Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Intensity-Modulated Radiotherapy with Concomitant Boost After Breast Conserving Surgery: A Phase I–II Trial
Authors Macchia G , Cilla S, Buwenge M , Zamagni A , Ammendolia I, Zamagni C, Frezza GP , Valentini V , Deodato F , Morganti AG
Received 8 May 2020
Accepted for publication 10 July 2020
Published 12 November 2020 Volume 2020:12 Pages 243—249
DOI https://doi.org/10.2147/BCTT.S261587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Gabriella Macchia,1 Savino Cilla,2 Milly Buwenge,3 Alice Zamagni,3 Ilario Ammendolia,3 Claudio Zamagni,4 Giovanni P Frezza,5 Vincenzo Valentini,6,7 Francesco Deodato,1,* Alessio G Morganti3,*
1Radiotherapy Unit, Gemelli Molise Hospital, Università Cattolica Del Sacro Cuore, Campobasso, Italy; 2Medical Physics Unit, Gemelli Molise Hospital, Università Cattolica Del Sacro Cuore, Campobasso, Italy; 3Radiation Oncology Center, Department of Experimental, Diagnostic and Specialty Medicine ‑ DIMES, University of Bologna, S. Orsola-Malpighi Hospital, Bologna, Italy; 4Addarii Medical Oncology Unit, S. Orsola-Malpighi Hospital, Bologna, Italy; 5Radiation Oncology Unit, Bellaria Hospital, Bologna, Italy; 6Dipartimento di Scienze Radiologiche, Radioterapiche ed Ematologiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, UOC di Radioterapia, Rome, Italy; 7Istituto di Radiologia, Università Cattolica Del Sacro Cuore, Rome, Italy
*These authors contributed equally to this work
Correspondence: Milly Buwenge
Radiation Oncology Unit, Department of Experimental, Diagnostic and Specialty Medicine – DIMES, University of Bologna, S. Orsola-Malpighi Hospital, Via Giuseppe Massarenti 9, 40138, Bologna
Tel +39 0512143564
Fax +39 0516364336
Email [email protected]
Purpose: A concomitant boost (CB) in patients treated with postoperative radiotherapy after conservative surgery of invasive breast cancer (BC) has been suggested for treatment time reduction and therapy intensification. The aim of this analysis was to assess long-term tolerability of a CB in patients treated with postoperative intensity Modulated Accelerated RAdiotherapy (MARA).
Patients and Methods: In this phase I–II trial, 321 patients with intermediate-high risk BC (pT1-4 with at least one of the following characteristics: pre or perimenopausal status, pN2-3, positive or close margins) were enrolled. Patients were treated with forward-planned intensity modulated radiotherapy (IMRT) and CB. A total dose of 50 Gy (2 Gy/fraction) and 60 Gy (2.4 Gy/fraction) was prescribed to the whole breast and the tumor bed, respectively. The potential impact of hypertension, diabetes, smoking habit, alcohol consumption, chemotherapy, and hormone therapy on both skin and subcutaneous late toxicity-free survival (LTFS) was evaluated. Survival curves were calculated using the Kaplan–Meier method.
Results: Median follow-up was 52 months (range: 3– 115). Regional node irradiation, adjuvant chemotherapy and hormonal therapy were prescribed to 29.3%, 65.4% and 81.0% of patients, respectively. Five-year G2 and G3 skin LTFS were 95.6% and 100.0%, respectively. Five-year G2 and G3 subcutaneous LTFS were 80.0% and 98.6%, respectively. Only diabetes showed a significant correlation with worse G3 subcutaneous LTFS (p: 0.024). Five-year loco-regional control, metastasis-free survival, disease-free survival, and overall survival were 98.0%, 91.8%, 89.7% and 96.3%, respectively.
Conclusion: IMRT combined with CB was associated with a low risk of > G2 late toxicities (0.0% and 1.4% for skin and subcutaneous tissue, respectively). The cumulative actuarial incidence of local recurrences was 2.0% despite the exclusion of low-risk patients. Our results suggest that CB is safe and effective in patients with intermediate-high risk BC.
Trial Registration: ClinicalTrials.gov: NCT03471741.
