Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Interactive Effect of IGF2BP2 rs4402960 Variant, Smoking and Type 2 Diabetes
Authors Nfor ON, Ndzinisa NB, Tsai MH, Hsiao CH, Liaw YP
Received 30 October 2020
Accepted for publication 10 December 2020
Published 30 December 2020 Volume 2020:13 Pages 5097—5102
DOI https://doi.org/10.2147/DMSO.S289642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Oswald Ndi Nfor,1 Nokuphila Balindile Ndzinisa,1 Meng‑Hsiun Tsai,2 Chih-Hsuan Hsiao,1 Yung-Po Liaw1,3
1Department of Public Health and Institute of Public Health, Chung Shan Medical University, Taichung City 40201, Taiwan; 2Department of Management Information Systems, National Chung Hsing University, Taichung, Taiwan; 3Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung City 40201, Taiwan
Correspondence: Yung-Po Liaw
Department of Public Health and Institute of Public Health, Chung Shan Medical University, No. 110, Sec. 1 Jianguo N. Road, Taichung City 40201, Taiwan
Tel +886-4-24730022 ext.11838
Fax +886-4-23248179
Email [email protected]
Purpose: Genetic and environmental factors are related to type 2 diabetes (T2D). Genetic modifiers of T2D have not been widely determined among smoking individuals. In this population-based study, we investigated the interactive association between rs4402960 polymorphism of the insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) gene and smoking with T2D among Taiwanese adults.
Materials and Methods: We obtained genetic data collected between 2008 and 2018 for 22,039 participants (aged 30– 70 years) from the Taiwan Biobank (TWB) database. These data were analyzed using the t-test, Chi-square (χ2) test, and multiple logistic regression.
Results: The mean ages for participants with and without diabetes were 58.11± 8.75 and 48.58± 11, respectively. Compared with the rs4402960 GG genotype, the odds ratio (OR) for T2D was 1.261 among GT and 1.545 among TT genotype individuals (p< 0.05). Current smokers compared to nonsmokers were associated with a higher risk of T2D (OR=1.266, p=0.0404). There was a significant interaction between the IGF2BP2 rs4402960 variant and smoking on T2D (p = 0.0497). After stratification by rs4402960 genotypes and smoking status, the OR was substantial only in current smokers with GG genotype (OR, 1.663, p = 0.0008).
Conclusion: This population-based study indicated that the risk for T2D was stronger among current smoking rs4402960 GG individuals recruited between 2008 and 2019 in Taiwan.
Keywords: polymorphism, metabolic syndrome, lifestyle, diabetes
Introduction
Type 2 diabetes remains a significant issue of public health.1 The global prevalence of the disease was 9.3% in 2019 and may likely be 10.9% by 2045.2 Between 2005 and 2014, Li and his colleagues observed a slight decline in diabetes mortality from 3.45% to 3% in Taiwan even though its prevalence keeps rising.3 T2D is still among the most prevalent chronic diseases in Taiwan4 and is more predominant among men.5 To a certain degree, the higher prevalence of the disease in men may be attributed to smoking6 as most men in Taiwan are smokers.7
So far, several studies have previously focused on host genetic factors associated with diseases,8 including T2D. Risk factors for T2D are both modifiable and non-modifiable.1 Some of the modifiable factors linked to this condition include smoking, physical inactivity, overweight/obesity, and abnormal cholesterol levels.9 Active smoking predicted an increase in the risk of T2D,10 even though not all smokers develop the disease. A genome-environment-wide-interaction study suggested possible mechanisms that link T2D to cigarette smoking.11
To our knowledge, significant efforts have been made to investigate how T2D is affected by smoking or genetic loci in different populations. However, the interactive effect of both variables on the disease has not been sufficiently investigated. So far, the latest findings from a Mendelian study provided genetic evidence to support the link between T2D and smoking.12 More results from the study indicated that gene-smoking interactions for T2D had focused mainly on variants in CYP2A6, CHRNA4, HNF1A, and APOC3 genes. It is worth noting that genetic loci in these genes have been associated either with smoking behavior, T2D, or other metabolic traits.
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) is one gene whose variants have also shown associations with T2D.13 This gene plays an essential role in embryogenesis and pancreatic development.14,15 Among the IGF2BP2 variants related to T2D, rs4402960 has shown strong associations, particularly among Europeans and East and South Asian populations,16,17 even though contrary findings have also been reported among Han Chinese populations.18 As far as we know, this variant has not been investigated based on smoking status. Considering that genetic factors may modify the effects of smoking on T2D, we performed analyses to identify the interactive effect of IGF2BP2 rs4402960 and cigarette smoking on the risk of T2D using data from participants in TWB.
