Back to Journals » Cancer Management and Research » Volume 12
Intercalary Allograft to Reconstruct Large-Segment Diaphysis Defects After Resection of Lower Extremity Malignant Bone Tumor
Authors Liu Q , He H, Duan Z , Zeng H, Yuan Y, Wang Z, Luo W
Received 15 April 2020
Accepted for publication 13 May 2020
Published 8 June 2020 Volume 2020:12 Pages 4299—4308
DOI https://doi.org/10.2147/CMAR.S257564
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Qing Liu,1– 3 Hongbo He,1 Zhixi Duan,1,4 Hao Zeng,1 Yuhao Yuan,1 Zhiwei Wang,1 Wei Luo1
1Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Department of Spine Surgery, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 3Molecular Oncology Laboratory, Department of Orthopaedic Surgery and Rehabilitation Medicine, The University of Chicago Medical Center, Chicago, IL, USA; 4Department of Orthopedics, The Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Wei Luo
Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Tel +86 15116329088
Email [email protected]
Aim: To evaluate the clinical effect of intercalary allograft transplantation and reconstruction in the treatment of diaphyseal defect after resection of lower extremity malignant bone tumor.
Methods: Clinical data of 17 patients diagnosed with malignant lower-limb bone tumors and having undergone segmental allograft reconstruction with a mean follow-up of 49.8 (26– 78) months were included. Segmental allografts of average 17-cm length preserved by deep-freezing were used and fixed using intramedullary nail, double plate, and intramedullary nail and plate combination in 2, 5, and 10 patients, respectively. Host–donor junctions were perfectly and roughly matched in 5 and 12 patients, respectively. Allograft union, local recurrence, and complications were assessed using clinical and radiological tests. Allograft union was evaluated using the International Society of Limb Salvage (ISOLS) scoring system. The functional prognosis was evaluated using the Musculoskeletal Tumour Society (MSTS) scoring system.
Results: Intercalary allograft reconstruction of femoral shaft, tibial shaft, and distal tibia with ankle arthrodesis was performed in eight, four, and five patients, respectively. Two patients had local recurrence and underwent amputation; one died of metastasis. Host–donor junctions in two patients showed nonunion; 12 patients achieved bone union. The average union time was 12.1 months. No allograft fracture or infection occurred. Union rates were 100% and 88.2% at metaphyseal and diaphyseal junctions, respectively. Healing time differed significantly between the precisely and roughly matched groups (p< 0.01). The incidence of nonunion was higher after intramedullary nailing than after the other two methods (p< 0.05). The mean MSTS score was 24.2 (14– 29) at the end of follow-up.
Conclusion: Intercalary allograft transplantation is an effective strategy for diaphyseal defect following post-tumor resection in the lower extremity. Good bone healing after allograft reconstruction is achieved with stable internal fixation and perfectly matched host–donor interfaces.
Keywords: intercalary allograft, malignant bone tumor, bone healing, lower extremity, internal fixation
Introduction
With the development of neoadjuvant chemotherapy, radiotherapy and targeted drug therapy in the clinical treatment of malignant bone tumors, limb salvage surgery has become the most important surgical method for malignant bone tumors. Limb salvage surgery includes two key techniques: radical resection of tumors and effective reconstruction of bone defects.1 The commonly used methods for the reconstruction of bone defect after resection of tumor segment include artificial prosthesis,2 allogeneic bone,3,4 devitalized bone5,6 and so on. The resection of malignant bone tumors located in the diaphysis tends to preserve their own joints, which is different from the metaphysis, but in many cases the residual bone at both ends is not enough to stabilize the metal prosthesis, which brings great difficulties to limb salvage.
Many literatures have reported7–13 that intercalary allograft reconstructions following resection of malignant bone tumors in limb salvage is an alternative method, which can preserve joint function to the largest extend by maintaining articular cartilage and ligaments, and even preserving adjacent growth plates in adolescent patients. However, the potential complications such as nonunion, infection, allograft fracture, internal fixator failure, immune response and so on limit its widespread use.10,14,15 Furthermore, there is disagreement regarding its clinical efficacy, with some studies showing very high failure rates to recommend this method.3,14,16 The prognosis of this technique at different limb sites also varies.15 Therefore, studies with a higher number of cases are required for more accurate conclusions.
