Back to Journals » Journal of Inflammation Research » Volume 16
Interaction Between Blood Vasculatures and Lymphatic Vasculatures During Inflammation
Authors Wang SS, Zhu XX, Wu XY, Zhang WW, Ding YD, Jin SW, Zhang PH
Received 29 March 2023
Accepted for publication 21 July 2023
Published 4 August 2023 Volume 2023:16 Pages 3271—3281
DOI https://doi.org/10.2147/JIR.S414891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Shun-Shun Wang,1,2,* Xin-Xu Zhu,1,2,* Xin-Yi Wu,1,2,* Wen-Wu Zhang,1,2 Yang-Dong Ding,1,2 Sheng-Wei Jin,1,2 Pu-Hong Zhang1,2
1Department of Anesthesia and Critical Care, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Zhejiang, People’s Republic of China; 2Key Laboratory of Anesthesiology of Zhejiang Province, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sheng-Wei Jin; Pu-Hong Zhang, Department of Anesthesia and Critical Care, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 109 Xueyuan Road, Wenzhou, Zhejiang Province, 325027, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Physiological activity cannot be regulated without the blood and lymphatic vasculatures, which play complementary roles in maintaining the body’s homeostasis and immune responses. Inflammation is the body’s initial response to pathological injury and is responsible for protecting the body, removing damaged tissues, and restoring and maintaining homeostasis in the body. A growing number of researches have shown that blood and lymphatic vessels play an essential role in a variety of inflammatory diseases. In the inflammatory state, the permeability of blood vessels and lymphatic vessels is altered, and angiogenesis and lymphangiogenesis subsequently occur. The blood vascular and lymphatic vascular systems interact to determine the development or resolution of inflammation. In this review, we discuss the changes that occur in the blood vascular and lymphatic vascular systems of several organs during inflammation, describe the different scenarios of angiogenesis and lymphangiogenesis at different sites of inflammation, and demonstrate the prospect of targeting the blood vasculature and lymphatic vasculature systems to limit the development of inflammation and promote the resolution of inflammation in inflammatory diseases.
Keywords: inflammation, blood vessels, lymphatic vessels, lymphangiogenesis, angiogenesis
Introduction
The inflammatory response occurs when the body’s tissues are damaged or destroyed and is characterized primarily by the activation of immune and non-immune cells with the aim of eliminating risk factors and damaged tissues. Infection with abundant pathogens (such as bacteria, viruses, fungi, etc.) or trauma caused by various injuries can also cause inflammatory responses.1 Acute inflammation is activated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and terminated by the body’s homeostatic regulatory mechanisms.2 If the regulatory mechanisms of inflammation are impaired or the causes are not eliminated, the inflammation may become chronic and affect multiple organs throughout the body. Chronic systemic inflammation is also associated with aging; the presence of certain psychological, social, and environmental factors and associated intracellular substances released by dead cells can also mediate the expression of proinflammatory genes in immune cells.2,3
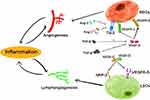
Functional and morphological changes in the blood and lymphatic vasculatures occur in a variety of inflammatory diseases. Vascular endothelial cells are extensively damaged by the action of vasoactive mediators produced in inflammatory responses, and endothelial barrier dysfunction leads to microcirculation failure and tissue edema. In inflammatory states, the body activates a variety of mechanisms to maintain homeostasis and repair blood vessels, including the activation and release of endothelial progenitor cells (EPCs) in the bone marrow and vascular wall niches to repair blood vessels.4 This also involves angiogenesis and lymphangiogenesis, which are fundamental changes in the development of inflammatory diseases (Figure 1). Angiogenesis has been defined as the formation of new blood vessels from preexisting blood vessels. The prevalence of angiogenesis in the inflammatory response suggests that the two phenomena are inextricably linked, with angiogenesis exacerbating the development of chronic inflammation and inflammation mediating higher levels of angiogenesis.5 Angiogenesis has a specific and unique molecular mechanism, and the substances involved in this process include the vascular endothelial growth factor (VEGF) family and angiopoietin (Ang) family. Among the VEGF family, VEGF-A is considered to be the most critical growth factor in the Angiogenesis process.6 Receptors for VEGF-A include vascular endothelial growth factor receptor (VEGFR)-1, which is widely expressed in endothelial cells, and VEGFR-2, which binds more preferentially to VEGF-A in healthy state.7 VEGFR-2 expression is relatively limited, present in blood vascular endothelial cells (BECs), and expresses high affinity for VEGF-A, an important receptor responsible for signaling during angiogenesis in inflammatory diseases.5 The Ang family involved in angiogenesis includes Ang-1, Ang-2, Ang-3, and Ang-4, among which Ang-1 and Ang-2 play antagonistic roles. Ang-1 is expressed in vascular smooth muscle cells and perivascular cells by activating tyrosine kinase binding to immunoglobulin-like and EGF-like domains (Tie)-2 receptor. It binds tightly to Tie-2 at endothelial cell junctions and improves vascular stability. Ang-2 is expressed by endothelial cells and acts as an antagonist of the Activating effect of Ang-1 on Tie2 in the presence of Ang-1, blocking the transmission of Tie2 activation signals and reducing vascular stability. In inflammatory diseases, Ang-2 is increased, mediating endothelial cell activation, and causing angiogenesis.8 In addition, transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF) have an effect on angiogenesis.5