Materials and Methods
Data Source
Established in 2005, TWB is a research database that aims to collect genetic data from about 200,000 ethnic Taiwanese adults (aged 30–70 years) with no history of cancer.19 These data, especially on local chronic diseases will contribute to enhance knowledge on their interaction with genes and the environment.20 Information on participants’ recruitment is obtained from various media sources such as websites, television, posters, and brochures. Participants are eligible for enrollment if they meet the requirements listed on the TWB website. Eligible volunteers provided their contact information and are later contacted to confirm their willingness to participate in the project. They are assigned to specific recruitment centers. So far, TBW has 29 recruiting centers.
Study Participants and Identification of T2D
We obtained TWB data from 22,091 participants recruited between 2008 and 2018. Participants were between 30 and 70 years old. During recruitment, each participant had completed questionnaires covering a wide range of medical, social, and lifestyle information and provided blood samples for DNA analysis. The TWB research team obtained ethical approval for the program from the Ethics and Governance Council of Taiwan Biobank and the Institutional Review Board on Biomedical Science Research/IRB-BM in Academia Sinica.21 Data from 52 participants were incomplete and hence were excluded. In the final analysis, data were from 22,039 participants (11,049 women and 10,990 men). We identified T2D based on (1) a fasting glucose level of ≥126 mg/dl; (2) a glycosylated hemoglobin A1c value of at least 6.5%; or (3) self-reported diabetes.22 We defined nonsmokers as people with no history of smoking. Former smokers were those who never smoked in the past 6 months. Current smokers were people that smoked continuously for at least 6 months and were still smoking during the assessment.
Every participant provided informed consent before trained staff collected their data. This study was approved by the Institutional Review Board of Chung Shan Medical University.
Variant Selection and Genotyping
Using web-based search engines (such as SNPedia, Google Scholar, PubMed, and others), we selected two genetic variants, rs4402960 and rs1470579 of the IGF2BP2 gene, which have shown strong associations with T2D. Of these SNPs, rs4402960 was the only SNP that passed the quality control test, hence it was used in our final analysis. Genotyping was done at the National Center for Genome Medicine in Academia Sinica using the Axiom™ Genome-Wide ASI Array.
Statistical Analysis
We expressed the results of the statistical analysis as a mean ± standard deviation (SD). We used the Student’s t-test and the χ2 test to compare characteristics among participants and multiple logistic regression analyses to estimate the ORs for T2D. We performed these analyses using PLINK v1.09 and SAS 9.4 (SAS Institute, Cary, NC) software. We exclude SNPs with 1) p-value for the Hardy-Weinberg equilibrium test <0.001, 2) low call rate (<95%), and 3) minor allele frequency of <0.05.
Results
Of the overall participants (n = 22,039), 1030 had diabetes (Table 1). Baseline characteristics differed significantly between those with and without T2D (p<0.05). The genotype distribution of the rs4402960 variant among patients with T2D was 52.72% for GG, 40.39% for GT, and 6.89% for TT, respectively. Compared with the rs4402960 GG genotype (Table 2), the OR for T2D was 1.261 (p<0.0013) in GT and 1.545 (p<0.0020) in TT genotype individuals. Current smokers compared to nonsmokers were associated with a higher risk of T2D (OR=1.266, p = 0.0404). The OR was 2.285 (p<0.0001) in obese individuals and 3.394 (p<0.0001) in those with hyperlipidemia. There was a significant interaction between the rs4402960 variant and smoking on T2D (p = 0.0497). After stratification by rs4402960 genotypes and smoking status (Table 3), the OR was significant only in current smokers with GG genotype (OR, 1.663, p = 0.0008). There was no association between T2D and smoking among GT and TT genotype individuals (p>0.05). Hyperlipidemia remained a risk factor for T2D, no matter the genotype. The OR was 3.550 in GG, 3.283 in GT, and 2.957 in TT individuals, respectively (p<0.05). However, obesity remained a risk factor for T2D only among those with GG (OR, 2.13) and GT (OR, 2.634) genotype, respectively (p<0.0001).
