Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Intimate Partner Violence During Pregnancy and Maternal Morbidity in South Ethiopia: A Cohort Study
Authors Utaile MM, Ahmed AA, Yalew AW
Received 1 June 2023
Accepted for publication 18 August 2023
Published 30 August 2023 Volume 2023:16 Pages 2577—2592
DOI https://doi.org/10.2147/JMDH.S421208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mesfin Mamo Utaile,1,2 Ahmed Ali Ahmed,2 Alemayehu Worku Yalew2
1Department of Public Health, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia; 2Department of Preventive Medicine, School of Public Health, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Mesfin Mamo Utaile, Tel +251 911 92 79 89, Email [email protected]
Purpose: Intimate partner violence during pregnancy is a universal public health problem. However, its link with maternal morbidity is not well understood in Ethiopia. Thus, the study assessed its effect on maternal morbidity during delivery and postpartum in South Ethiopia.
Methods: A prospective cohort study was conducted among 1535 pregnant women. Pregnant women with intimate partner violence during pregnancy were enrolled as the “exposed group”, and pregnant women without intimate partner violence were registered as the “unexposed group”. A total of 711 exposed and 774 unexposed women were included in the analysis of this study. Data were collected using an interviewer-administered questionnaire. Data entry and analysis were done in STATA Version 14. A generalized linear model with a log link function using the binreg command was applied to examine the effect of intimate partner violence on maternal morbidity.
Results: The level of maternal morbidity during delivery and postpartum was higher among women with intimate partner violence than women without intimate partner violence (34.0% vs 26.6%). After adjusting for confounders, women with intimate partner violence during pregnancy were more likely to experience maternal morbidity than women without intimate partner violence (aRR=4.45; 95% CI: 3.15, 6.28). Psychological violence was also identified as a risk factor for maternal morbidity (aRR=2.17; 95% CI: 1.76, 2.67). Likewise, women with physical violence were more likely to experience maternal morbidity than those without physical violence (aRR=1.31; 95% CI: 1.12, 1.53).
Conclusion: The current study demonstrated a higher level of maternal morbidity among women with intimate partner violence. Psychological violence, physical violence, and intimate partner violence during pregnancy were found to increase the risk of maternal morbidity. Strengthening the prevention and prompt management of intimate partner violence during pregnancy may significantly reduce the incidence of maternal morbidity.
Keywords: intimate partner, violence, morbidity, cohort, Ethiopia
Introduction
Maternal morbidity is a neglected agenda in maternal health.1 Notably, insufficient attention has been given to maternal morbidity in resource-poor settings. Globally, for every woman who dies of maternal causes, an estimated 20–30 women experience acute or chronic morbidity.2 Based on the spectrum, the status of women’s reproductive health starts with a healthy pregnancy continues with morbidity, severe morbidity, near miss, and ends with maternal mortality. Several studies were conducted on maternal near miss and severe maternal morbidity (SMM). However, maternal morbidity, which is none or less in severity than SMM, has received less attention.3 The World Health Organization (WHO) defines maternal morbidity as any health condition attributed to and/or aggravated by pregnancy and childbirth that negatively affects a woman’s well-being and/or functioning.4
Maternal morbidity is attributed to many factors, such as poor health services, and economic and socio-cultural factors.5 According to the Maternal Morbidity Working Group’s (MMWG) report, one of the risk factors for maternal morbidity is violence against women (VAW).6 Intimate partner violence (IPV) is the most common form of VAW.7 IPV can occur at any stage, even during pregnancy.7 IPV during pregnancy may contribute for the occurrence of IPV at any time following delivery. Thus, it is considered as a risk factor for the continuity of different forms of IPV.8–10 IPV during pregnancy affects the health of women and fetuses, with a high burden in developing countries.11 Preventing and reducing VAW and maternal mortality are among the Sustainable Development Goals (SDGs).12 Some studies have reported the morbid and mortal consequences of VAW on reproductive-age women.11,13,14 Therefore, addressing IPV and its linkages with maternal morbidity is crucial to achieving international and national goals related to maternal health.15
The effects of IPV during pregnancy on maternal outcomes are multifaceted and largely preventable.16 A complex interaction exists between IPV during pregnancy and maternal morbidity. Studies conducted in developed countries have indicated that IPV during pregnancy is associated with adverse maternal health outcomes.6,17 The proposed mechanism that links IPV during pregnancy and maternal morbidity may include direct physical, mental, and behavioral pathways.18
IPV substantially impacts a woman’s health and quality of life.7,19,20 The negative consequences of IPV include physical disorders, sexually transmitted infections, various mental health disorders, late and insufficient prenatal care, maternal mal-behaviors, and maternal depression.14,21–23 These effects are amplified in pregnancy, with an increased risk of materno-fetal outcomes.24,25
In various studies, the risk of maternal morbidity was significantly greater among women who reported IPV. IPV has an association with maternal morbidities such as preterm labour, premature rupture of the membrane, infection, hyperemesis, substance abuse, bleeding during pregnancy, poor nutrition, and inadequate weight gain.16,22,26,27 It may also lead to unintended pregnancies, induced abortions, and gynaecological problems.28
Nevertheless, most studies substantiating the effects of IPV were employed in the general women population and developed countries. Little is known about the impact of IPV on maternal morbidity among pregnant women in developing countries. Despite their availability, evidence on the effects of IPV related to maternal morbidities has not been consistent across populations and varies across social and cultural settings.29 Research evidence on the link between IPV during pregnancy and maternal complications is scarce in resource-constrained settings with high maternal morbidity and mortality.