Keywords: breast cancer, adjuvant radiotherapy, concomitant boost, IMRT, toxicity
Introduction
Around 276,500 new breast cancer (BC) cases are expected in 2020 in the USA with an estimated 90.0% 5-year survival.1 After breast conserving surgery, whole breast irradiation (WBI) is the standard of care based on the results from randomized trials.2,3
Current international guidelines4,5 recommend the delivery of a boost on the tumor bed in women ≤50 years, with high-grade disease, focal positive margins and other evidence of aggressive disease (such as extensive ductal carcinoma in situ component or lymphovascular invasion), based on the results of the EORTC trial.6
In order to improve WBI tolerability, intensity-modulated radiotherapy (IMRT) has been investigated in the conservative treatment of BC, showing reduced acute skin toxicity and improved long-term cosmesis.7 With IMRT, the homogeneity of dose distribution can be improved and a simultaneous integrated boost (SIB) can be delivered. Therefore, IMRT-SIB has been used in BC patients with the advantages of reduced overall treatment time and delivery of a higher dose per fraction on the tumor bed.8–10
Moreover, the use of a concomitant boost (CB) is equivalent to the delivery of an accelerated and hypofractionated dose on the tumor bed. This radiotherapy regimen is theoretically associated with higher efficacy (assuming an α/β value of 4 for BC) although a higher risk of late toxicity could be expected. However, evidence on the tolerability of CB in this setting is scarce.8–10
Therefore, to clarify this topic, the aim of this study was to prospectively evaluate late toxicity-free survival (LTFS) in a large cohort of patients with intermediate-high risk of recurrence treated with IMRT and CB.
Patients and Methods
A phase I–II trial was carried out on patients with histologically confirmed invasive BC and intermediate-high risk of recurrence who were treated according to the Modulated Accelerated RAdiotherapy (MARA-2) institutional protocol. The inclusion criteria were breast conserving surgery patients with invasive pT1-4 BC diagnosis with one or more of the following characteristics: pre or perimenopausal status, pN2a or pN3a, and focal positive (defined as ink on tumor) or close margins (≤2 mm). Exclusion criteria were distant metastasis, involvement of supraclavicular lymph nodes, involvement of internal mammary nodes, and pregnancy.
Endpoints
The primary objective of the study was to analyze LTFS (cutaneous and subcutaneous). Secondary objectives were local control (LC), disease-free survival (DFS), metastasis-free survival (MFS) and overall survival (OS). The study design was previously described.11
Treatment Planning
Patients were immobilized in supine position with an alpha-cradle system during radiotherapy planning and delivery. Computed tomography simulation slices were taken at 5-mm intervals from the larynx to the upper abdomen. The planning target volumes (PTV1 and PTV2) were defined as the relative clinical target volumes 1 and 2 (CTV1: tumor bed; CTV2: whole breast tissue minus skin) plus a three-dimensional 8-mm margins for set-up uncertainties. The skin (external 5 mm) was not included in the CTV2 except for tumors staged pT4 due to cutaneous infiltration. A single isocenter technique was used.
In patients undergoing prophylactic lymph node irradiation, the supraclavicular volume was irradiated cranially to the isocenter with 2 opposed beams. Instead, in the same patients, the breast volume was irradiated caudally to the isocenter with the tangential technique.
Dose prescription and specification were based on the ICRU 62 report.12 The simplified IMRT technique was planned using two conformed tangential beams (field in field technique) with two different photons energies (6 MV, 15 MV). The details of this treatment technique have been previously described.11 The entire breast volume was included in the first segment (6 MV photons beam). The thickest region of the breast was included in the second segment (15 MV photons) which was conformed to increase the dose to the deepest part of the PTV (generally under-dosed in the absence of filters) while sparing the most superficial part of the breast. The PTV1 was treated using two 3D-conformal tangential photon beams with standard MLC and wedge filters at the same time of PTV2.