Intercalary allograft has been used for diaphyseal defect reconstruction following malignant bone tumor resection at our center for 10 years. During long-term follow-up, we found differences in the clinical efficacy of this technique between application in the upper and lower extremity; however, the sample size of the upper-limb group was insufficient to evaluate curative outcomes. In this study, we retrospectively analyzed clinical data of patients who underwent lower-limb diaphyseal reconstruction with intercalary allografts following primary malignant bone tumor resection, objectively evaluated the oncology prognosis and functional prognosis, and analyzed the prognostic factors to provide evidence support for clinical treatment.
Patients and Methods
Patients
Patients with primary malignant bone tumors of lower extremities who underwent long-segment allograft bone transplantation in our Bone Tumor Center between August 2012 and November 2017 were enrolled in the study. There were 17 patients, nine males and eight females, with a mean age of 24.9 years (14 to 66). Diagnoses were confirmed on preoperative histopathological examination and included osteosarcoma (OS), chondrosarcoma (CS), ameloblastoma (AB), undifferentiated pleomorphic sarcoma (UDPS), and Ewing sarcoma (ES) in 12, 1, 1, 1, and 2 patients, respectively. The inclusion criteria were as follows: 1. Histopathological confirmed primary malignant bone tumors; 2. Tumors located in the lower extremity shaft, without involving important blood vessels and nerves; 3. Limb-salvage conditions for repairing bone defects with large allografts are available. 4. The patient’s case data are complete and long-term follow-up is obtained. All patients underwent radiography, computed tomography (CT), magnetic resonance imaging (MRI) and bone scans preoperatively, and routine needle biopsy was performed to make a definite diagnosis. Neoadjuvant chemotherapy, post-operative chemotherapy, post-operative radiotherapy and targeted drug therapy were administered as needed. The clinical data including sex, age, location of tumors, length of bone grafts, surgical methods and types of internal fixation were recorded and analyzed (Supplement 1). The grade of tumors was assessed by Enneking staging of malignant bone tumors.17 The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Xiangya Hospital. All patients participating in the study received informed consent and signed consent from the patient or their legal guardians.
Treatment
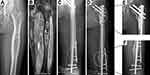
Patients with OS and ES received neoadjuvant chemotherapy and post-operative chemotherapy; patients with UDPS received routine post-operative chemotherapy; patients with CS and AB underwent only surgical treatment. The resection margin was determined using enhanced MRI images; intraoperative frozen-section biopsy was performed to confirm tumor negativity of margins of the extracted bone. A 3D-printed osteotomy guide plate was used in some patients (Figure 1).
We performed gradient rewarming of cryopreserved segmental bone allografts (Osteolink, Hubei, China) preoperatively. Allografts were successively immersed in sterilized water followed by in hydrogen peroxide for 30 and 15 min, respectively, then rinse thoroughly with sterilized water to remove residual bone marrow and to reduce the immunogenicity. The isometric bone allograft for reconstruction was obtained according to bone defect length following tumor resection (Figure 1B and C), and osteotomy interfaces at the host–donor junctions matched well (Figure 1F). The patients fixed with intramedullary nails expanded the marrow of the allograft, while the patients fixed with double steel plates injected with bone cement to strengthen the allograft. In five patients, we prepared two sets of identical osteotomy guide plates by 3D printing to ensure the accurate matching of host–donor junctions, one set for tumor osteotomy and the other for allograft osteotomy (Figure 1A and C). In our opinion, the coincidence area between the allograft and the host is more than 90% of the cross-section of the allograft, which is an accurate match. The shape and size of the tumor bone and allograft intercepted by the guide plate are almost similar (Figure 1D and E). Thereafter, allograft fixation and soft-tissue reconstruction were performed. Allografts were fixed using intramedullary nail (Stryker, Michigan, USA), a double plate (Stryker, Michigan, USA), and an intramedullary nail and plate combination in 2, 5, and 10 patients, respectively. Soft-tissue reconstruction via transposition of muscle (eg, sartorius and the medial head of gastrocnemius) flaps for good tissue coverage of allografts in situ.