In inflamed tissues, lymphatic vessels are responsible for transporting soluble antigens and antigen-presenting cells to lymph nodes (LNs). Lymphangiogenesis is present at the site of inflammatory injury in most inflammatory diseases. Although the VEGF family is also involved in lymphangiogenesis, which may be related to VEGF-C and VEGF-D.9 In chronic inflammatory conditions, such as in a mouse model of chronic colitis, VEGF-C enhances local lymphatic drainage and leads to the normalization of gut microbiota.10 The high expression of VEGF-C and VEGF-D at the sites of inflammatory injury may be induced by macrophages11 and mast cells12 among others. VEGFR-3 is widely expressed in lymphatic endothelial cells (LECs) and can be activated by VEGF-C and VEGF-D, inducing LEC proliferation and migration. Blocking VEGFR-3 signaling can reduce lymphangiogenesis and aggravate the risk of inflammatory edema.13,14 In addition, activating the nuclear factor-kappa B (NF-κB) signaling pathway in inflammation can also upregulate VEGF-C expression15 and mediate the upregulation of VEGFR −3 through LECs, increasing its affinity for VEGF-C and VEGF-D.16 Neuropilins (NRPs) are also involved in the development of the vascular and lymphatic systems, in which NRP-2 levels can be maintained continuously under inflammatory conditions and are involved in the regulation of inflammatory diseases.17 NRP2 is expressed on LECs, can also bind to VEGF-C and VEGF-D, increasing the sensitivity of LECs to VEGF-C and VEGF-D, and participates in lymphangiogenesis as a coreceptor for VEGFR-3.18 Changes in the blood and lymphatic vasculatures may be different when inflammation occurs at different sites.
The Skin
The skin is the largest organ in the human body. Due to its direct contact with the environment, it is required to perform functions such as regulating body temperature, providing a protective barrier against external pathogenic factors, and participating in the regulation of the immune system. In skin-related diseases, especially inflammatory or proliferative diseases, changes in the vasculature and lymphatic vessels cannot be ignored. The interaction between vascular system and lymphatic system maintains the homeostasis of the skin. Cutaneous neovascularization and lymphangiogenesis were previously thought to be associated with cancer spread,19 but an increasing number of studies have shown that they also play an essential role in the development of chronic skin inflammation. The structural differences between BECs and LECs in the skin determine their functional differences. Resting cutaneous vessels consist of a continuous monolayer of endothelial cells closely connected with pericytes. The overlapping layers of LECs and the lack of tightly connected cells facilitate the absorption of relevant components in the tissue fluid.20 When chronic skin inflammation occurs, endothelial cells are activated by inflammatory mediators, including VEGF-A and TNF-α, which induce vascular and lymphatic remodeling, resulting in increased VEGFR-1 and VEGFR-2 expression in BECs and increased vascular permeability and responsiveness to leukocytes. By contrast, VEGFR-2 and VEGFR-3 expression is increased in LECs, and the overlap structure of LECs is altered possibly because of increased interstitial fluid pressure due to edema caused by the hyperpermeability of the vascular system.20,21
A variety of inflammatory skin lesions are associated with structural or functional changes in the vascular and lymphatic systems, including but not limited to ultraviolet damage, psoriasis, and rosacea.22–24 Vasculature changes are a major feature of the pathogenesis of psoriasis, including the widened and distorted capillary structures in the dermis where psoriasis damages and the formation of glomerulus-like structures by distorted capillaries.25 In addition to the lesion site, there is a varying degree of increased permeability of the surrounding normal skin vessels, which plays an important role in the migration of inflammatory cells. Another major alteration of the vascular system in psoriasis is angiogenesis, which is associated with the rise of multiple pro-angiogenic cytokines, including VEGF, endothelial cell-stimulating angiogenesis factor (ESAF), TNF, TGF, hypoxia-inducible factor (HIF), and PDGF.26 VEGF-A is probably the most important growth factor involved in the changes of the lymphatic and vascular systems during skin inflammation. It can directly activate blood vessels and lymphatic vessels. VEGF-A is highly expressed in psoriasis and is a key component leading to vascular and lymphatic abnormalities.20,27 Treatment with anti-VEGF and its receptor is effective in inhibiting the progression of skin inflammation and the infiltration of inflammatory cells in mice with psoriasis.28 Lymphatic system changes in psoriasis have not attracted much attention compared with vascular system changes, but the lymphatic system also has great research potential. Lymphatic vessels at the lesion site are hyperplastic and dilated, with highly permeable lymphatic vessels and increased drainage of inflammatory cells.29 Similarly, anti-VEGF-A and anti-VEGF-A receptors inhibit lymphatic proliferation and expansion.30 However, in the cutaneous lymphatic system, the VEGF-C and VEGF-D secreted by mast cells and macrophages may be more important than VEGF-A in mediating lymphatic remodeling.31,32 The pathological features of psoriasis also include the excessive proliferation and immature differentiation of epidermal keratinocytes, with new blood vessels providing access to nutrients for the abnormally proliferating keratinocytes. The