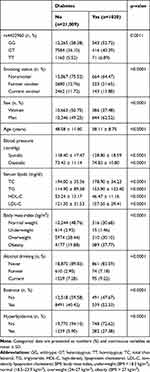 |
Table 1 Demographic Characteristics According to Disease Status |
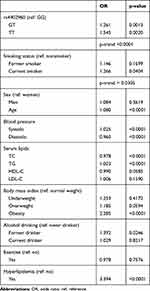 |
Table 2 Odds Ratios for T2D Among Overall Participants |
 |
Table 3 Odds Ratios for Type 2 Diabetes Based on rs4402960 Genotypes |
Discussion
In the current study, we investigated how the association between T2D and smoking vary based on the IGF2BP2 rs4402960 genotypes. As far as we know, prior studies in Taiwan have not assessed this interactive association. Using TWB data, we found that current smokers compared to nonsmokers were associated with a higher risk of T2D. According to Maddatu and his team, previous population-based studies have observed similar findings.23 We also found that rs4402960 GT and TT individuals were more likely to have T2D than GG individuals. This observation is similar to those that have been previously reported.16,24
When we performed the test for interaction for the rs4402960 variant and smoking, we observed that only the GG genotype was associated with T2D and specifically in current smokers (OR, 1.663, p = 0.0008). A recent replication study in Taiwan showed that genetic susceptibility to diabetes might be intensified by active smoking.21 Most recently, genome-environment-wide interactive studies on T2D also provided evidence for genetic interactions with active smoking.11 Despite these observations, the probable biologic pathways or regulatory mechanisms to clarify these associations remain to be determined.
Our general model showed that hyperlipidemia and obesity were substantial risk factors for T2D. These metabolic abnormalities are known to cause T2D. Obese smokers were observed with a higher risk and incidence of T2D.25 After stratification by rs4402960 genotypes, we found that obesity remained a risk factor for T2D only among those with GG and GT genotypes, In contrast, hyperlipidemia was a significant risk factor for T2D irrespective of the genotype.
The IGF2BP2 gene is greatly expressed in the pancreatic islets. The interactive effects between polymorphisms in this gene and T2D have been linked to the insulin-like growth factor 2 (IGF2) and insulin pathways.24 IGF2 activity also appears to be influenced by tobacco smoking.26 IGF2BP2 rs4402960 has been associated with reduced first-phase insulin secretion.24,27
Importantly, we found that the GT and TT genotypes were independent risk factors for T2D in Taiwanese adults. These are similar to those previously reported in European, East Asian, and South Asian populations.16 Contrary to expectation, when we tested for interaction between the rs4402960 variant and smoking, we found that the GG but not GT and TT genotype enhanced T2D risk among current smokers. The mechanism behind this observed association is not clear. However, there is a possibility that smoking might have either obscured or played a protective role in the association between T2D and the GT and TT genotype. Future studies would help to validate these findings. The main strength of our study is the large dataset. However, we collected participants’ information using TWB questionnaires; hence there was a possibility of bias.
Conclusion
We found that the rs4402960 variant of the IGF2BP2 gene had an interaction with smoking on T2D risk from this population-based study. We observed that only current smokers with the GG genotype had a greater chance of developing T2D. These findings extend knowledge of gene-environment interactions and their role in the etiology of T2D.
Funding
This study was supported by the Ministry of Science and Technology (MOST 107-2627-M-040-002, MOST 108-2621-M-040-001, MOST 109 −2121-M-040-002) and the National Chung Hsing University/Chung Shan Medical University Collaborative Research Grants (NCHU-CSMU 10707).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–236. doi:10.1038/nrendo.2011.183
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Li H-Y, Wu Y-L, Te Tu S, Hwu C-M, Liu J-S, Chuang L-M. Trends of mortality in diabetic patients in Taiwan: a nationwide survey in 2005–2014. J Formos Med Assoc. 2019;118:S83–S89. doi:10.1016/j.jfma.2019.07.008
4. Chang C-J, Lu F-H, Yang Y-C, et al. Epidemiologic study of type 2 diabetes in Taiwan. Diabetes Res Clin Pract. 2000;50:S49–S59. doi:10.1016/S0168-8227(00)00179-0