Prophylactic use of antibiotics for 7 days and a negative pressure drain were placed in situ until the drainage volume decreased to <10 mL/day. The operated limb was fixed with an external gypsum cast for 4 weeks. Patients were started on isometric muscle exercises immediately after the operation, and flexion and extension exercises of adjacent joints were initiated on cast removal. Patients were allowed non-weight-bearing ambulation after eight postoperative weeks but not partial weight-bearing until there was radiological evidence of graft incorporation, which usually occurred >3 months postoperatively.
Follow-Up and Evaluation
Follow-up radiographs were obtained at 6 and 12 weeks post-surgery and then at every 3-month intervals for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Follow-up included physical examination, chest CT, and X-ray of the operated site. Tumor recurrence was suspected in case of the resurgence of clinical symptoms or on abnormality detection on plain radiographs. Allograft union was evaluated using ISOLS scoring system.18 At the final follow-up, bone union was defined by an ISOLS score >30, while non-union was either defined as host–donor interface nonunion detected radiologically, or ISOLS score <30. The functional outcome was assessed using the MSTS scoring system.19
Statistical Analysis
The data were analyzed using SPSS version 20 (SPSS Inc., Chicago, Illinois). The measurement data are expressed as mean ± standard deviation. The interface matching, complications and other counting data were expressed by specific values. The follow-up data were analyzed by paired T-test, independent-samples T-test and Chi-square test; P-value<0.05 was considered statistically significant.
Results
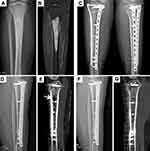
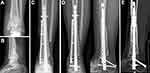
We performed intercalary allograft reconstruction of the femoral and tibial shafts in seven and four patients (Figures 2 and 3), respectively; another four patients underwent distal tibial reconstruction with ankle arthrodesis (Figure 4). The average allogenic bone graft length used in this group was 17 (range: 12.5–24.5) cm. No procedure-related operative complications were noted. The mean follow-up time was 49.8 months (26 to 78).
Oncology Prognosis
Up to the last follow-up, 13 cases survived without tumors, 4 cases survived with tumors and was treated with oral-targeted drugs for lung metastasis, and 1 case died of lung metastasis of osteosarcoma. Among them, two cases received amputation because of local recurrence after bone healing.
Healing of Host–Donor Junctions
Among the 34 host–donor junctions of 17 cases, 32 junctions had bone healing, of which five cases had accurate osteotomy with a 3D printing guide plate (Figure 2). The healing time was 6–22 months, with an average of 12.1 months. The healing time of metaphysis (9.1 mon) was significantly shorter than that of bone shaft (16.3 mon) (P < 0.05) (Table 1) and the use of osteotomy guide plate can achieve accurate matching to shorten the healing time, indicating that the more prone the metaphysis to bone healing (Figure 4). Host–donor junctions in two patients showed nonunion. The probability of nonunion was 6.77% in patients receiving chemotherapy (Table 2).
 |
Table 1 Summary Data of Patient Information |
 |
Table 2 Analysis of Related Factors of Bone Healing |
Functional Prognosis and Complications
Neither infection nor allograft fracture occurred in this group. Although four patients developed postoperative immune response, mainly manifested as fever and wound swelling of unknown cause, the symptoms abated with low-dose dexamethasone administration. There was no loosening or breaking of internal fixators, and limb salvage rate was 94.1% at the end of the final follow-up. Postoperative limb function according to the MSTS system averaged 24.2±5.3. Excellent and good ISOLS scores were achieved in 76.5% of patients (Table 1).