Radiotherapy
Radiotherapy started ≥3 weeks after completion of chemotherapy. The prescribed dose to the whole breast (PTV2) was 50 Gy with a concomitant boost (CB) of 10 Gy in 0.40 Gy/fraction (total: 25 fractions) to the PTV1. Before radiotherapy treatment, the whole procedure of planning was verified by carrying-out several independent checks.13 Prior to delivery of each radiotherapy daily fraction, a set-up verification was performed using an Electronic Portal Imaging Device.14
Follow-Up
Standard clinical examinations started 3 weeks after the end of radiotherapy, then visits were planned every 6 months for the first 3 years and annually thereafter. Bilateral mammography was required every 12 months. Late toxicity was evaluated at every visit by two expert physicians (GM, AGM) using the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) criteria (Table 1).15 Late toxicity was defined as any toxicity occurring after 3 months from the start date of radiotherapy. Only toxicity on the breast was evaluated in this study while toxicity detected at the level of supraclavicular area was not considered being not related to the delivery of a CB. Differentiation between skin toxicity from breast treatment and from supraclavicular node treatment was shown to be easy. Distinction was simplified by the permanent tattooing performed at the level of the isocenter. According to the used technique, this was placed at the field junction.
 |
Table 1 Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer Scale Late Toxicity Scale |
Statistical Analysis
LC, DFS, and MFS were calculated from the start date of radiotherapy until the date of the first clinical or radiological confirmation of disease recurrence. Actuarial curves were calculated using the Kaplan–Meier method.16 In the descriptive tables, statistical summaries were expressed as numbers (with percentages) or medians (with ranges) for continuous variables while absolute and relative frequencies for categorical ones. Using the Log rank test, the impact on LTFS on the following parameters was analyzed:17 hypertension, diabetes, alcohol consumption, smoking habit, chemotherapy, and hormone therapy. Hypertension, diabetes, smoking habits, chemotherapy and hormone therapy were simply categorized as ‘yes’ or “no” while we considered patients who took at least one dose of alcohol per day as alcohol users. We defined as statistically significant a two-sided p-value < 0.05. Statistical analysis was performed using IBM SPSS Statistic for Windows version 23.0 (Released 2015. Armonk, NY: IBM Corp.). A multivariate analysis based on the Cox proportional-hazards regression model was not performed to estimate hazard ratios of cutaneous and subcutaneous toxicities due to lack of statistical significance (p < 0.05) or trends (p < 0.1) at univariate analysis, in all but one single parameter.
Ethical Issues
All patients signed a written informed consent before enrolment. This trial (UCSC-CB-2003/05, MARA-2 study) was approved by the local review board (Fondazione Giovanni Paolo II Ethics Committee) and was in accordance with the Helsinki Declaration. The study is registered in an international public registry (ClinicalTrials.gov: NCT03471741).
Results
Patients’ Characteristics
A total of 321 patients were included in this study between June 2004 and November 2014. Median follow-up was 52 months (range: 3–115 months). In Tables 2 and 3 patients and treatment characteristics are shown in detail, respectively. Eighteen patients had diabetes and 85 patients had hypertension. In addition, 53 patients were smokers and 99 patients were regular consumers of alcohol. Supraclavicular prophylactic node irradiation was performed in 29.3% of patients. No patient received prophylactic irradiation of the internal mammary lymph nodes. Adjuvant chemotherapy and hormonal therapy were prescribed to 65.4% and 81.0% of patients, respectively.
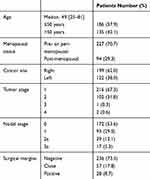 |
Table 2 Patients’ Characteristics |
 |
Table 3 Treatment Characteristics |
Toxicity and Outcome
Acute skin toxicity was scored as grade 0–1 in 277 patients (86.3%), grade 2 in 42 patients (13.1%), and grade 3 in 2 patients (0.6%).
Five-year grade 2 and grade 3 skin LTFS were 95.6% and 100.0%, respectively. Five-year grade 2 and grade 3 subcutaneous LTFS were 80.0% and 98.6%, respectively. All cases of grade 3 subcutaneous late toxicity were represented by severe fibrosis. No cases of rib fracture were recorded.
