
Inclusion Criteria
- Patients who were diagnosed with HCC via one of the following methods: (a) imaging features of lesions on computed tomography (CT) or magnetic resonance imaging (MRI) complying with the 2018 version of LI-RADS or (b) histopathologically confirmed HCC lesions.
- Eastern Cooperative Oncology Group (ECOG) performance score of 0–1;
- International normalized ratio <1.5 and platelet count >50*109;
- Compensated liver function (Child-Pugh class A or B);
- No refractory ascites or renal failure;
Exclusion Criteria
- Unsuitable psychosocial condition;
- Main portal venous tumor thrombus (PVTT);
- Presence of comorbidities such as cardiovascular disease;
- Periodic administration of NSAIDs or steroids;
- Age <18 years;
- The expected dose of Lipiodol used was less than 10 mL.
Dropout Criteria
- Death during the study;
- Patients who were lost to follow-up;
- Patients who were required to stop treatment or withdrew written informed consent.
Withdrawal Criteria
- Patients found not to conform to inclusion criteria after enrollment;
- Poorly compliant patients who could not cooperate with physicians to complete the study.
Sample Size
The sample size was determined to be sufficient using GPower 3.1.9.2 computer programming software. A priori power analysis indicated that a total of 70 participants were needed for a medium-size effect d (0.7) with α=0.05 and power set at 0.8 for the two independent groups.
Procedures
All interventions for each patient were discussed in a multidisciplinary tumor board before treatment. The TACE procedure was performed under DSA (GE-IGS-530, GE Healthcare, the United States) by two interventional radiologists with 15 and 19 years of experience in liver interventions. After arteriography of the celiac trunk and superior mesenteric artery, a 2.2-French coaxial microcatheter (Carnelian, Tokai Medical Products, Japan) was used to catheterize the main tumor-feeding arteries. TACE was performed under moderate sedation with midazolam and fentanyl tailored individually to body habitus and drug tolerance. Prophylactic intravenous dexamethasone (10 mg) was used to reduce the incidence of TACE-induced nausea/vomiting. Super-selective chemoembolization was performed to prevent non-target embolization. After catheterization, patients in Groups A and B received lidocaine as previously decided during the study design. 10–20 mL of Lipiodol was injected with a median dose of 10 mL, and 40–80 mg of epirubicin was injected with a median dose of 60 mg. Gelfoam slurries were injected to embolize the proximal tumor feeders in all patients. The technical endpoint of TACE was defined as the reduction in arterial inflow to the tumor and tumor devascularization. All the TACE procedures were technically successful according to the Society of Interventional Radiology (SIR) guidelines.18
Efficacy Assessment
Visual analog scale (VAS), which ranges from 0 to 10, was used to assess the pain intensity of each patient at specific time intervals: before TACE (Tbefore), during TACE (T0), 4 h after TACE (T4), 8 h after TACE (T8), 24 h after TACE (T24), 48 h after TACE (T48), and 72 h after TACE (T72). To further evaluate the analgesic results, pain scores of 0–3 were categorized as mild pain; 4–6, moderate pain; and 7–10, severe pain. Pain scores of more than six were considered meaningful, requiring medical treatment to reduce pain. If needed, 100 mg tramadol was used for analgesia, as for severe pain cases refractory to tramadol, 100 mg pethidine was used. Pain scores at the designated time points and opioid analgesics were used within 72 h after the procedure was recorded.
Safety Assessment
A safety assessment was performed by the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Patients that experienced adverse events related to TACE, such as nausea, vomiting, constipation, insomnia, and fever, within 72 h after the procedure were assessed and compared between two groups.
Study Endpoint
The primary outcome was a pain score at each time point in the two groups. The secondary outcomes were the dosage of opioid analgesic intake and the percentage of adverse events after TACE in the two groups.
Statistical Analysis
The data were expressed as mean with standard deviation (SD), median with interquartile range (IQR), or frequency. Pearson’s chi-squared test or Fisher’s exact test was used to compare categorical variables, while the independent-samples t-test or Mann–Whitney U-test was used to compare numerical variables. Linear regression was conducted to analyze the correlation among T0, T4, T8, and T24 pain intensity of the HCC patients. All statistical analyses were performed with SPSS version 20 (International Business Machines Corporation, the United States), and P < 0.05 was considered statistically significant.