function of keratinocytes also includes the secretion of VEGF-C and VEGF-A, which affect the vascular and lymphatic systems.20 Keratinocyte differentiation is affected by the bioactive lipid, sphingosine-1-phosphate (S1P), which is secreted by activated platelets, blood cells, and vascular endothelial cells. S1P receptor is expressed on macrophages. Under inflammatory conditions, S1P receptor 1 (S1PR1) can regulate angiogenesis and lymphangiogenesis by mediating the expression of VEGF-A, VEGF-C, and their receptors.33 S1P may also affect the NF-κB signaling pathway by promoting TNF-α and IL-β secretion by LECs.34 The loss of myeloid S1PR1 can induce angiogenesis, inhibit lymphangiogenesis, and promote skin inflammation. These findings provide potential ways to treat inflammation by affecting the vascular and lymphatic systems.33 An increasing number of studies have focused on interfering with the cytokine network and keratinocytes that are closely related to angiogenesis for the treatment of psoriasis.27,35
The Cornea
The cornea is a transparent disk-shaped structure located at the front of the eye wall. The cornea of the human eye normally has no blood and lymphatic vessels to better let light pass through. The absence of blood and lymphatic vessels in the cornea is essential for maintaining clarity of vision and range of vision. This characteristic of the cornea is associated with many natural inhibitors of hemangiogenesis and the endogenous selective inhibitor of lymphatic vessels. Soluble VEGFR-1 (sVEGFR-1) is one of the key antiangiogenic molecules,36,37 and soluble VEGFR-2 is one of the key antilymphangiogenic molecules.38 sVEGFR-1, a soluble isoform of VEGFR-1, is present in the extracellular matrix (ECM) and acts as a decoy receptor for VEGF-A to sequester VEGF-A; thus, it has an anti-angiogenic capacity.39
However, when corneal inflammation is caused by a variety of factors (including but not limited to microorganisms, transplantation, tumor, and degeneration), inflammation can induce angiogenesis and lymphangiogenesis, which can improve the immune defense function of the cornea and speed up the repair of the cornea. In addition, the expression of several anti-angiogenesis factors is inhibited during inflammation. The expression of VEGF-A receptors, VEGFR1 and VEGFR2, is further increased in BECs during inflammation. Their activation promotes vascular leakage and the release of BECs from blood vessels;40 BECs release proteolytic enzymes that degrade the ECM around the cornea, paving the way for BECs to enter and cross the corneal matrix. BECs move to the inflammatory site through chemotaxis, forming new vascular lumens, and forming vascular branches, leading to angiogenesis.41 Corneal lymphangiogenesis is closely related to angiogenesis, and they have similar or different molecular mechanisms.42 The formation of corneal lymphatic vessels is associated with the VEGF-C expression induced by interleukin (IL)-1β and tumor necrosis factor (TNF)-α.43
In the corneal inflammatory response, angiogenesis and lymphangiogenesis are the two indispensable parts of immune response. Neovascularization provides immune effector cells with the ability to enter the cornea, whereas lymphangiogenesis accelerates the entry of corneal antigens into the LNs and provides an outlet for corneal immunity.
The Heart
Cardiac inflammation includes pericarditis, myocarditis, and endocarditis. Myocarditis is an inflammatory disease of the heart muscle, which can be caused by a variety of infectious factors, but the most common infectious agent is virus.44 The visceral vascular system plays an important role in the pathogenesis of infectious myocarditis. Intracardial microvessels and epicardial coronary arteries act as barriers to the entry of blood-borne pathogens into the heart and are an important part of the immune response to cardiac inflammation. Moreover, the vascular system is a prime target for infection. In infectious myocarditis, the endothelial cells of cardiac microvessels are the first barrier to pathogens of blood-borne infectious diseases. During the development of myocarditis, endothelial cells of cardiac microvessels can be infected by a variety of viruses, including dengue virus45 and hantavirus.46 Infection of cardiac microvascular endothelial cells results in endothelial cell damage and increased activation or permeability. The induction of inflammatory response may be related to the expression of adhesion molecules at the site of infection and injury. Intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 are increased in patients with lymphocytic myocarditis.47,48 Increased expression of endothelial adhesion molecules may also be associated with increased proinflammatory cytokines. For example, increased expression of the adhesion molecule ICAM-1 is associated with increased TNF-α and IL-1β levels.49 In addition, pathogens that cause inflammation also promote the expression of endothelial cell adhesion molecules, creating a positive feedback that accelerates inflammation.50
Studies on the lymphatic system in myocarditis are relatively few. Myocardial edema and the infiltration of lymphocytes and macrophages are manifestations of myocarditis51 and may be related to disorders of the lymphatic system. The cardiac lymphatic network is distributed between layers of the cardiac wall, and the lymphatic fluid of the endocardium flows through the myocardium, reaches the epicardium, and finally drains to the right lymphatic duct.52 Infection leads to acute myocarditis and increased TNF-α and IL-1β levels in the plasma circulation owing to endothelial cell damage which persist throughout the infection.53 These inflammatory mediators can influence or negatively affect the pattern of lymphatic contraction,54,55 resulting in impaired lymphatic collection.