Discussion
The reconstruction methods for diaphyseal defects after primary malignant bone tumors resection include artificial prosthesis replacement,2 autogenous bone transplantation,20,21 allogeneic bone transplantation,9,22,23 masquelet technique,24 bone lengthening,25 autogenous devitalized bone reimplantation,5 autogenous fibula composite allogeneic bone transplantation,26 and so on. Rates and durations of survival, impact on the next steps of treatment, and possible complications should be considered while choosing the reconstruction method. Sometimes the tumors are located in the diaphysis, and the residual bone after segmental resection cannot be effectively fixed by the artificial prosthesis, so amputation is necessary. Allogeneic bone is a bone defect repair material with good histocompatibility, high mechanical stability, strong bone conduction, and excellent osteoinductive ability, which can last a lifetime once it survives. It is an excellent choice in the treatment of limb salvage and nonunion.4,9,27–31 Rollo G et al.28–31 reported that the use of allograft in the treatment of aseptic nonunion has achieved good results, and it also has significant advantages in fracture revision surgery. However, its application has been limited because of potential complications and controversial prognosis.10,14,16,32
Segmental allograft healing implies host–donor junction healing. Large segmental allografts cannot be completely transformed into host bone, which relies on the callus formed and induced osteogenesis by the host to gradually completes the creeping substitution process.11,13,33 The reported rate of nonunion in large bone allograft reconstruction is 9%~63%, with an average of 34%.34 Aponte TL35 reported a 13% incidence rate of nonunion after interfemoral allograft transplantation with diaphyseal junction showing higher nonunion than metaphyseal junction. Other studies reported nonunion incidence rates of 40% after allogenic bone transplantation for bone defect repair and simple intramedullary nailing or using >10 cm bone grafts increases the nonunion risk.11,15 A comparative analysis of allogenic and autogenous bone transplantation for repairing distal tibial bone defects showed higher complications of allogenic bone transplantation, with nonunion incidence reaching 27%.22 Muscolo DL et al1 used allogenic bone grafts to repair tibial and femoral interpositional bone defects and found that 37% patients needed reoperation because of complications, while 41% experienced nonunion. Although vascularized fibula and massive bone allograft can effectively accelerate bone healing,36 the extension of the operation time, the inability to use intramedullary nail fixation and the influence of chemotherapy on blood vessels limit its application in our study. In this study, the overall healing rate of 88.2%, and the average healing time was 12.1 months, which was shorter than the previous reports.11 The healing time of metaphysis was significantly shorter than that of bone shaft and the use of osteotomy guide plate can achieve precise matching to shorten the healing time. It is suggested that the successful rate of repairing bone defect with large bone allograft in lower limbs is higher, which may be related to more stress stimulation in lower limbs, and the accurate matching of graft and host junctional surfaces increases the contact area leading to satisfactory prognosis.
3D printing osteotomy guide plate is an excellent assistant for precise osteotomy, which can design accurate osteotomy according to the preoperative design and achieve a perfect match with the allograft. As the shaft is rigidity and incompressible, we usually use wire saw, pendulum saw, ultrasonic scalpel and other methods to cut off or meticulous treat the allograft. But in the actual operation process, the subjective error is inevitable. When the force line seems to be correct, it usually appears that the allograft has good contact with the host bone, but in fact, most of the surfaces are not in contact with each other, only some points are well matched, and there are still small gaps, which is no problem for simple fracture healing. However, for large-segment allograft, these gaps may lead to an imbalance of stress stimulation and affect healing. In order to solve this problem, we carefully selected the grafts according to the length and diameter of the resected bone, and the same size osteotomy guide plate was used to cut the transplanted bone. Surprisingly, the osteotomy surface matched very well. This method is very simple, but the exact result needs to be confirmed by further large samples.