Understanding the association between IPV during pregnancy and maternal morbidity is paramount for developing and implementing interventions to prevent maternal morbidity and mortality. To the best of our knowledge, this is the first study on the link between IPV during pregnancy and maternal morbidity in the Ethiopian community. Despite the prevalence of IPV during pregnancy, the intersection of IPV during pregnancy and maternal morbidity has not been widely examined or documented in Ethiopia, particularly in the study area. Therefore, this study aimed to shed light on the association between IPV during pregnancy and maternal morbidity (complications) occurring at the time of delivery through the postpartum period (in the first 42 days after childbirth).
Materials and Methods
Study Setting, Design and Period
This community-based prospective cohort study was conducted in Gammo Goffa Zone, South Ethiopia, from July 2020 to March 2021. Gammo Goffa Zone is one of the 14 zones of the Southern Nations, Nationalities, and Peoples Regional (SNNPR) State of Ethiopia, located 505 km south of Addis Ababa, the capital city of Ethiopia. The zone has 15 rural districts designated as “Woredas” and two town administrations. As projected from the 2007 national census, the zone has a total population of 2,043,668, of which 1,001,397 (49%) were males and 1,042,271 (51%) were females. The zone currently has 553 functioning health institutions (2 general hospitals, 5 primary hospitals, 75 health centers, and 471 health posts).
Study Population
In this prospective cohort study, pregnant women identified during the study period were included in the follow-up. The study participants were enrolled at baseline and followed up until 42 days of the postpartum period. At baseline, pregnant women were screened for their exposure status. Those with the experience of IPV were considered exposed, while those without IPV were taken as unexposed. In both groups, women who reported having a pregnancy of 24 weeks or more were included in the study in order to get adequate sample, as the probability of experiencing IPV has been reported in prior studies to be higher among those women.11,30,31
Sample Size Determination
The sample size was determined using Epi-Info V.7.1.1.14 by considering two sample comparisons of proportions. Maternal morbidity was the outcome variable, and IPV during pregnancy was the primary exposure variable. Since there was no previous study in the country on the difference in the rate of maternal morbidity with IPV during pregnancy, the proportion of women with maternal morbidity among the unexposed (women without experiencing IPV during pregnancy) was assumed based on an estimation from other sources. According to the WHO, an estimated 15% of women are expected to experience at least one complication.32 Therefore, 15% was chosen among the unexposed group (P1 = 0.15).32 At the same time, the proportion of women who experienced maternal morbidity among the exposed (experienced IPV during pregnancy) was estimated to be 25% (P2 = 0.25) to detect a 10% difference. Furthermore, a level of confidence of 95%, a power of 80%, and exposed to the unexposed ratio of 1:3 (r = 3) was used. A design effect of 2 was considered because of the multistage clustered sampling techniques. Finally, a possible follow-up loss of 10% was added. Based on the above assumptions, the sample size was estimated to be 381 women exposed to IPV and 1142 mothers unexposed to IPV, with a total sample size of 1523 pregnant women. However, this study was part of a large study in which 1535 pregnant women were followed up. Thus, to increase the power of the study, all available samples, 1535 (exposed=735 and unexposed=800), were followed-up. Finally, after excluding loss-to-follow-up, 1485 women (711 with IPV and 774 without IPV) were included in the current analysis.
Sampling Procedure
In this study, a multi-stage cluster sampling technique was employed to identify the study subjects. By considering time and logistics, six districts were selected randomly from the 15 rural districts (the “Woredas”). All the selected districts were stratified into urban and rural “kebeles” (a kebele is the smallest administrative unit considered a cluster in this study). Then, a simple random sampling method was employed to select three rural kebeles and one urban “kebele” from each selected district. There are two town administrations (Arba Minch and Sawla), each with 11 and 6 kebeles, and all were purposefully included. A total of 41 clusters were selected randomly. Then, for all the selected kebeles, pregnant women were enumerated. Those who were selected randomly joined the follow-up scheme. In this follow-up, a group of pregnant women with IPV (exposed) and without IPV (unexposed) were identified at the baseline survey and followed up for the outcomes of interest till 42 days of the postpartum period (Figure 1).
 |
Figure 1 Schematic presentation of sampling procedure for the cohort study on the effect of IPV during pregnancy on maternal morbidity during delivery and postpartum. |
Measurements
The outcome variable for this study was maternal morbidity during delivery and postpartum. Maternal morbidities are obstetric complications that occur during pregnancy through the postpartum period of 42 days. In this study, maternal morbidity was considered when women reported their experience of at least one form of morbidity (among the most common forms: haemorrhage, prolonged labour, the premature rupture of membrane, convulsion, severe headache, and sepsis or high fever) during delivery and postpartum periods. The data on maternal morbidity were collected just at the end of the postpartum period (42 days after delivery).