The Central Nervous System
Glial cells in the brain parenchyma play an important role in the inflammatory response of the central nervous system (CNS). Microglia sense PAMPs and endogenous risk signals through Toll-like receptors in CNS lesions.56 Astrocytes are also involved in immune response to CNS injury and survive and function well in CNS inflammation.57 Lymphatic vessels were previously believed to be found only in the CNS, cartilage, bone marrow, thymus, and teeth. However, this idea has been questioned, because the clearance of excess fluid and macromolecules and tissue homeostasis must depend on lymphatic circulation. If the CNS lacks lymphatic vessels, as this idea suggests, then how the brain interstitial fluid (ISF) removes unwanted fluid and molecules becomes a puzzle.58 In a transgenic mouse model expressing VEGF-C/D trap, although the dural lymphatic vessels were completely aplastic, brain ISF pressure and water content were not affected, probably due to the presence of the dural lymphatic network. The presence of these lymphatic structures also provides a possible pathway for CSF drainage and immune cells to leave the central system for circulation during CNS inflammation.59
The blood–brain barrier (BBB) is the main barrier between the blood and brain. The structural composition and maintenance of the vascular system of the BBB require specialized endothelial cells, pericytes, perivascular microglia, and astrocytes, among which specialized endothelial cells are the most important. Together with glial cells in the brain, the BBB prevents immune cells from invading the CNS parenchyma during CNS inflammation and, unlike inflammation in other organs, the BBB is not angiogenic.60 Under normal circumstances, endothelial cells are considered to be the major component in the regulation of vascular hemodynamics and vascular permeability.61 When inflammation or injury occurs in the CNS, PAMPs activate brain endothelial cells; vascular permeability increases; and leukocytes interact with activated endothelial cells, attach to and roll around endothelial cells, search for interendothelial connections, and migrate across the endothelial barrier to the locally inflamed sites of the CNS with chemokine mediation (selectin and integrin).60 Culture of cerebral capillary endothelial cells in a specific environment can improve the self-protective ability of endothelial cells to maintain normal signaling pathways in response to LPS-induced infection60. Under physiological conditions, a very small number of leukocytes are found in the CNS. When CNS inflammation occurs, such as viral infection, leukocytes migrate to the CNS through the vascular system, including the epithelial monolayers of choroid plexus, meningeal vascular branches, and capillary posterior venules.62 In addition, the potential export of immune cells in the CNS may be related to the lymphatic structures mentioned above. At present, these potential lymphatic structures are still the focus of current research and need to be further understood.63,64
The Intestine
Similar to most other tissues in the body, the human digestive tract contains vascular and lymphatic systems. The two systems are responsible for absorbing various substances in the tissue fluid, but their roles are different. The solubility of substances determines which system they are associated with. The vascular system absorbs water-soluble substances, such as glucose, amino acids, and short-chain fatty acids. The lymphatic system is more responsible for the absorption of lipophilic substances.65 The lipids absorbed by intestinal cells assemble into chylomicrons (CMs), which enter the bloodstream through intestinal lymphatics known as lacteals.66 VEGF-A signal is closely related to the absorptive function of intestinal lymphatic vessels. The high expression of VEGF-A signals can inhibit CM uptake but can increase the permeability of the vascular system by opening the cell–cell connections that are closed under physiological conditions.66
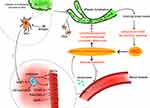
In inflammatory bowel disease (IBD), the vascular and lymphatic systems coordinate the transport of antigens and immune cells. The vascular system participates in the distribution and entry of immune cells into the inflammatory site, and leukocytes and antibodies at the site of inflammation are cleared through lymphatic vessels to control the development of inflammation.67 The vascular system is also responsible for carrying out biological functions, such as blood flow and tissue homeostasis. In IBD, activated endothelial cells are also involved in leukocyte recruitment, which limits the early development of inflammation.68 However, when the vascular system overloads the inflammatory burden and the lymphatic system fails to output enough to meet the body’s needs, these systemic disorders promote the development of abnormal inflammation and more lymphocyte activation.69 Under normal conditions, the adhesion process of intestinal leukocytes exists in two ways: (1) The CD11a/CD18 of leukocytes is involved and binds to the ICAM-1 of endothelial cells or (2) It binds to VCAM-1 or mucosal addressin cell adhesion molecule (MADCAM)-1via α4β7 integrin. The expression of MADCAM-1, ICAM-1, and VCAM-1 is enhanced in the vascular system of patients with IBD, further increasing the recruitment of leukocytes to the intestine. Inflammatory cells at the inflammatory site of intestinal inflammation are involved in angiogenesis through the production of angiogenic factors, which are also associated with the upregulation of VEGF due to hypoxia at the inflammatory site.70 These newly formed blood vessels are highly permeable and immature, increasing the number of inflammatory cells at the inflammatory site and contributing to IBD progression.67 IBD presents morphological and functional changes in the lymphatic system. Lymphatic obstruction and edema may occur in the early stage of inflammation and further lead to lymphatic dilation.71 However, lymphangiogenesis plays a positive role in improving inflammation and regulating the immune and metabolic activities of the body.16
Dendritic cells (DCs) in inflammatory tissues play an important role in intestinal inflammation. DCs carry antigens through lymphatic vessels to drain lymph nodes for antigen presentation and to determine immune response or immunosuppression.72 In addition, DCs induce the increased expression of adhesion molecules on lymphocytes, such as α4β7 integrin, which further promotes lymphocyte recruitment to inflammatory sites.72 Studies have shown that blocking the α4β7 and αEβ7 expressed by dendritic cell subsets can improve intestinal inflammation and reduce lymphocyte activation in patients with IBD.69 Changes in the blood and lymphatic vasculatures of patients with IBD are shown in Figure 2.