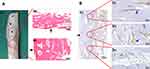
In this study, one patient underwent amputation due to recurrence, but imaging showed that the host–donor bone interface had healed. Histopathological analysis of host–donor junction (post-amputation) showed the formation of complete osseous junctions and many new haversian systems at the host–donor interface (Figure 5). The number of cells in the lacunae and bone mass at host bone junction were greater than that at the allograft (Figure 5A). Immunohistochemical results with CD31 staining revealed several blood vessels in the lacunae of the graft–host interface and the host bone but none in the allograft (Figure 5B). These findings indicated that bone healing occurred mainly at the interface, while the vascularization of allograft was difficult to achieve (Figure 5). Thus, once graft–host junction has healed, local bone strength is restored and increases over time. Allografts can last a lifetime, which is their biggest advantage as compared to prosthesis replacement.
The complications of intercalary allograft transplantation are also troubling. Allograft fracture,10,16,37-39 deep infection and immune response have been reported in previous studies, and the prognosis is not optimistic. Allograft fractures are difficult to heal because of their lack of biological activity and revision surgery is often inevitable. Fortunately, there were no allograft fractures in this study. The application of high-strength internal fixation may be a critical factor in preventing allograft fractures, which theoretically should be the standard procedure used for segmental allograft transplantation. Infection after allograft transplantation is a catastrophic complication. Removal of the allograft or amputation, if necessary, is often the only option.14,26 Studies14,40 have shown that radiotherapy and chemotherapy may increase the probability of postoperative infection. Surprisingly, although 88% (15/17) of our patients received chemotherapy, no postoperative infection occurred in this group, with only 2 patients showing delayed wound healing, possibly due to good soft-tissue coverage of the allogenic bone and adequate drainage of the wound postoperatively.
Our findings indicate the obvious advantages of using intercalary allograft for lower extremity diaphyseal defect reconstruction after tumor resection, but some details must be strictly grasped: ① tumor resection must be thorough to achieve good oncological prognosis; ② long term and absolute stable internal fixation is the guarantee of successful allograft transplantation; ③ increased osteotomy surface contact at host–donor junctions to the maximum extent possible and compressed contact surfaces effectively accelerate bone healing and prevent bone resorption; ④ adequate soft-tissue coverage to provide a good osteoinductive environment and reduce the risk of infection; ⑤precise preparation of allograft and adequate drainage facilitated postoperatively are also important.
Our study has certain limitations. First, the sample size was small to determine the incidence of complications accurately. Second, this was a retrospective study, and direct or randomized comparisons were not performed. Lastly, follow-up of some patients was inadequate to assess long-term complications. We hereby analyze the results of the application of this technique in lower limbs, hoping to provide help for clinical treatment. We also look forward to large sample, multi-center, prospective studies to obtain more accurate results.
Conclusion
Intercalary allograft transplantation is effective for diaphyseal defect following post-tumor resection in the lower extremity. Good bone healing after allograft reconstruction is achieved with stable internal fixation and perfectly matched host–donor interfaces. Optimizing the details of the treatment can effectively reduce the occurrence of postoperative complications and achieve a better prognosis.
Abbreviations
CT, computed tomography; MRI, magnetic resonance imaging; ISOLS, International Society of Limb Salvage; MSTS, Musculoskeletal Tumor Society; OS, osteosarcoma; CS, chondrosarcoma; AB, ameloblastoma; UDPS, undifferentiated pleomorphic sarcoma; ES, Ewing sarcoma.