5. Chen K-T, Chen C-J, Gregg EW, Engelgau MM, Narayan KV. Prevalence of type 2 diabetes mellitus in Taiwan: ethnic variation and risk factors. Diabetes Res Clin Pract. 2001;51(1):59–66. doi:10.1016/S0168-8227(00)00200-X
6. Wannamethee SG, Shaper AG, Perry IJ. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care. 2001;24(9):1590–1595. doi:10.2337/diacare.24.9.1590
7. Wen C, Levy D, Cheng TY, Hsu C, Tsai S. Smoking behaviour in Taiwan, 2001. Tob Control. 2005;14(suppl1):i51–i55. doi:10.1136/tc.2004.008011
8. Moffitt TE, Caspi A, Rutter M. Measured gene-environment interactions in psychopathology: concepts, research strategies, and implications for research, intervention, and public understanding of genetics. Perspect Psychol Sci. 2006;1(1):5–27. doi:10.1111/j.1745-6916.2006.00002.x
9. Gillett M, Royle P, Snaith A, et al. Modifiable risk factors for type 2 diabetes mellitus. In: Non-Pharmacological Interventions to Reduce the Risk of Diabetes in People with Impaired Glucose Regulation: A Systematic Review and Economic Evaluation. NIHR Journals Library; 2012;16(33):15–26. doi:10.3310/hta16330
10. Yeh H-C, Duncan BB, Schmidt MI, Wang N-Y, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152(1):10–17. doi:10.7326/0003-4819-152-1-201001050-00005
11. Wu P, Rybin D, Bielak LF, et al. Smoking-by-genotype interaction in type 2 diabetes risk and fasting glucose. PLoS One. 2020;15(5):e0230815. doi:10.1371/journal.pone.0230815
12. Yuan S, Larsson SC. A causal relationship between cigarette smoking and type 2 diabetes mellitus: a Mendelian randomization study. Sci Rep. 2019;9(1):1–4. doi:10.1038/s41598-019-56014-9
13. Rao P, Wang H, Fang H, et al. Association between IGF2BP2 polymorphisms and type 2 diabetes mellitus: a case-control study and meta-analysis. Int J Environ Res Public Health. 2016;13(6):574. doi:10.3390/ijerph13060574
14. Li Z, Gilbert JA, Zhang Y, et al. An HMGA2-IGF2BP2 axis regulates myoblast proliferation and myogenesis. Dev Cell. 2012;23(6):1176–1188. doi:10.1016/j.devcel.2012.10.019
15. Christiansen J, Kolte AM, Nielsen FC, Nielsen FC. IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J Mol Endocrinol. 2009;43(5):187–195. doi:10.1677/JME-09-0016
16. Jia H, Yu L, Jiang Z, Ji Q. Association between IGF2BP2 rs4402960 polymorphism and risk of type 2 diabetes mellitus: a meta-analysis. Arch Med Res. 2011;42(5):361–367. doi:10.1016/j.arcmed.2011.08.001
17. Zhang S-M, Xiao J-Z, Qian R, et al. Replication of association study between type 2 diabetes mellitus andIGF2BP2in Han Chinese population. Chin Med J. 2013;126(21):4013–4018.
18. Wu -H-H, Liu N-J, Yang Z, et al. IGF2BP2 and obesity interaction analysis for type 2 diabetes mellitus in Chinese Han population. Eur J Med Res. 2014;19(1):40. doi:10.1186/2047-783X-19-40
19. Biobank T. Taiwan Biobank. 2015.
20. Fan C-T, Lin J-C, Lee C-H Taiwan Biobank: a project aiming to aid Taiwan’s transition into a biomedical island. 2008.
21. Lin W-Y, Liu Y-L, Yang AC, Tsai S-J, Kuo P-H. Active cigarette smoking is associated with an exacerbation of genetic susceptibility to diabetes. Diabetes. 2020;69:2819–2829. doi:10.2337/db20-0156
22. Nfor ON, Wu M-F, Lee C-T, et al. Body mass index modulates the association between CDKAL1 rs10946398 variant and type 2 diabetes among Taiwanese women. Sci Rep. 2018;8(1):1–7. doi:10.1038/s41598-018-31415-4
23. Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101–107. doi:10.1016/j.trsl.2017.02.004
24. Huang Q, Yin J-Y, Dai X-P, et al. IGF2BP2 variations influence repaglinide response and risk of type 2 diabetes in Chinese population. Acta Pharmacol Sin. 2010;31(6):709–717. doi:10.1038/aps.2010.47
25. Luo W, Guo Z, Wu M, Hao C, Zhou Z, Yao X. Interaction of smoking and obesity on type 2 diabetes risk in a Chinese cohort. Physiol Behav. 2015;139:240–243. doi:10.1016/j.physbeh.2014.11.038
26. Boo H-J, Min H-Y, Jang H-J, et al. The tobacco-specific carcinogen-operated calcium channel promotes lung tumorigenesis via IGF2 exocytosis in lung epithelial cells. Nat Commun. 2016;7(1):1–16. doi:10.1038/ncomms12961
27. Groenewoud M, Dekker J, Fritsche A, et al. Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia. 2008;51(9):1659–1663. doi:10.1007/s00125-008-1083-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