Platelets are associated with lymphangiogenesis in the mouse model of colitis; they are able to migrate to lymphatic vessels and exert an effect on LECs, ultimately reducing the level of lymphangiogenesis and aggravating the development of colitis.73 The VEGF-C/VEGFR-3 signaling pathway is also a hot topic in colitis treatment.74
The Bone
A fine network of lymphatic vessels is present throughout the body, but it was previously thought that normal bone tissue was devoid of lymphatic tissue and that lymphatic vessel growth in bone could be detrimental, as in Gorham-Stout syndrome (Gorham syndrome), which manifests as an increase in lymphatic vessels and fibrous tissue in the bone.75 And some studies have come to a different conclusion. For example, India ink injected into the bone marrow cavity was able to reach the lymph nodes after two weeks; high molecular weight ferritin and horseradish peroxide injected into the bone marrow were able to reach the periosteal surface, suggesting a connecting path between these two regions. The blood vessels and lymphatic vessels of bone tissue are elusive due to the technical difficulties of imaging calcified tissue. In recent years, high-resolution 3D imaging techniques have enabled tremendous progress in the study of blood and lymph in bone tissue,76,77 which has revealed the presence of lymphatic vessels within the bone.
Previous studies reported that blood vessels in the skeleton are highly sensitive and severely affected during radiation and bone marrow ablation.78 It has now been found that skeletal lymphatic vessels dilate in response to stresses such as irradiation and that they are able to secrete CXCL12 to promote hematopoietic stem cell regeneration and also act on Myh11+/CXCR4+ pericytes to maintain skeletal regeneration. This study suggests that lymphangiogenesis is a therapeutic pathway to stimulate blood and bone regeneration. Studies have also shown a relationship between angiogenesis, lymphangiogenesis and aging. Endothelial cell-specific knockdown of VEGFR2 in young mice leads to increased senescence, and these studies suggest that vascular damage precedes other characteristic senescent cellular events and that vascular changes can induce other cellular senescence events. Senescence also inhibits the skeletal lymphatic vessel response to stressful conditions.
Promising Approach
Existing research confirms that improving lymphatic circulation can actively remove pro-inflammatory mediators, inflammatory cells and tissue edema fluid, accelerate the restoration of damaged tissues, effectively alleviate the process of myocardial infarction, Alzheimer’s disease, ulcerative colitis, periodontitis and other diseases, and is a natural channel for “inflammation resolution”.14,79–82 Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection.83–85 Inflammatory storm brought about by a dysregulated inflammatory response is one of the most critical factors affecting the prognosis of patients with sepsis. The structural destruction of lymphatic vessels during sepsis, significant thrombosis and leakage, and dysfunctional lymphatic reflux were found in our laboratory preliminarily to be important causes of impaired inflammatory regression. Then it may be a very effective inflammatory treatment to promote inflammation regression by improving lymphatic reflux function, actively removing pro-inflammatory mediators, inflammatory cells and tissue edema fluid, and accelerating the restoration of the function of damaged organs.86,87
Conclusion
At present, various problems accompanying the inflammatory process still dominate the high morbidity and mortality of patients in clinical practice.2 Most of the previous studies have focused on reducing the release of inflammatory mediators or improving the immune function of the body for the treatment of inflammatory diseases. Here, we summarize the changes in blood and lymphatic vasculatures and their interactions in certain sites of inflammation. We believe that targeting the blood and lymphatic vasculatures is a promising approach for inflammation treatment. However, research on blood vessels and lymphatic vessels in inflammation has not yet reached an expected level; in particular, lymphatic vessels have been relatively little studied in the inflammatory response. In the therapeutic prospect, targeting the activation of lymphatic vessels to limit the occurrence and progression of inflammatory diseases has potential value, and the mechanisms of blood and lymphatic vessels in inflammation is worthy of further study. Finally, whether therapeutic approaches targeting blood and lymphatic vessels in inflammation are safe and effective deserves to be answered by further studies.
Data Sharing Statement
The authors declare that all data supporting the findings of this study are available within the article or from the corresponding author (Sheng-Wei Jin or Pu-Hong Zhang).