Results
Lidocaine Concentrations in VX2 Tumor Tissues and Peripheral Blood
The time–concentration curves indicated that the concentrations of lidocaine in tumor tissues were higher in experimental group at T0.5 (P=0.004), T1 (P=0.038), T4 (P=0.036), and T8 (P=0.029) than in control group (Table 1). There was no difference in concentrations of lidocaine in tumor tissues after 24 h between the experimental and control groups. In peripheral blood, the concentrations of lidocaine were higher in control group at T0.5 (P=0.005) than in experimental group and the concentrations of lidocaine were not significantly different between the two groups at T1, T4, T8, and T24 (Table 1). The time–concentration curves of lidocaine in VX2 tumor tissues and peripheral blood are shown in Figure 3.
 |
Table 1 Time-Concentration of Lidocaine in Tumor Tissues and Peripheral Blood at Different Time Points |
 |
Figure 3 Time–concentration curves of lidocaine in tumor tissues (A) and peripheral blood (B). (*P value < 0.05). |
Patients’ Basic Information
The flowchart of the study population is shown in Figure 4. No patient dropped out or withdrew from the study. Finally, a total of 70 HCC patients who underwent TACE were enrolled. Each group (A and B) consisted of 35 patients. Preoperative data of groups A and B consisted of age, gender, Child-Pugh class, underlying liver disease, tumor distribution, number of lesions, the largest tumor diameter, the lipiodol dose, the epirubicin dose, BCLC staging, and alpha-fetoprotein (Table 2). No statistical difference was found in preoperative data between the two groups (P-value>0.05).
 |
Table 2 Demographics of HCC Patients Receiving TACE |
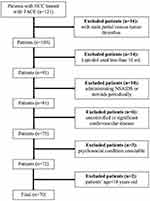 |
Figure 4 Flowchart of the study population. |
Comparison of VAS Scores and Analgesia Intake Between the Two Groups
There was no difference in the pain scores at baseline between the two groups (P=0.237). However, the pain scores in Group B were significantly lower at T0 (P=0.006), T4 (P=0.001), T8 (P=0.002), and T24 (P=0.005) after TACE than in Group A (Figure 5). In addition, mild pain was significantly higher in group B than in group A at T0 (P=0.001), T4 (P=0.007), T8 (P<0.001), and T24 (P=0.024). The pain scores were not significantly different between the two groups at T48 (P=0.565) and T72 (P=0.460), and the postoperative length of hospitalization was not significantly different between the two groups (P=0.891). The dose of tramadol used in Group B was lower than in Group A (P =0.021), and the dose of pethidine used was comparable between the two groups (P=0.204). The detailed pain scores and pain degree distribution in two groups are listed in Tables 3 and 4.
 |
Table 3 Distribution of VAS Scores and Analgesic Consumption |
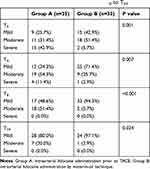 |
Table 4 Distribution of Pain Degree During T0 to T24 |
Incidence of Adverse Events After TACE
Incidence of the adverse events after TACE was compared between groups A and B: Nausea/Vomiting, 11 (31.43%) vs 6 (17.14%), P value 0.163; Fever, 8 (22.86%) vs 6 (17.14%), P value 0.550; Insomnia, 3 (8.57%) vs 3 (8.57%), P value 1.000; Constipation, 4 (11.43%) vs 5 (14.29%), P value 0.721. Overall, the incidence of adverse events was comparable between groups A and B (Table 5).
 |
Table 5 Percentages of Patients with TACE-Related Side Effect |
Correlation Among the Pain Intensity of T0, T4, T8, and T24
Linear regression showed that pain intensity of T0 (pain intensity during TACE) was highly correlated with T4 (R2=0.587, P<0.001), T8 (R2=0.287, P<0.001), and T24 (R2=0.384, P<0.001). The detailed content is shown in Figure 6.
Discussion
The in vivo study demonstrated that lidocaine could be slowly released from the lidocaine–epirubicin–lipiodol emulsion and the randomized clinical trial showed that pain scores were significantly lower in patients who received intraarterial lidocaine injection by the W/O technique at T0, T4, T8, and T24 after the procedure compared to patients who received an intraarterial bolus injection of lidocaine. Moreover, a comparison of the pain degree showed that a greater percentage of patients in group B experienced mild pain at T0, T4, T8, and T24 after the procedure than group A. However, no significant difference in pain scores was observed at T48 and T72 between the two groups. The present study’s findings are highly significant for clinical practice as post-TACE pain has been reported to occur within 12 to 24 h after the procedure by a previous study.19 In addition, our study also substantiated that pain intensity during TACE was highly correlated with pain intensity 4, 8, and 24 h after TACE, which indicated that achieving excellent pain control during the TACE procedure would lead to lower postoperative pain.