The morbidities were defined based on the WHO guideline for monitoring emergency obstetric care and other literature.32–35 Accordingly, haemorrhage was defined as any of the following: postpartum bleeding that required treatment, retained placenta, vaginal bleeding above 500 mL after childbirth, more than one pad soaked in blood in 5 minutes, and wetting of cloths. Prolonged labour refers to any of the following: >12 h after the first stage of labour, >1 h after the second stage of labour, labour duration greater than 24 hours from the onset of mild pains to the birth of the baby, cephalo-pelvic disproportion, and malpresentation. Premature rupture of the membrane (PROM) is defined as rupture of the membrane at any time before the onset of uterine contractions. Convulsion was defined as the occurrence of fits or seizures. It was used to represent eclampsia. Postpartum sepsis (infection) is defined as puerperal sepsis (fever lasting more than 24 hours after delivery) and any of the following signs and symptoms: lower abdominal pain, purulent, offensive vaginal discharge, and a tender uterus.
The primary explanatory variable for this study was IPV during pregnancy. It was defined as the experience of at least one act of any form of violence (psychological, physical, or sexual violence) by women perpetrated by their current or most recent partners during the current pregnancy period. The other variables were individual and household characteristics such as socio-demography, wealth index, and obstetric characteristics. The wealth index was computed using principal component analysis (PCA).
Data Collection Tools and Methods
Data were collected face-to-face using an interviewer-administered, pretested questionnaire. The questionnaire was adapted from the WHO multi-country study of the VAW questionnaire,17 the Ethiopian Demographic and Health Survey (EDHS),36 and other related literature. The questionnaire was prepared in English, translated to the local language (Amharic), and back-translated to English by another person to ensure its consistency and accuracy. Health extension workers were recruited, trained, and deployed for data collection. Trained supervisors and principal investigators supervised the data collection process. The supervisors were degree-holders with knowledge and skills in maternal health care. The data collectors and supervisors were recruited based on their eloquence in local languages, qualifications, and experience in data collection. The research team adopted the WHO’s practical guide for researching VAW.7,37 Furthermore, we did not encounter any disruption during the study period due to COVID-19 because there was no strict lockdown or shutdown in Ethiopia and the disease incidence was very slow.
Data Analysis
After the data were coded and entered into EpiData v. 3.1, were exported to STATA 14 for cleaning, editing, and analysis. Descriptive statistics were computed and presented. Socioeconomic quintiles were determined using principal component analysis (PCA). The socio-demographic characteristics and outcome variable (maternal morbidity during delivery and postpartum periods) were compared between women who experienced IPV during pregnancy and those who had not experienced IPV (exposed and unexposed women).
A generalized linear model with a log link function using the binreg command was applied to examine the effect of IPV during pregnancy on maternal morbidity during delivery and postpartum periods. Bivariate analysis was done using cross-tabulation to see associations between each independent variable and maternal morbidity. All variables in crude analysis with p < 0.25 were considered candidates for the final model. Variables such as place of residence, wealth index, maternal education, at least one ANC, four or more ANC visits, parity and place of delivery were used in the adjustment of the final model.
Multicollinearity between the independent variables was assessed using the variance inflation factor (VIF). As all included variables had VIF < 10, no multicollinearity was detected. After adjusted for confounders, a risk ratio or relative risk (RR) with 95% confidence interval was calculated, and a p-value of less than 0.05 was considered for statistical significance.
Results
Response Rate
A total of 1535 pregnant women (735 with IPV and 800 without IPV) were registered and completed the first evaluation of this study. However, 1485 mothers (711 with IPV and 774 without IPV) completed the second evaluation and were included in the analysis, with a response rate of 96.7%. The loss to follow-up for the remaining 50 (3.3%) was due to travelling to other places to seek social support during delivery and postpartum. The detailed process and flow of the study are demonstrated below (Figure 2).
 |
Figure 2 A flow diagram of the overall study process. |
Socio-Demographic Characteristics
A total of 307 (43.2%) pregnant women with IPV and 316 (40.8%) without IPV were rural residents. The majority (85.95%) of women with IPV and without IPV (87.6%) were aged 20–34. The mean (SD) age of women with IPV and without IPV was 26.1(4.7) and 26.4 (4.7) years, respectively. Gammo was the dominant ethnic group among women with IPV (51.9%) and without IPV (62.7%). Protestant Christianity was the leading religion in both groups. More than ninety-five percent of the respondents in both groups were married at the time of the interview. Less than one percent of women with IPV and 20% without IPV attended tertiary education. The majority of women in both groups were homemakers. Nearly half of the partners/husbands of women in both groups were in the age group of 25–34. More than one-third of partners attended tertiary education. The data also indicated that one-third of partners had secondary education. Farming was the leading occupation of partners among women with IPV (40.8%) and without IPV (46.8%) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Respondents in Gammo Goffa Zone, South Ethiopia, July 2020 to March 2021 |
Obstetric Characteristics of Respondents
More than three fourth of women with IPV and without IPV were multigravida. About half of the respondents had one or two deliveries. Around half of the respondents had an inter-birth interval of less than 24 months. A total of 690(97.0%) women with IPV and 741(95.9%) women without IPV had at least one ANC visit during the current pregnancy. However, only 435(62.5%) women with IPV and 514(68.6%) women without IPV had four or more ANC visits. Among the respondents, 666(93.7%) women who experienced IPV and 708(91.6%) women who did not experience IPV had given birth at health facilities (Table 2).