Acknowledgments
We gratefully acknowledge the team of the Scientific Research Center of Wenzhou Medical University for their technical and scientific expertise.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82270098, 82202378). China postdoctoral Science Foundation (2021M702502). Youth Scientific Research innovation Project of Wenzhou Medical University (KYYW202130).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Singh N, Baby D, Rajguru J, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
2. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0
3. Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374(6571):1076–1080. doi:10.1126/science.abi5934
4. Shi X, Seidle KA, Simms KJ, et al. Endothelial progenitor cells in the host defense response. Pharmacol Ther. 2023;241:108315. doi:10.1016/j.pharmthera.2022.108315
5. Lee HJ, Hong YJ, Kim M. Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci. 2021;22:12035.
6. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
7. Ling M, Lai X, Quan L, et al. Knockdown of VEGFB/VEGFR1 signaling promotes white adipose tissue browning and skeletal muscle development. Int J Mol Sci. 2022;23(14):7524. doi:10.3390/ijms23147524
8. Wang R, Yang M, Jiang L, Huang M. Role of angiopoietin-tie axis in vascular and lymphatic systems and therapeutic interventions. Pharmacol Res. 2022;182:106331. doi:10.1016/j.phrs.2022.106331
9. Secker GA, Harvey NL. VEGFR signaling during lymphatic vascular development: from progenitor cells to functional vessels. Dev Dyn. 2015;244(3):323–331. doi:10.1002/dvdy.24227
10. Wang X, Zhao J, Qin L. VEGF-C mediated enhancement of lymphatic drainage reduces intestinal inflammation by regulating IL-9/IL-17 balance and improving gut microbiota in experimental chronic colitis. Am J Transl Res. 2017;9(11):4772–4784.
11. Miyazaki T, Miyazaki A. Hypercholesterolemia and lymphatic defects: the chicken or the egg?. Front Cardiovasc Med. 2021;8:701229. doi:10.3389/fcvm.2021.701229
12. Sammarco G, Varricchi G, Ferraro V, et al. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int J Mol Sci. 2019;20(9):2106. doi:10.3390/ijms20092106
13. Wang XL, Zhao J, Qin L, Qiao M. Promoting inflammatory lymphangiogenesis by vascular endothelial growth factor-C (VEGF-C) aggravated intestinal inflammation in mice with experimental acute colitis. Braz J Med Biol Res. 2016;49(5):e4738. doi:10.1590/1414-431x20154738
14. D’Alessio S, Correale C, Tacconi C, et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124(9):3863–3878. doi:10.1172/JCI72189
15. Li CZ, Jiang X-J, Lin B, et al. RIP1 regulates TNF-α-mediated lymphangiogenesis and lymphatic metastasis in gallbladder cancer by modulating the NF-κB-VEGF-C pathway. Onco Targets Ther. 2018;11:2875–2890. doi:10.2147/OTT.S159026
16. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi:10.1016/j.cell.2010.01.045
17. Immormino RM, Jania CM, Tilley SL, Moran TP. Neuropilin-2 regulates airway inflammation in a neutrophilic asthma model. Immun Inflamm Dis. 2022;10(3):e575. doi:10.1002/iid3.575
18. Islam R, Mishra J, Bodas S, et al. Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance. Cancer Metastasis Rev. 2022;41(3):771–787. doi:10.1007/s10555-022-10048-0
19. Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13(8a):1405–1416. doi:10.1111/j.1582-4934.2009.00834.x
20. Huggenberger R, Detmar M. The cutaneous vascular system in chronic skin inflammation. J Invest Dermatol Symp Proc. 2011;15(1):24–32. doi:10.1038/jidsymp.2011.5
21. Bowlin A, Roys H, Wanjala H, et al. Hypoxia-inducible factor signaling in macrophages promotes lymphangiogenesis in leishmania major infection. Infect Immun. 2021;89(8):e0012421. doi:10.1128/IAI.00124-21
22. Xie X, Wang Y, Xia Y, Mao Y. Overexpressed vascular endothelial growth factor in adipose derived stem cells attenuates fibroblasts and skin injuries by ultraviolet radiation. Biosci Rep. 2019;39(7). doi:10.1042/BSR20190433
23. He Y, Kim J, Tacconi C, et al. Mediators of capillary-to-venule conversion in the chronic inflammatory skin disease psoriasis. J Invest Dermatol. 2022;142(12):3313–3326.e13. doi:10.1016/j.jid.2022.05.1089
24. Rodrigues-Braz D, Zhao M, Yesilirmak N, et al. Cutaneous and ocular rosacea: common and specific physiopathogenic mechanisms and study models. Mol Vis. 2021;27:323–353.