We evaluated at univariate analysis the potential impact of hypertension, diabetes, smoking habits, alcohol consumption, chemotherapy, and hormone therapy on both skin and subcutaneous LTFS. Aside from diabetes, none of the analyzed factors showed a significant correlation or a trend with any grade of late toxicity. Five-year subcutaneous grade 3 LTFS was 93.8% and 98.9% in diabetic and non-diabetic patients, respectively (p: 0.024). Finally, 5-year LC, MFS, DFS, and OS were 98.0%, 91.8%, 89.8%, and 96.3%, respectively.
Discussion
The combination of CB with conventional or hypofractionated WBI in intermediate-high risk BC following breast conserving surgery is potentially useful. In fact, it allows time reduction and dose escalation in the higher risk area (and therefore a higher radiobiological effect on the tumor bed). Moreover, IMRT technique allows an improvement of dosimetric parameters and reduction of doses at organs at risk also in WBI setting.9 However, the optimal schedule for WBI plus CB is still not defined. Some studies that tested this combination with different techniques and doses reported satisfactory results in terms of clinical outcome and toxicity.18–29
In our study, we analyzed LTFS in 321 patients with intermediate-high risk BC treated using a simplified forward-planned IMRT technique plus CB [whole breast: 50 Gy (2 Gy/fraction); tumor bed: 60 Gy (2.4 Gy/fraction)].
Our analysis has several limitations such as the lack of assessment of pain in the irradiated site, of patients reported outcome measures, and of the cosmetic outcome. In addition, the risk classification adopted by us was based on a subjective judgment and not on recognized international guidelines. Moreover, since the study design dates to the beginning of 2003, we used a toxicity scale now considered obsolete (RTOG/EORTC) and this limits the possibilities of comparison with more recent experiences. Finally, for the same reason, the study was based on a conventional dose fractionation and therefore our conclusions cannot be generalized to hypofractionated regimens, that are now considered as the standard treatment option in this setting. We found a statistically significant correlation between grade 3 subcutaneous late toxicity and diabetes. However, this result should be considered with caution due to the small number of observed events (3 cases of G3 subcutaneous toxicity) and the small number of diabetic patients.18 We did not register any other significant correlation with potential risk factors (hypertension, smoking habits, alcohol consumption, chemotherapy, and hormone therapy). Particularly, the lack of correlation between radiotherapy induced toxicity and chemotherapy is consistent with literature data.20,26,30,31
Five-year LTFS was high considering skin toxicity (G2 and G3 LTFS: 95.6% and 100.0%, respectively) and reasonable for subcutaneous toxicity (G2 and G3 LTFS: 80.0% and 98.6%, respectively). A treatment modality quite similar to ours (conventionally fractionated WBI plus CB) was used by Bantema-Joppe and colleagues19 and by Alford and colleagues,25 who reported lower rates of G2 subcutaneous toxicity (8.5% and 1.9%, respectively) compared to the current series, as shown in Table 4. However, these results are not completely comparable with ours due to the different grading systems used (RTOG/EORTC versus CTCAE v3.0). In fact, the grade definition for this toxicity do not completely correspond among the two systems. Furthermore, the median follow-up of our series was longer compared to the other authors and this may have impacted on the detection of moderate fibrosis. Moreover, in the cited studies, the toxicity rates were reported as crude values while in our analysis they are actuarial. However, beyond the methodological differences, the recorded results suggest that the use of CB does not significantly worsen the toxicity rates.
 |
Table 4 Late Toxicity in Selected Studies |
Although conventional fractionation is still an option accepted by international guidelines,4 in recent decades hypofractionated regimens have been used more frequently in WBI after conservative surgery, as mentioned above. However, it should be noted that our study was designed in early 2003, when the evidence on the efficacy and tolerability of hypofractionation in this setting was limited. Hamilton and colleagues10 analyzed the available data on toxicity in patients treated with hypofractionated WBI and CB. They reported G2 late fibrosis rates ranging from 1% to 9% and G2 late telangiectasia rates ranging from 1% to 6%. G3 fibrosis was never reported using this fractionation schedule and telangiectasia was 0% in all studies but one (4%).23 These results seem to suggest that even the combination of hypofractionated WBI with CB is tolerable in terms of late effects.