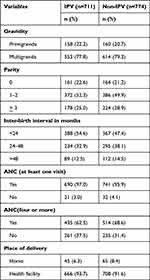 |
Table 2 Obstetric Characteristics of Respondents by Their IPV Status in Gammo Goffa Zone, South Ethiopia, July 2020 to March 2021 |
The Level of Maternal Morbidity Among Respondents
A total of 242 (34.0%) women with IPV during pregnancy and 206 (26.6%) women without IPV developed at least one form of maternal morbidity during delivery and postpartum. The most commonly encountered morbidities were haemorrhage (8.9% vs 6.7%) and severe headache (5.9% vs 6.0%) among exposed and unexposed women. The proportion of convulsion among women with IPV was 3.7% and 2.5% among women without IPV. Furthermore, 6.8% of women with IPV and 4.1% without IPV had high-grade fever. Prolonged labour was encountered by 5.8% with IPV and 5.0% without IPV. Moreover, PROM occurred in 3.2% with IPV and 3.6% without IPV, respectively (Table 3).
 |
Table 3 Frequency Distribution of Self-Reported Maternal Morbidity Among Respondents by Their IPV Status During Delivery and Postpartum Periods, Gammo Goffa, South Ethiopia, July 2020 to March 2021 |
The Effect of Intimate Partner Violence During Pregnancy on Maternal Morbidity
In the adjusted final model, some socio-demographic, obstetric, and IPV-related variables were found to be important determinants of maternal morbidity during delivery and postpartum.
Among socio-demographic variables, place of residence, wealth index, and maternal education were found to have a statistically significant association with maternal morbidity during delivery and postpartum. Women in rural areas were less likely to experience maternal morbidity compared to urban women (aRR = 0.56; 95% CI: 0.46, 0.69). Compared to respondents from the poorest household, those from the middle (aRR = 1.38; 95% CI: 1.03, 1.86) and the richest households (aRR = 1.37; 95% CI: 1.03, 1.83) were more likely to report maternal morbidity. Having a primary (1–8 grades) education (aRR = 0.81; 95% CI: 0.66, 0.99) showed a 19% reduction in the occurrence of maternal morbidity compared to not having a formal education.
Among obstetric variables, four or more antenatal care (ANC) visits, place of delivery, and parity were found to have a statistically significant association with maternal morbidity during delivery and postpartum periods. Mothers who experienced four or more deliveries (aRR = 0.62; 95% CI: 0.49, 0.78) were less likely to develop maternal morbidity than primiparous mothers. Similarly, women with four or more ANC visits (aRR = 0.68; 95% CI: 0.58, 0.79) were less likely to experience maternal morbidity. Giving birth at home was found to be associated with a lower probability of experiencing maternal morbidity during delivery and postpartum compared to giving birth at a health facility in both the unadjusted and adjusted analyses (aRR = 0.46; 95% CI: 0.28, 0.77).
Among violence-related variables, psychological violence, physical violence, and IPV during pregnancy were identified as predictors of maternal morbidity during delivery and postpartum. Women with psychological violence had a 2.17 times higher risk of maternal morbidity (aRR = 2.17; 95% CI: 1.76, 2.67) compared to women without psychological violence. Similarly, women with physical violence were 1.31 times more likely to experience maternal morbidity (aRR = 1.31; 95% CI: 1.12, 1.53) than those without physical violence. A significant association was observed between sexual violence and maternal morbidity in the bivariate analysis (cRR = 3.49; 95% CI: 3.05, 3.98). However, it disappeared in the multivariable analysis (aRR = 1.06; 95% CI: 0.95, 1.19). IPV during pregnancy was found to increase the likelihood of experiencing maternal morbidity significantly as compared to non-IPV during pregnancy (aRR = 4.45; 95% CI: 3.15, 6.28) (Table 4).
 |
Table 4 The Effect of IPV During Pregnancy on Maternal Morbidity During Delivery and Postpartum, Gammo Goffa Zone, South Ethiopia, July 2020 to March 2021 |
Discussion
Studies on the link between IPV during pregnancy and maternal morbidity in developing countries are limited, and if available, they usually describe the life-threatening morbidities diagnosed at the hospital level. This is the first community-based follow-up study in Ethiopia that has examined the effect of IPV on self-reported maternal morbidity. In the current study, 34.0% of women with IPV and 26.6% without IPV experienced at least one form of maternal morbidity during delivery and postpartum periods. The finding of this study is consistent with a study conducted in Peru.38 This may be explained by the fact that pregnancy is a particularly vulnerable period for women, which may increase susceptibility to the potential effects of IPV on women’s pregnancy and childbirth-related morbidity.