Ethical Review Committee Statement
This study has been approved by the Research Ethics Committee of Xiangya Hospital.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Muscolo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426(426):97–102. doi:10.1097/01.blo.0000141652.93178.10
2. Benevenia J, Kirchner R, Patterson F, et al. Outcomes of a modular intercalary endoprosthesis as treatment for segmental defects of the femur, tibia, and humerus. Clin Orthop Relat Res. 2016;474(2):539–548. doi:10.1007/s11999-015-4588-z
3. Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324(324):86–97. doi:10.1097/00003086-199603000-00011
4. Aponte-Tinao L, Ayerza MA, Muscolo DL, Farfalli GL. Survival, recurrence, and function after epiphyseal preservation and allograft reconstruction in osteosarcoma of the knee. Clin Orthop Relat Res. 2015;473(5):1789–1796. doi:10.1007/s11999-014-4028-5
5. Chen TH, Chen WM, Huang CK. Reconstruction after intercalary resection of malignant bone tumours: comparison between segmental allograft and extracorporeally-irradiated autograft. J Bone Joint Surg Br. 2005;87-B(5):704–709. doi:10.1302/0301-620X.87B5.15491
6. Chen C, Garlich J, Vincent K, Brien E. Postoperative complications with cryotherapy in bone tumors. J Bone Oncol. 2017;7:13–17. doi:10.1016/j.jbo.2017.04.002
7. Aponte-Tinao LA, Ritacco LE, Albergo JI, Ayerza MA, Muscolo DL, Farfalli GL. The principles and applications of fresh frozen allografts to bone and joint reconstruction. Orthop Clin North Am. 2014;45(2):257–269. doi:10.1016/j.ocl.2013.12.008
8. Lee SH, Han SS, Yoo BM, Kim JW. Outcomes of locking plate fixation with fibular allograft augmentation for proximal humeral fractures in osteoporotic patients: comparison with locking plate fixation alone. Bone Joint J. 2019;101-B(3):260–265. doi:10.1302/0301-620X.101B3.BJJ-2018-0802.R1
9. Gupta S, Kafchinski LA, Gundle KR, et al. Intercalary allograft augmented with intramedullary cement and plate fixation is a reliable solution after resection of a diaphyseal tumour. Bone Joint J. 2017;99-B(7):973–978. doi:10.1302/0301-620X.99B7.BJJ-2016-0996
10. Ogilvie CM, Crawford EA, Hosalkar HS, King JJ, Lackman RD. Long-term results for limb salvage with osteoarticular allograft reconstruction. Clin Orthop Relat Res. 2009;467(10):2685–2690. doi:10.1007/s11999-009-0726-9
11. Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83(7):971–986. doi:10.2106/00004623-200107000-00001
12. Alman BA, De Bari A, Krajbich JI. Massive allografts in the treatment of osteosarcoma and ewing sarcoma in children and adolescents. J Bone Joint Surg Am. 1995;77(1):54–64. doi:10.2106/00004623-199501000-00008
13. Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73(8):1123–1142. doi:10.2106/00004623-199173080-00002
14. Mankin HJ, Hornicek FJ, Raskin KA, Infection in massive bone allografts. Clin Orthop Relat Res. 2005;(432):210–216. doi:10.1097/01.blo.0000150371.77314.52
15. Bus MP, Dijkstra PD, van de Sande MA, et al. Intercalary allograft reconstructions following resection of primary bone tumors: a nationwide multicenter study. J Bone Joint Surg Am. 2014;96(4):e26. doi:10.2106/JBJS.M.00655
16. Sorger JI, Hornicek FJ, Zavatta M, et al. Allograft fractures revisited. Clin Orthop Relat Res. 2001;382(382):66–74. doi:10.1097/00003086-200101000-00011
17. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120.
18. GLASSER D, LANGLAIS F. The ISOLS Radiological Implants Evaluation System. In Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Berlin: Springer-Verlag; 1991.
19. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;(286):241–246.
20. Hierner R, Wood MB. Comparison of vascularised iliac crest and vascularised fibula transfer for reconstruction of segmental and partial bone defects in long bones of the lower extremity. Microsurg. 1995;16(12):818–826. doi:10.1002/micr.1920161209
21. Qu H, Guo W, Yang R, et al. Reconstruction of segmental bone defect of long bones after tumor resection by devitalized tumor-bearing bone. World J Surg Oncol. 2015;13(1):282. doi:10.1186/s12957-015-0694-3
22. Zhao ZQ, Yan TQ, Guo W, Yang RL, Tang XD, Yang Y. Surgical treatment of primary malignant tumours of the distal tibia. Bone Joint J. 2018;100-B(12):1633–1639. doi:10.1302/0301-620X.100B12.BJJ-2018-0779.R1
23. Muscolo DL, Ayerza MA, Aponte-Tinao L, Farfalli G. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res. 2008;466(8):1856–1862. doi:10.1007/s11999-008-0303-7
24. Masquelet AC, Kishi T, Benko PE. Very long-term results of post-traumatic bone defect reconstruction by the induced membrane technique. Orthop Traumatol Surg Res. 2019;105(1):159–166. doi:10.1016/j.otsr.2018.11.012
25. Eralp L, Kocaoglu M, Rashid H. Reconstruction of segmental bone defects due to chronic osteomyelitis with use of an external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2007;89 Suppl 2(2):183–195.