Finally, our study showed satisfactory results in terms of LC and OS, particularly if we consider the selection criteria (intermediate-high BC). A comparison with similar studies is reported in Table 5. Bantema-Joppe and colleagues reported 3.3% and 93.3% 5-year incidence of local failure and OS, respectively.22 McDonald and colleagues reported 2.9% and 97.0% 3-year incidence of local failure and OS, respectively.18 In our series, the 5-year incidence of local failure and OS rates were 2.0% and 96.3%, respectively. Overall, the results achieved by CB in terms of outcome, especially LC, seem to be positive.
 |
Table 5 Outcome Results in Selected Studies |
Conclusion
In summary, compared to sequential boost, CB have some theoretical advantages like the reduced treatment time and improved radiobiological effects in the higher risk area. Our results suggest that CB is tolerable and effective in reducing local recurrence rate in patients with intermediate-high risk BC. However, the optimal technique and fractionation is still not defined. The results from ongoing randomized controlled trials (such as RTOG1005, IMPORT HIGH, IMRT MC-2 trials) will further clarify this topic.
Dedication
This paper is dedicated to our colleague Cinzia Digesù (1971–2015) who passed away when the study was under development and whose contribution to this trial was invaluable.
Data Sharing Statement
The data sets used and/or analyzed during the current study (de-identified patients characteristics and outcomes) are available from the corresponding author after publication and for the duration of 5 years on reasonable request. No other documents will be made available.
Disclosure
Claudio Zamagni reports grants, personal fees, and non-financial support from Roche, Novartis, AstraZeneca, and Pfizer, grants from Eisai, PharmaMar, Celgene, lilly, Amgen, Pierre Fabre, Takeda, TEVA, Medivation, Array BioPharma, Abbvie, Morphotek, Synthon, Seattle Genetics, Daiichi and Sankyo, grants and personal fees from Tesaro, personal fees from QuintilesIMS, and travel accommodation and research funding from Istituto Gentili, outside the submitted work.
Alessio G. Morganti reports grants from Elekta, Beyer, and IGEA and personal fees from Astellas and Alfa-Sigma, outside the submitted work.
The authors report no other potential conflicts of interest in this work.
References
1. Female Breast Cancer - Cancer Stat Facts. Available from: https://seer.cancer.gov/statfacts/html/breast.html.
2. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi:10.1056/NEJMoa022152
3. Veronesi U, Cascinell N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi:10.1056/NEJMoa020989
4. Macdonald S, Oncology R, General M. Breast cancer. Available from: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf
5. Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi:10.1093/annonc/mdv298
6. Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised Phase 3 trial. Lancet Oncol. 2015;16:47–56. doi:10.1016/S1470-2045(14)71156-8
7. Buwenge M, Cammelli S, Ammendolia I, et al. Intensity modulated radiation therapy for breast cancer: current perspectives. Breast Cancer. 2017;9:121–126.
8. Freedman GM, White JR, Arthur DW, Allen Li X, Vicini FA. Accelerated fractionation with a concurrent boost for early stage breast cancer. Radiother Oncol. 2013;106(1):15–20. doi:10.1016/j.radonc.2012.12.001
9. Franco P, Cante D, Sciacero P, Girelli G, La Porta MR, Ricardi U. Tumor bed boost integration during whole breast radiotherapy: A review of the current evidence. Breast Care. 2015;10:44–49. doi:10.1159/000369845
10. Hamilton DG, Bale R, Jones C, et al. Impact of tumour bed boost integration on acute and late toxicity in patients with breast cancer: A systematic review. Breast. 2016;27:126–135. doi:10.1016/j.breast.2016.03.002
11. Morganti AG, Cilla S, Valentini V, et al. Phase I-II studies on accelerated IMRT in breast carcinoma: technical comparison and acute toxicity in 332 patients. Radiother Oncol. 2009;90:86–92. doi:10.1016/j.radonc.2008.10.017
12. International Commission on Radiation Units and Measurements. ICRU Report 62. Prescribing, Recording, and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). Bethesda, MD: ICRU; 1999.