This study identified socio-demographic and economic variables such as place of residence, wealth index, and maternal education as risk factors for maternal morbidity. This is in line with a study done in Brazil.39 However, the current finding is not in agreement with studies done in India, Rwanda, and Uganda.33,40–42 This may be explained by the fact that women in urban areas have more access to skilled care, media, and health information, which might have increased their level of risk perception and thereby increased their capabilities of identifying and reporting morbidities. The other possible explanation is that the poor may not have enough access to information on maternal complications, so they may not perceive or be aware of the complications they experienced and may not report them adequately. This may also be explained by the fact that better education increases access to health information and risk perception, which in turn increases their use of health care services that can help them prevent maternal morbidity.
Among obstetric variables, four or more ANC visits, place of delivery, and parity were found to have a statistically significant association with maternal morbidity during delivery and postpartum. This is consistent with the findings of studies done in Uganda43 and Brazil.44 This may be explained by the fact that during four or more ANC visits, the health conditions of mothers can be screened and treated earlier. Furthermore, women who attend more ANC sessions have better access to health information, which can help them avoid certain morbidities. After controlling for confounders, giving birth at home indicated significant negative association with reported morbidity. This is consistent with a prior study conducted in Ethiopia. This may be explained by the fact that women who give birth at home may have less access to health information so that they may not know and report some kind of morbidity than their counterparts.33
Among violence-related variables, psychological violence, physical violence, and IPV during pregnancy were identified as predictors of maternal morbidity during delivery and postpartum. However, sexual violence during pregnancy was not significantly associated with maternal morbidity during delivery and postpartum. Women with psychological (emotional) violence had a higher risk of maternal morbidity than women without psychological violence. This finding is supported by evidence generated from studies conducted before.26,38 This may be explained by the fact that women affected by emotional violence may develop high levels of anxiety and depression, which often lead to alcohol and drug abuse. This could lead to the occurrence of adverse obstetric outcomes.45,46 However, anxiety and depression may not always lead to substance abuse (alcohol and drug abuse).47,48 Moreover, anxiety and depression, commonly co-occur with alcohol use disorder.49
Likewise, women who experienced physical violence during pregnancy were more likely to experience maternal morbidity than those who did not. Similar findings have been reported in previous studies conducted in Bangladesh45 and Saudi Arabia.50 This may be due to the close association between physical violence during pregnancy and premature labour, pre-term delivery, and haemorrhage due to abruptio-placenta and abdominal trauma. The other possible explanation may be violence-related stress, which may play an essential role in causing adverse maternal outcomes through both behavioral and biological pathways.46 Furthermore, prenatal stress caused by exposure to IPV during pregnancy may lead to adverse maternal outcomes such as miscarriage, preterm labor, and premature rupture of membranes. These associations have been supported by various prior studies.23,46,51
In Bangladesh studies,26,45 women who experienced sexual IPV during pregnancy were at increased risk of experiencing maternal morbidity. In contrast, findings from the current study do not document a significant association between sexual violence and maternal morbidity. The discrepancy may be due to variations in study settings, study designs, and measurement of violence and maternal morbidity.
In this study, the overall IPV during pregnancy was found to increase the likelihood of experiencing maternal morbidity significantly compared to non-IPV during pregnancy. This is consistent with other prior studies conducted in some African countries like Nigeria52 and Egypt.53 It is also consistent with previous studies conducted elsewhere.23,46,51,54 The observed associations between IPV and maternal morbidity could be attributable to direct injury (mediated through psychosocial, emotional, and physical stress, depression, and low self-esteem) from IPV during pregnancy. It may also be linked with indirect pathways, as victims of IPV may be less likely to access prenatal care and adopt unhealthy behaviors (increased alcohol use, cigarette smoking, and drug use).38,51,54 Moreover, stress may affect maternal behavior associated with IPV during pregnancy, which in turn may have an adverse impact on maternal and fetal outcomes.50,51,53
Strengths and Limitations
Since this study was a prospective follow-up study, it has minimized recall bias. It also used large sample size that resulted in high power. Our study findings should also be considered under certain limitations. Data on the experience of IPV and maternal morbidity in this study were collected through self-report, which could be subject to social desirability bias. Social desirability bias may be more serious for sensitive issues like IPV (all forms) and could lead to the under-reporting of these experiences. To minimize such biases, data collectors and supervisors were given extensive training to collect data on IPV and maternal morbidity. Moreover, an exhaustive list of signs and symptoms of common maternal morbidities was included in the questionnaire and pretested before use. Compared to medical examinations, the reliability of self-reported morbidities may be limited. However, in settings where women do not access and use maternal services easily and adequately, studies on self-reported morbidity can provide useful evidence on women’s lived experiences and maternal morbidities. Additionally, analyzing IPV during pregnancy as a risk factor for continuum of violence during postpartum and remaining life was not the focus of this particular study.