Importantly, we found that patients who received lidocaine administered by the W/O technique required less tramadol than patients who received a bolus injection of lidocaine, suggesting that lidocaine administration by the W/O technique may reduce the dosage of opioid analgesic used. It should be noted that the postoperative length of hospitalization was not significantly different between the two groups in our study, which is inconsistent with findings of a previous study12 that showed post-TACE pain was associated with an extended hospital stay. The median postoperative length of hospitalization in this study was 4 days in both groups while the pain scores of two groups decreased rapidly and with no difference 2 days after TACE procedure. Therefore, it might explain why post-TACE pain was not associated with an extended hospital stay in our study.
To date, although the etiology of TACE-related pain is not well understood, several scientific hypotheses have been presented, such as acute ischemia of the liver parenchyma, distention of the liver capsule, tumor necrosis, and gallbladder ischemia secondary to inadvertent embolization of the cystic artery.20–22 Currently, different methods have been suggested for the management of intra- and post-TACE pain. Guo et al found that pre-TACE parecoxib- and sufentanil-based multimodal analgesia was safe, efficient, and cost-effective for post-TACE pain control in HCC patients.11 Oxycodone hydrochloride controlled-release tablets have been reported to be safe, efficient, and cost-effective for controlling post-TACE pain in HCC patients by Zhou et al.23 Lee et al suggested that it was reasonable to routinely perform pre-TACE lidocaine administration to reduce the incidence of post-TACE pain and the amount of analgesic consumption.16 Although accumulating evidence demonstrated success in controlling TACE-related pain could reduce opioid analgesic use and improve patient quality of life, there is still considerable heterogeneity in drug selection and the associated duration of action and route of administration.24 Lidocaine is a local analgesic with an onset of action ranging from 45 to 90 s and 10 to 20 min of clinical effectiveness. Given that the elimination half-life of lidocaine is approximately 90 min to 120 min24 and relatively quick, sustained release of lidocaine is essential for long-term post-TACE pain control and, as seen in our study, could be achieved by the W/O technique. In contrast to Lee et al’s study, we found that intraarterial lidocaine administration by the W/O technique could achieve slow release of lidocaine and decreased pain intensity more effectively with lower VAS and a higher percentage of patients experiencing mild pain after TACE than intraarterial lidocaine administration without the W/O technique.
To the best of our knowledge, this is the first to document the successful use of a lidocaine–epirubicin–lipiodol emulsion to achieve pain control in intra- and post-TACE patients with longer-lasting pain relief. Furthermore, this study also confirmed the sustained release of lidocaine in vivo, which explains the efficacy of the lidocaine–epirubicin–lipiodol emulsion in attenuating intra- and post-TACE pain within 24 h after TACE.
The present study has some limitations. First, the post-TACE pain intensity might have been influenced by opioid analgesics, including tramadol and pethidine. Indeed, the use of opioid analgesics can rapidly decrease VAS scores and thus cause statistical bias. Second, the use of VAS could be another source of bias. In the present study, assessments of pain scores were conducted in patients who had no previous experience of VAS use; thus, their assessments might have varied during the course of treatment. Third, the long-term outcomes of the two groups were not assessed in the present study. However, lidocaine administration in W/O method might decrease the antitumor effect by reducing the concentration of the anti-cancer component. Accordingly, further studies are essential to substantiate our study findings.
Conclusions
In conclusion, this study demonstrated the effectiveness and safety of intraarterial lidocaine administration by the W/O technique in controlling intra- and post-TACE pain.
Data Sharing Statement
Data are available upon reasonable request from Tian-Cheng Wang.
Acknowledgments
We would like to thank the English editing service provided by Freescience Editorial Team. Tian-Cheng Wang and Shi Zhou are co-correspondence authors for this study.
Funding
This work was supported by the Fund of High-Level Innovation Talents (No. 2015-4029), the Base of International Pharmaceutical Science and Technology Cooperation of Guizhou Province (No. 2017-5802).
Disclosure
The authors declare that there is no conflict of interest.