25. Stinco G, Buligan C, Maione V, Valent F, Patrone P. Videocapillaroscopic findings in the microcirculation of the psoriatic plaque during etanercept therapy. Clin Exp Dermatol. 2013;38(6):633–637. doi:10.1111/ced.12036
26. Micali G, Lacarrubba F, Musumeci ML, Massimino D, Nasca MR. Cutaneous vascular patterns in psoriasis. Int J Dermatol. 2010;49(3):249–256. doi:10.1111/j.1365-4632.2009.04287.x
27. Luengas‐Martinez A, Paus R, Young HS. Antivascular endothelial growth factor-a therapy: a novel personalized treatment approach for psoriasis. Br J Dermatol. 2022;186(5):782–791. doi:10.1111/bjd.20940
28. Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF. Systemic anti-VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci U S A. 2009;106(50):21264–21269. doi:10.1073/pnas.0907550106
29. Meier TO, Kovacicova L, Huggenberger R, et al. Increased permeability of cutaneous lymphatic capillaries and enhanced blood flow in psoriatic plaques. Dermatology. 2013;227(2):118–125. doi:10.1159/000351878
30. Granata F, Frattini A, Loffredo S, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010;184(9):5232–5241. doi:10.4049/jimmunol.0902501
31. Schwager S, Detmar M. Inflammation and lymphatic function. Front Immunol. 2019;10:308. doi:10.3389/fimmu.2019.00308
32. Moustou AE, Alexandrou P, Stratigos AJ, et al. Expression of lymphatic markers and lymphatic growth factors in psoriasis before and after anti-TNF treatment. An Bras Dermatol. 2014;89(6):891–897. doi:10.1590/abd1806-4841.20143210
33. Syed SN, Raue R, Weigert A, von Knethen A, Brune B. Macrophage S1PR1 signaling alters angiogenesis and lymphangiogenesis during skin inflammation. Cells. 2019;8(8):785. doi:10.3390/cells8080785
34. Zheng Z, Zeng Y-Z, Ren K, et al. S1P promotes inflammation-induced tube formation by HLECs via the S1PR1/NF-κB pathway. Int Immunopharmacol. 2019;66:224–235. doi:10.1016/j.intimp.2018.11.032
35. Wang J, Liu L, Sun X-Y, et al. Evidence and potential mechanism of action of lithospermum erythrorhizon and its active components for psoriasis. Front Pharmacol. 2022;13:781850. doi:10.3389/fphar.2022.781850
36. Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–997. doi:10.1038/nature05249
37. Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2006;104:264–302.
38. Albuquerque RJC, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15(9):1023–1030. doi:10.1038/nm.2018
39. Ceci C, Atzori MG, Lacal PM, Graziani G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: experimental evidence in different metastatic cancer models. Int J Mol Sci. 2020;21(4):1388. doi:10.3390/ijms21041388
40. Penn JS, Madan A, Caldwell RB, et al. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–371. doi:10.1016/j.preteyeres.2008.05.001
41. Nicholas MP, Mysore N. Corneal neovascularization. Exp Eye Res. 2021;202:108363. doi:10.1016/j.exer.2020.108363
42. Lee HK, Lee SM, Lee DI. Corneal lymphangiogenesis: current pathophysiological understandings and its functional role in ocular surface disease. Int J Mol Sci. 2021;22:11628.
43. Chung E-S, Saban DR, Chauhan SK, Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Visual Sci. 2009;50(4):1613–1618. doi:10.1167/iovs.08-2212
44. Martinez NE, Sato F, Kawai E, et al. Regulatory T cells and Th17 cells in viral infections: implications for multiple sclerosis and myocarditis. Future Virol. 2012;7:593–608. doi:10.2217/fvl.12.44
45. Povoa TF, Alves AMB, Oliveira CAB, et al. The pathology of severe dengue in multiple organs of human fatal cases: histopathology, ultrastructure and virus replication. PLoS One. 2014;9(4):e83386. doi:10.1371/journal.pone.0083386
46. Saggioro FP, Rossi M, Duarte M, et al. Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantavirus pulmonary syndrome. J Infect Dis. 2007;195(10):1541–1549. doi:10.1086/513874
47. Ino T, Kishiro M, Okubo M, et al. Late, persistent expressions of ICAM-1 and VCAM-1 on myocardial tissue in children with lymphocytic myocarditis. Cardiovasc Res. 1997;34(2):323–328. doi:10.1016/S0008-6363(97)00002-3
48. Benvenuti LA, Higuchi ML, Reis MM. Upregulation of adhesion molecules and class I HLA in the myocardium of chronic chagasic cardiomyopathy and heart allograft rejection, but not in dilated cardiomyopathy. Cardiovasc Pathol. 2000;9(2):111–117. doi:10.1016/S1054-8807(00)00027-2
49. Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99(8):1091–1100. doi:10.1161/01.CIR.99.8.1091
50. Woudstra L, Juffermans LJM, van Rossum AC, Niessen HWM, Krijnen PAJ. Infectious myocarditis: the role of the cardiac vasculature. Heart Fail Rev. 2018;23(4):583–595. doi:10.1007/s10741-018-9688-x
51. Zagrosek A, Wassmuth R, Abdel-Aty H, et al. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis – a CMR study. J Cardiovasc Magn Reson. 2008;10(1):19. doi:10.1186/1532-429X-10-19
52. Shimada T, Noguchi T, Takita K, Kitamura H, Nakamura M. Morphology of lymphatics of the mammalian heart with special reference to the architecture and distribution of the subepicardial lymphatic system. Acta Anat. 1989;136(1):16–20. doi:10.1159/000146791
53. Zhang L, Han B, Liu H, et al. Circular RNA circACSL1 aggravated myocardial inflammation and myocardial injury by sponging miR-8055 and regulating MAPK14 expression. Cell Death Dis. 2021;12(5):487. doi:10.1038/s41419-021-03777-7
54. Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64(1):362–369. doi:10.1016/j.cyto.2013.05.015
55. Al-Kofahi M, Omura S, Tsunoda I, et al. IL-1β reduces cardiac lymphatic muscle contraction via COX-2 and PGE2 induction: potential role in myocarditis. Biomed Pharmacother. 2018;107:1591–1600. doi:10.1016/j.biopha.2018.08.004
56. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in toll-like receptor-mediated neuronal injury. Glia. 2010;58(3):253–263. doi:10.1002/glia.20928
57. Lu S-Z, Wu Y, Guo Y-S, et al. Inhibition of astrocytic DRD2 suppresses CNS inflammation in an animal model of multiple sclerosis. J Exp Med. 2022;219(9). doi:10.1084/jem.20210998
58. Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6_suppl_1):S93–S95. doi:10.1161/STROKEAHA.112.678698
59. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi:10.1084/jem.20142290
60. Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. 2017;101(5):1119–1132. doi:10.1189/jlb.3RU0816-352RR
61. Davidson SM. Endothelial mitochondria and heart disease. Cardiovasc Res. 2010;88(1):58–66. doi:10.1093/cvr/cvq195
62. Li X, Qi L, Yang D, et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat Neurosci. 2022;25(5):577–587. doi:10.1038/s41593-022-01063-z
63. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi:10.1038/nature14432
64. Hogan BM, Bower NI. Lymphatics and the brain: it’s time to go fishing. Circ Res. 2021;128(1):59–61. doi:10.1161/CIRCRESAHA.120.318496
65. DeSesso JM, Jacobson CF. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem Toxicol. 2001;39(3):209–228. doi:10.1016/S0278-6915(00)00136-8
66. Zhang F, Zarkada G, Han J, et al. Lacteal junction zippering protects against diet-induced obesity. Science. 2018;361(6402):599–603. doi:10.1126/science.aap9331
67. D’Alessio S, Tacconi C, Fiocchi C, Danese S. Advances in therapeutic interventions targeting the vascular and lymphatic endothelium in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29(6):608–613. doi:10.1097/MOG.0b013e328365d37c
68. Verstockt B, Vetrano S, Salas A, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2022;19(6):351–366. doi:10.1038/s41575-021-00574-7
69. Dai B, Hackney JA, Ichikawa R, et al. Dual targeting of lymphocyte homing and retention through α4β7 and αEβ7 inhibition in inflammatory bowel disease. Cell Rep Med. 2021;2(8):100381. doi:10.1016/j.xcrm.2021.100381
70. Martin-Rodriguez O, Gauthier T, Bonnefoy F, et al. Pro-resolving factors released by macrophages after efferocytosis promote mucosal wound healing in inflammatory bowel disease. Front Immunol. 2021;12:754475. doi:10.3389/fimmu.2021.754475
71. Zhang L, Ocansey DK, Liu L, et al. Implications of lymphatic alterations in the pathogenesis and treatment of inflammatory bowel disease. Biomed Pharmacother. 2021;140:111752. doi:10.1016/j.biopha.2021.111752
72. Hokari R, Tomioka A. The role of lymphatics in intestinal inflammation. Inflamm Regen. 2021;41(1):25. doi:10.1186/s41232-021-00175-6
73. Sato H, Higashiyama M, Hozumi H, et al. Platelet interaction with lymphatics aggravates intestinal inflammation by suppressing lymphangiogenesis. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G276–G285. doi:10.1152/ajpgi.00455.2015
74. Lee AS, Hur HJ, Sung MJ. The effect of artemisinin on inflammation-associated lymphangiogenesis in experimental acute colitis. Int J Mol Sci. 2020;21:8068.
75. Solorzano E, Alejo AL, Ball HC, et al. Osteopathy in complex lymphatic anomalies. Int J Mol Sci. 2022;23(15):8258. doi:10.3390/ijms23158258
76. Chen J, Sivan U, Tan SL, et al. High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci Adv. 2021;7:eabd7819.
77. Biswas L, Chen J, De Angelis J, et al. Lymphatic vessels in bone support regeneration after injury. Cell. 2023;186(2):382–397.e324. doi:10.1016/j.cell.2022.12.031
78. Stucker S, Chen J, Watt FE, Kusumbe AP. Bone angiogenesis and vascular niche remodeling in stress, aging, and diseases. Front Cell Dev Biol. 2020;8:602269. doi:10.3389/fcell.2020.602269
79. Klotz L, Norman S, Vieira JM, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522(7554):62–67. doi:10.1038/nature14483
80. Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. doi:10.1038/s41586-018-0368-8
81. Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, et al. Enhancing renal lymphatic expansion prevents hypertension in mice. Circ Res. 2018;122(8):1094–1101. doi:10.1161/CIRCRESAHA.118.312765
82. Wang H, Chen Y, Li W, et al. Effect of VEGFC on lymph flow and inflammation-induced alveolar bone loss. J Pathol. 2020;251(3):323–335. doi:10.1002/path.5456
83. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
84. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–787. doi:10.1001/jama.2016.0289
85. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
86. Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi:10.1038/nature02238
87. Wang Q, Zhang H-W, Mei H-X, et al. MCTR1 enhances the resolution of lipopolysaccharide-induced lung injury through STAT6-mediated resident M2 alveolar macrophage polarization in mice. J Cell Mol Med. 2020;24(17):9646–9657. doi:10.1111/jcmm.15481
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.