Conclusion
The objective of the current study was to assess the effect of IPV during pregnancy on maternal morbidity during delivery and postpartum. The finding of the study demonstrated a higher level of maternal morbidity during delivery and postpartum (34.0%) among women with IPV during pregnancy compared to women without IPV (26.6%). The study also found that psychological violence, physical violence, and IPV during pregnancy significantly affect the development of maternal morbidity during delivery and postpartum. Place of residence, wealth index, maternal education, four or more ANC visits, parity, and place of birth, were also identified as factors associated with maternal morbidity. Health programmers in the health care delivery system should use this evidence to improve maternal health outcomes. In addition, healthcare providers, particularly community health workers, should strengthen the prevention, early screening, and prompt management of IPV during pregnancy to reduce the incidence of adverse maternal outcomes.
Data Sharing Statement
The datasets used and / or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was conducted in compliance with the ethical principles outlined by Helsinki declaration.55 Accordingly, the study was approved for scientific and ethical integrity by the Institutional Review Board (IRB) of the College of Health Sciences of Addis Ababa University (Protocol number: 106/19/SPH). All necessary permissions were obtained from all local administrators. Written informed consent was sought from every study participant before actual data collection. For women under the age of 18, consent was obtained from their parents. The study strictly followed the WHO guideline on ethical issues related to violence research.37 All interviews were conducted in complete privacy. Confidentiality was maintained by removing any identifier from the questionnaire. Data collectors were trained to provide necessary health information based on the needs of the study participants, but not an intervention. Furthermore, during data collection, women who were identified as sick were advised to seek examinations and treatment at nearby health facilities.
Acknowledgments
We are very grateful to Addis Ababa University for funding the data collection of this study. Thanks to Gamo Goffa Zone Department of Health and data collectors for their technical assistance. We are also indebted to the study participants for their willingness to participate in the research.
Author Contributions
All authors have made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; have drafted, revised or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was obtained from any funding agency. The data collection was conducted using a small grant provided by Addis Ababa University. The university has no role in the design of the study, collection, analysis and interpretation of the data. The finding and conclusion of the study reflect the view of the authors only.
Disclosure
The authors declare that they have no competing interests.
References
1. Koblinsky M, Chowdhury ME, Moran A, Ronsmans C. Maternal morbidity and disability and their consequences: neglected agenda in maternal health. J Heal Popul Nutr. 2012;30(2):124–130.
2. Firoz T, Chou D, Von Dadelszen P, Agrawal P, Vanderkruik R, Tunçalp O. Measuring maternal health: focus on maternal morbidity. Bull World Heal Organ. 2013;91(10):794–796. doi:10.2471/BLT.13.117564
3. Vanderkruik RC, Tunçalp Ö, Chou D, Say L. Framing maternal morbidity: WHO scoping exercise. BMC Pregnancy Childbirth. 2013;13:213. doi:10.1186/1471-2393-13-213
4. Chou D, Tunçalp Ö, Firoz T, Barreix M, Filippi V, Von Dadelszen P. Constructing maternal morbidity – towards a standard tool to measure and monitor maternal health beyond mortality. BMC Pregnancy Childbirth. 2016;16:45. doi:10.1186/s12884-015-0789-4
5. The World Bank. Reproductive, Maternal, Newborn, and Child Health. Washington, DC, USA: The World Bank; 2016.
6. Say L, Barreix M, Chou D, et al. Maternal morbidity measurement tool pilot: study protocol. Reprod Health. 2016;13(1). doi:10.1186/s12978-016-0164-6
7. García-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts C. WHO multi-country study on women’s health and domestic violence against women; 2005. Available from: http://whqlibdoc.who.int/publications/2005/924159358X_eng.pdf.
8. Kita S, Chan KL, Tobe H, et al. A follow-up study on the continuity and spillover effects of intimate partner violence during pregnancy on postnatal child abuse. J Interpers Violence. 2021;36(13–14):NP6904–27. doi:10.1177/0886260518821460
9. Koenig LJ, Whitaker DJ, Royce RA, Wilson TE, Ethier K, Fernandez MI. Physical and sexual violence during pregnancy and after delivery: a prospective multistate study of women with or at risk for HIV infection. Am J Public Health. 2006;96(6):1052–1059. doi:10.2105/AJPH.2005.067744
10. Abubakari A, Mbwambo J, Mahenge B, Sto H, Jahn A. Physical, sexual, emotional and economic intimate partner violence and controlling behaviors during pregnancy and postpartum among women in Dar es Salaam, Tanzania. PLoS One. 2016;11(10):e0164376. doi:10.1371/journal.pone.0164376
11. Gashaw BT, Schei B, Magnus JH. Social ecological factors and intimate partner violence in pregnancy. PLoS One. 2018;13(3):e0194681. doi:10.1371/journal.pone.0194681
12. United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. United Nations General Assembly; 2015.
13. Faramarzi M, Esmaelzadeh S, Mosavi S. Prevalence, maternal complications and birth outcome of physical, sexual and emotional domestic violence during pregnancy. Acta Med Iran. 2005;43(2):115–122.