References
1. Mokrane FZ, Lu L, Vavasseur A, et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol. 2020;30(1):558–570. doi:10.1007/s00330-019-06347-w
2. Zhang Z, Zhou Y, Yang J, et al. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: a meta analysis. BMC Cancer. 2019;19(1):511. doi:10.1186/s12885-019-5735-9
3. Kim W, Cho SK, Shin SW, et al. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol. 2019;44(6):2283–2292. doi:10.1007/s00261-019-01952-1
4. Eilard MS, Andersson M, Naredi P, et al. A prospective clinical trial on sorafenib treatment of hepatocellular carcinoma before liver transplantation. BMC Cancer. 2019;19(1):568. doi:10.1186/s12885-019-5760-8
5. Peng Z, Chen S, Xiao H, et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology. 2019;292(1):237–247. doi:10.1148/radiol.2019181818
6. Amorim J, Franca M, Perez-Girbes A, et al. Critical review of HCC imaging in the multidisciplinary setting: treatment allocation and evaluation of response. Abdom Radiol. 2020;45(10):3119–3128. doi:10.1007/s00261-020-02470-1
7. Kudo M, Han K, Ye S, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi:10.1159/000507370
8. Lee DH, Lee JM, Kim PN, et al. Whole tumor ablation of locally recurred hepatocellular carcinoma including retained iodized oil after transarterial chemoembolization improves progression-free survival. Eur Radiol. 2019;29(9):5052–5062. doi:10.1007/s00330-018-5993-y
9. Piscaglia F, Ogasawara S. Patient selection for transarterial chemoembolization in hepatocellular carcinoma: importance of benefit/risk assessment. Liver Cancer. 2018;7(1):104–119. doi:10.1159/000485471
10. Ogasawara S, Chiba T, Ooka Y, et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67(2):575–585. doi:10.1002/hep.29403
11. Guo J, Zhao L, Rao Y, et al. Novel multimodal analgesia regimen improves post-TACE pain in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2018;17(6):510–516. doi:10.1016/j.hbpd.2018.08.001
12. Andersen KJ, Grønbaek H, Villadsen GE, et al. Chemoembolization of intermediate stage hepatocellular carcinomas: results from a Nordic tertiary liver cancer center. Indian J Gastroenterol. 2014;33(4):322–329. doi:10.1007/s12664-013-0428-9
13. Blackburn H, West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer. Cancer Nurs. 2016;39(5):E1–E18. doi:10.1097/NCC.0000000000000302
14. Iolascon G, Moretti A. Pharmacotherapeutic options for complex regional pain syndrome. Expert Opin Pharmacother. 2019;20(11):1377–1386. doi:10.1080/14656566.2019.1612367
15. Zhou D, Wang L, Cui Q, et al. Repositioning lidocaine as an anticancer drug: the role beyond anesthesia. Front Cell Dev Biol. 2020;8:565. doi:10.3389/fcell.2020.00565
16. Lee SH, Hahn ST, Park SH. Intraarterial lidocaine administration for relief of pain resulting from transarterial chemoembolization of hepatocellular carcinoma: its effectiveness and optimal timing of administration. Cardiovasc Intervent Radiol. 2001;24(6):368–371. doi:10.1007/s00270-001-0073-z
17. Yang ZW, Zou RH, Zheng Y, et al. Lipiodol deposition in portal vein tumour thrombus predicts treatment outcome in HCC patients after transarterial chemoembolization. Eur Radiol. 2019;29(11):5752–5762. doi:10.1007/s00330-019-06157-0
18. Gaba RC, Lewandowski RJ, Hickey R, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016;27(4):457–473. doi:10.1016/j.jvir.2015.12.752
19. Wang ZX, Liu SL, Sun CH, et al. Psychological intervention reduces postembolization pain during hepatic arterial chemoembolization therapy: a complementary approach to drug analgesia. World J Gastroenterol. 2008;14(6):931–935. doi:10.3748/wjg.14.931
20. Lima M, Dutra S, Gomes FV, et al. Risk factors for the development of postembolization syndrome after transarterial chemoembolization for hepatocellular carcinoma treatment. Acta Med Port. 2018;31(1):22–29. doi:10.20344/amp.8976
21. Benzakoun J, Ronot M, Lagadec M, et al. Risks factors for severe pain after selective liver transarterial chemoembolization. Liver Int. 2017;37(4):583–591. doi:10.1111/liv.13235
22. Khalaf MH, Sundaram V, Mohammed MAA, et al. A predictive model for postembolization syndrome after transarterial hepatic chemoembolization of hepatocellular carcinoma. Radiology. 2019;290(1):254–261. doi:10.1148/radiol.2018180257
23. Zhou B, Wang JH, Yan ZP, et al. Liver cancer: effects, safety, and cost-effectiveness of controlled-release oxycodone for pain control after TACE. Radiology. 2012;262(3):1014–1021. doi:10.1148/radiol.11110552
24. Lv N, Kong Y, Mu L, et al. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016;26(10):3492–3499. doi:10.1007/s00330-016-4207-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