26. Campanacci DA, Totti F, Puccini S, et al. Intercalary reconstruction of femur after tumour resection: is a vascularized fibular autograft plus allograft a long-lasting solution? Bone Joint J. 2018;100-B(3):378–386. doi:10.1302/0301-620X.100B3.BJJ-2017-0283.R2
27. Vicenti G, Maruccia M, Carrozzo M, Elia R, Giudice G, Moretti B. Free vascularized osteoseptocutaneous fibular flap for radius shaft nonunion: the final solution when the iliac crest autograft fails. A case report. Injury. 2018;49:S63–S70. doi:10.1016/j.injury.2018.11.030
28. Rollo G, Prkic A, Bisaccia M, et al. Grafting and fixation after aseptic non-union of the humeral shaft: a case series. J Clin Orthop Trauma. 2020;11(Suppl 1):S51–S55. doi:10.1016/j.jcot.2019.08.020
29. Rollo G, Bonura EM, Huri G, et al. Standard plating vs. cortical strut and plating for periprosthetic knee fractures: a multicentre experience. Med Glas (Zenica). 2020;17(1).
30. Rollo G, Pichierri P, Grubor P, et al. The challenge of nonunion and malunion in distal femur surgical revision. Med Glas (Zenica). 2019;16(2).
31. Rollo G, Tartaglia N, Falzarano G, et al. The challenge of non-union in subtrochanteric fractures with breakage of intramedullary nail: evaluation of outcomes in surgery revision with angled blade plate and allograft bone strut. Eur J Trauma Emerg Surg. 2017;43(6):853–861. doi:10.1007/s00068-016-0755-5
32. Ramseier LE, Malinin TI, Temple HT, Mnaymneh WA, Exner GU. Allograft reconstruction for bone sarcoma of the tibia in the growing child. J Bone Joint Surg Br. 2006;88-B(1):95–99. doi:10.1302/0301-620X.88B1.16253
33. Vander GR. The effect of internal fixation on the healing of large allografts. J Bone Joint Surg Am. 1994;76(5):657–663. doi:10.2106/00004623-199405000-00005
34. Donati D, Capanna R, Campanacci D, et al. The use of massive bone allografts for intercalary reconstruction and arthrodeses after tumor resection. A multicentric European study. Chir Organi Mov. 1993;78(2):81–94.
35. Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res. 2012;470(3):728–734. doi:10.1007/s11999-011-1952-5
36. Errani C, Ceruso M, Donati DM, Manfrini M. Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur J Orthop Surg Traumatol. 2019;29(2):307–311. doi:10.1007/s00590-018-2360-2
37. Bus MP, van de Sande MA, Taminiau AH, Dijkstra PD. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone Joint J. 2017;99-B(4):522–530. doi:10.1302/0301-620X.99B4.BJJ-2016-0443.R2
38. Asavamongkolkul A, Waikakul S. Plate fixation technique for reducing osteoarticular allograft fracture: a preliminary report. J Med Assoc Thai. 2016;99(10):1110–1118.
39. Bullens PH, Minderhoud NM, de Waal MM, Veth RP, Buma P, Schreuder HW. Survival of massive allografts in segmental oncological bone defect reconstructions. Int Orthop. 2009;33(3):757–760. doi:10.1007/s00264-008-0700-2
40. Wu C, Hsieh P, Fan JJ, Shih H, Chen C, Hu C. A positive bacterial culture from allograft bone at implantation does not correlate with subsequent surgical site infection. Bone Joint J. 2015;97-B(3):427–431. doi:10.1302/0301-620X.97B3.34600
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.