14. Miura A, Fujiwara T. Intimate partner violence during pregnancy and postpartum depression in Japan: a cross-sectional study. Front Public Heal. 2017;5(1):81. doi:10.3389/fpubh.2017.00081
15. United Nations. The Sustainable Development Goals Report. United Nations; 2016:1–56.
16. Alhusen JL, Ray E, Sharps P, Bullock L. Intimate partner violence during pregnancy: maternal and neonatal outcomes. J Womens Health. 2015;24(1):100–106. doi:10.1089/jwh.2014.4872
17. Silverman JG, Balaiah D, Ritter J, et al. Maternal morbidity associated with violence and maltreatment from husbands and in-laws: findings from Indian slum communities. BMC Reprod Heal. 2016;13:109. doi:10.1186/s12978-016-0223-z
18. Bailey BA. Partner violence during pregnancy: prevalence, effects, screening, and management. Dovepress Int J Women’s Heal. 2010;2:183–197. doi:10.2147/IJWH.S8632
19. Gupta RK, Langer B, Singh P, Kumari R, Akhter N, Gupta R. Domestic violence in rural currently married women: effects on utilization of reproductive and maternal health services. Int J Reprod Contraception Obstet Gynecol. 2018;7(2):602–607. doi:10.18203/2320-1770.ijrcog20180179
20. World Health Organization. Global and Regional Estimates of Violence Against Women: Prevalence and Health Effects of Intimate Partner Violence and Non-Partner Sexual Violence. World Health Organization; 2013:57.
21. Quintanilla BPA, Pollock WE, McDonald SJ, Taft AJ. Impact of violence against women on severe acute maternal morbidity in the intensive care unit, including neonatal outcomes: a case-control study protocol in a tertiary healthcare facility in Lima, Peru. BMJ Open. 2018;8(3):e020147 doi:10.1136/bmjopen-2017-020147.
22. Kearney MH, Haggerty LA, Munro BH, Hawkins JW. Birth outcomes and maternal morbidity in abused pregnant women with public versus private health insurance. J Nurs Scholarsh an off Publ Sigma Theta Tau Int Honor Soc Nurs. 2003;35(4):345–349.
23. Ibrahim ZM, Ahmed WAS, Hagras AM, Hagras AM. Intimate partner violence among Egyptian pregnant women: incidence, risk factors, and adverse maternal and fetal outcomes. Clin Exp Obstet Gynecol. 2015;42(2):2012–2019. doi:10.12891/ceog1829.2015
24. Chisholm CA, Rn LB, Jef JE, Ii F. Intimate partner violence and pregnancy: screening and intervention. Am J Obstet Gynecol. 2017;217(2):145–149. doi:10.1016/j.ajog.2017.05.043
25. Janssen PA, Holt VL, Sugg NK, Emanuel I, Critchlow CM, Henderson AD. Intimate partner violence and adverse pregnancy outcomes: a population-based study. Am J Obstet Gynecol. 2003;188(5):1341–1347. doi:10.1067/mob.2003.274
26. Islam MJ, Broidy L, Baird KM, Mazerolle P. Intimate partner violence around the time of pregnancy and postpartum depression: the experience of women of Bangladesh. PLoS One. 2017;12(5):e0176211. doi:10.1371/journal.pone.0176211
27. Lövestad S, Löve J, Vaez M, Krantz G. Prevalence of intimate partner violence and its association with symptoms of depression; A cross-sectional study based on a female population sample in Sweden. BMC Public Health. 2017;17(1):335. doi:10.1186/s12889-017-4222-y
28. World Health Organization. Preventing Intimate Partner and Sexual Violence Against Women. Taking Action and Generating Evidence. World Health Organization; 2010.
29. UNICEF. Domestic Violence Against Women and Girls. UNICEF Innocenti Research Centre. Florence, Italy: UNICEF; 2000.
30. Tura G, Afework MF, Yalew AW. The effect of birth preparedness and complication readiness on skilled care use: a prospective follow-up study in Southwest Ethiopia. BMC Reprod Heal. 2014;11:60. doi:10.1186/1742-4755-11-60
31. Groves AK, Moodley D, McNaughton-Reyes L, et al. Prevalence and rates of intimate partner violence among South African women during pregnancy and the postpartum period. Matern Child Heal J. 2015;19(3):487–495. doi:10.1007/s10995-014-1528-6
32. World Health Organization. WHO, UNFPA, UNICEF, AMDD. Monitoring Emergency Obstetric Care: A Handbook. Geneva, Switzerland: World Health Organization; 2009.
33. Mekonnen A, Mahmoud E, Fantahun M, Hagos S, Assegid M. Maternal morbidity in Butajira and Wukro districts, North and South central Ethiopia. Ethiop Med J. 2013;51(4):239–248.
34. Gebeyehu A, Alemayehu W, Yalew W. The contributions of maternity care to reducing adverse pregnancy outcomes: a cohort Study in Dabat District, Northwest Ethiopia. Matern Child Heal J. 2013;18(6):1336–1344.