13. Morganti AG, Deodato F, Zizzari S, et al. Complexity index (COMIX) and not type of treatment predicts undetected errors in radiotherapy planning and delivery. Radiother Oncol. 2008;89:320–329. doi:10.1016/j.radonc.2008.07.009
14. Deodato F, Cilla S, Massaccesi M, et al. Daily on-line set-up correction in 3d-conformal radiotherapy: is it feasible? Tumori. 2012;98:441–444. doi:10.1177/030089161209800407
15. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C
16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi:10.1080/01621459.1958.10501452
17. Peto R, Peto J. Asymptotically efficient rank invariant procedures. J R Stat Soc. 1972;135:185–207.
18. McDonald MW, Godette KD, Whitaker DJ, Davis LW, Johnstone PAS. Three-year outcomes of breast intensity-modulated radiation therapy with simultaneous integrated boost. Int J Radiat Oncol Biol Phys. 2010;77:523–530. doi:10.1016/j.ijrobp.2009.05.042
19. Bantema-Joppe EJ, Schilstra C, De Bock GH, et al. Simultaneous integrated boost irradiation after breast-conserving surgery: physician-rated toxicity and cosmetic outcome at 30 months’ follow-up. Int J Radiat Oncol Biol Phys. 2012;83:e471–e477. doi:10.1016/j.ijrobp.2012.01.050
20. Corvò R, Ricchetti F, Doino D, et al. Adjuvant hypofractionated radiotherapy with weekly concomitant boost for women with early breast cancer: the clinical experience at Genoa university. Anticancer Res. 2010;30:4749–4753.
21. Teh AYM, Walsh L, Purdie TG, et al. Concomitant intensity modulated boost during whole breast hypofractionated radiotherapy - A feasibility and toxicity study. Radiother Oncol. 2012;102:89–95. doi:10.1016/j.radonc.2011.10.015
22. Bantema-Joppe EJ, Vredeveld EJ, De Bock GH, et al. Five year outcomes of hypofractionated simultaneous integrated boost irradiation in breast conserving therapy; Patterns of recurrence. Radiother Oncol. 2013;108:269–272. doi:10.1016/j.radonc.2013.08.037
23. Raza S, Lymberis SC, Ciervide R, et al. Comparison of acute and late toxicity of two regimens of 3- and 5-week concomitant boost prone IMRT to standard 6-week breast radiotherapy. Front Oncol. 2012;2:1–10.
24. Cante D, Franco P, Sciacero P, et al. Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the surgical bed after conserving surgery for early breast cancer. Med Oncol. 2013;30:518. doi:10.1007/s12032-013-0518-7
25. Alford SL, Prassas GN, Vogelesang CR, Leggett HJ, Hamilton CS. Adjuvant breast radiotherapy using a simultaneous integrated boost: clinical and dosimetric perspectives. J Med Imaging Radiat Oncol. 2013;57:222–229. doi:10.1111/j.1754-9485.2012.02473.x
26. Osa EOO, Dewyngaert K, Roses D, et al. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys. 2014;89:899–906. doi:10.1016/j.ijrobp.2014.03.036
27. Chadha M, Woode R, Sillanpaa J, et al. Early-stage breast cancer treated with 3-week accelerated whole-breast radiation therapy and concomitant boost. Int J Radiat Oncol Biol Phys. 2013;86:40–44. doi:10.1016/j.ijrobp.2012.11.010
28. Formenti SC, Gidea-Addeo D, Goldberg JD, et al. Phase I-II trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J Clin Oncol. 2007;25:2236–2242. doi:10.1200/JCO.2006.09.1041
29. Freedman GM, Anderson PR, Bleicher RJ, et al. Five-year local control in a Phase II study of hypofractionated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:888–893. doi:10.1016/j.ijrobp.2012.01.091
30. Cante D, Petrucci E, Sciacero P, et al. Ten-year results of accelerated hypofractionated adjuvant whole-breast radiation with concomitant boost to the lumpectomy cavity after conserving surgery for early breast cancer. Med Oncol. 2017;34:152. doi:10.1007/s12032-017-1020-4
31. De Rose F, Fogliata A, Franceschini D, et al. Hypofractionation with simultaneous boost in breast cancer patients receiving adjuvant chemotherapy: A prospective evaluation of a case series and review of the literature. Breast. 2018;42:31–37. doi:10.1016/j.breast.2018.08.098
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