35. Assefa NE, Berhe H, Girma F et al. Risk factors of premature rupture of membranes in public hospitals at Mekele city, Tigray, a case control study. BMC Pregnancy Childbirth. 2018;18 :386. doi:10.1186/s12884-018-2016-6
36. ICF. CSA [Ethiopia] and Ethiopia Demographic and Health Survey 2015. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Authority and ICF; 2016.
37. World Health Organization. WHO Ethical and Safety Recommendations for Researching, Documenting and Monitoring Sexual Violence in Emergencies. World Health Organization; 2007.
38. Sanchez SE, Qiu C, Perales MT, Lam N, Garcia P, Williams MA. Intimate partner violence (IPV) and preeclampsia among Peruvian women. Eur J Obstet Gynecol Reprod Biol. 2008;137:50–55. doi:10.1016/j.ejogrb.2007.05.013
39. Souza JP, Cecatti JG, Parpinelli MA, Sousa MH, Lago TG, Pacagnella RC. Maternal morbidity and near miss in the community: findings from the 2006 Brazilian demographic health survey. BJOG. 2010;117:1586–1592. doi:10.1111/j.1471-0528.2010.02746.x
40. Wandabwa JN. Investigation of risk factors for severe maternal morbidity and progression to mortality: a case control and follow up study in Mulago Hospital Complex Uganda [PhD thesis]. London School of Hygiene & Tropical Medicine; 2004.
41. Bang RA, Bang AT, Reddy MH, Deshmukh MD, Baitule SB, Filippi V. Maternal morbidity during labour and the puerperium in rural homes and the need for medical attention: a prospective observational study in Gadchiroli, India. An Int J Obstet Gynaecol. 2004;111:231–238. doi:10.1111/j.1471-0528.2004.00063.x
42. Habimana-kabano I, Broekhuis A, Hooimeijer P. Inter-pregnancy intervals and maternal morbidity: new evidence from Rwanda. Afr J Reprod Health. 2015;19(3):77–86.
43. Kiondo P, Wamuyu‐Maina G, Bimenya GS, Tumwesigye NM, Wandabwa JOP. Risk factors for pre‐eclampsia in Mulago Hospital Kampala Uganda. Trop Med Int Heal. 2012;17(4):480–487. doi:10.1111/j.1365-3156.2011.02926.x
44. José A, Pacheco C, Katz L, et al. Factors associated with severe maternal morbidity and near miss in the São Francisco Valley, Brazil: a retrospective, cohort study. BMC Pregnancy Childbirth. 2014;14(1):91. doi:10.1186/1471-2393-14-91
45. Ferdos J, Rahman MM, Jesmin SS, Rahman MA, Sasagawa T. Association between intimate partner violence during pregnancy and maternal pregnancy complications among recently delivered women in Bangladesh. Aggress Behav. 2018;44(3):294–305. doi:10.1002/ab.21752
46. Hassan M, Kashanian M, Hassan M, Roohi M, Yousefi H. Maternal outcomes of intimate partner violence during pregnancy: study in Iran. Elsevier Public Heal. 2014;128(5):410–415. doi:10.1016/j.puhe.2013.11.007
47. Shmulewitz D, Hasin DS. Risk factors for alcohol use among pregnant women, ages 15 – 44, in the United States, 2002 to 2017. Prev Med. 2019;124(2019):75–83. doi:10.1016/j.ypmed.2019.04.027
48. Conner KR, Pinquart M, Gamble SA. Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abus Treat. 2009;37(2):127–137. doi:10.1016/j.jsat.2008.11.007
49. Mchugh RK, Weiss RD. Alcohol use disorder and depressive disorders. Alcohol Res. 2019;40(1):1–8.
50. Rachana C, Suraiya K, Hisham A, Abdulaziz A, Hai A. Prevalence and complications of physical violence during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;103(1):26–29. doi:10.1016/S0301-2115(02)00022-2
51. Dhar D, Mcdougal L, Hay K, et al. Associations between intimate partner violence and reproductive and maternal health outcomes in Bihar, India: a cross- sectional study. BMC Reprod Heal. 2018;15:109. doi:10.1186/s12978-018-0551-2
52. Andersson N, Omer K, Caldwell D, et al. Male responsibility and maternal morbidity: a cross-sectional study in two Nigerian states. BMC Health Serv Res. 2011;11(Suppl 2):57. doi:10.1186/1472-6963-11-S2-S7
53. Elkhateeb R, Abdelmeged A, Ahmad S, et al. Impact of domestic violence against pregnant women in Minia governorate, Egypt: a cross sectional study. BMC Pregnancy Childbirth. 2021;21:535. doi:10.1186/s12884-021-03953-9
54. Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U. S. states: associations with maternal and neonatal health. Am J Obstet Gynecol. 2006;195:140–148. doi:10.1016/j.ajog.2005.12.052
55. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA. 2013;310(20):2191–2194. PMID: 24141714. doi:10.1001/jama.2013.281053
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
