Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Integration of Single-Cell Transcriptomics Data Reveal Differences in Cell Composition and Communication in Acne
Authors Zhao CX, Wang SL, Li HX, Li X
Received 18 September 2023
Accepted for publication 21 November 2023
Published 30 November 2023 Volume 2023:16 Pages 3413—3426
DOI https://doi.org/10.2147/CCID.S436776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Chen-Xi Zhao, Shi-Lei Wang, Hai-Xia Li, Xin Li
Department of Cosmetology and Dermatology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing, 400021, People’s Republic of China
Correspondence: Xin Li, Department of Cosmetology and Dermatology, Chongqing Hospital of Traditional Chinese, Medicine, Chongqing, 400021, People’s Republic of China, Tel +86 18680731024 ; +86 67063760, Email [email protected]
Purpose: Acne is a kind of hair follicle sebaceous inflammatory disease, which has a high incidence rate among adolescents. Comparative data on cells which beneficial for precise treatment of acne patients.
Patients and Methods: After integrating and removing the batch effect of single-cell transcriptomics data of acne patients and health skin, the dimensionality reduction clustering was performed and the change in characteristics of each cell group were analyzed. Further, cell communication differences between gender were analyzed by use Cellchat software.
Results: 70,189 cells were analyzed, and 11 cell groups were identified. The proportion of basal cells and macrophages in skin of acne patients are relatively high than that of skin in healthy people. The results of cell communication showed that the communication intensity of acne patients was significantly higher than that of healthy skin, and the endothelial cells showed a strong ability to receive signals. From the perspective of gender differences, the proportion of macrophages in male patients were higher than that in female patients, and there were a large number of basal cells in the lesion area of female patients. There are also have some specific immune response ligand-receptor regulatory signals in male patients.
Conclusion: There are significant differences in skin cell composition and cell communication patterns between acne patients and healthy people, especially reflected in gender differences. Basal cells, macrophages and endothelial cells can serve as key targets for acne treatment. The treatment methods for men and women should be more personalized.
Keywords: acne, single-cell, gender, cell communication, cell composition
Introduction
Acne is a chronic inflammatory disease of the hair follicle and sebaceous gland.1 It is often found on the face, chest and back, where sebum secretion is strong.2–4 According to statistics, acne patients account for about 5% of patients in the Department of Dermatology. Epidemiological survey shows that acne is more common in young people.5,6 About 80–90% of teenagers have acne, 15–30% of them need treatment, and 2–7% have left severe scars. Most patients will naturally relieve with age, but 10% of patients will continue to be over 25 years old.7–10 Because acne often occurs on the face, it affects the appearance of patients and has a significant impact on the physical and mental health of patients.11
The pathogenesis of acne is complex. Four factors or pathological processes, such as excessive secretion of lipids, abnormal keratinization of hair follicles and sebaceous ducts, the proliferation of Propionibacterium acne and inflammation, constitute the main process of acne.12,13 Under the influence of puberty male hormones, sebaceous glands secrete a large number of lipids, which is one of the preconditions for acne occurrence.14 The occurrence of acne is closely related to the level of sex hormones, in which androgen is considered to play the most important role.15 In addition, inflammation is an important pathological process of acne, and its clinical manifestations are papules, pustules and nodules.16 However, the occurrence and development of acne inflammation, especially the changes in the single cell level of patients, need to be further clarified.17 In recent years, single cell sequencing technology has developed rapidly, based on the above background information, this study uses public databases to download and re-analyzed single cell transcriptomics data of acne and that of healthy skin, hoping to find the key target of acne regulation from the single-cell transcriptome data.
Materials and Methods
Data Source
The original single cell transcriptomics data of acne patients were download from the GEO (https://www.ncbi.nlm.nih.gov/geo/) public database, which includes the data of six acne patients’ single-cell transcriptome (GSE175817).18 Each sample contains single-cell data of acne lesion area and surrounding normal non-lesion area. At the same time, we also collected the single cell transcriptome data of 7 normal healthy skin cells. Seven samples are from 5 different datasets (GSE130973,19 GSE181316,20 GSE156326,21 GSE176415,22 Tabib201823). It contains skin single-cell sequencing data of 3 healthy male and 4 healthy female donors (Table 1).
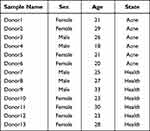 |
Table 1 Donor Information |
Data Integration, Quality Control and Removal of Batch Effects
We have conducted a strict review of each dataset, including the platform, the specific gender, the age to the integrity of the sample. For all datasets, the DoubletFinder software package (version 2.0.3) is used to remove double cells, and then each sample is extracted to create a seurat object separately using seurat package (version 4.1.1). Finally, all seurat objects are merged into one seurat object, and sample information and other contents are added to the metadata table The data characteristics of seurat objects were evaluated to measure the number of genes and cells as well as the expression of mitochondria, erythrocytes and ribosomes. The quality control was carried out according to the standards of the minimum gene of a single cell is 500, the maximum gene data of 6000, the number of mitochondrial genes less than 15% and the proportion of red blood cell genes less than 1%. The method of Harmony software package (version 0.1.0) was further used to remove the batch effect,24 and the sample distribution and lisi score of each cell cluster were used to check the removal of batch effect.
Dimension Reduction and Cell Clustering
The integrated seurat object which removes the batch effect is analyzed according to the seurat standard analysis process, including the use functions of NormalizeData, FindVariableFeatures and ScaleData, all of which use the default parameters of the function. In order to obtain the appropriate number of cell clusters, the clustree function (version 0.4.4) was used to plot the number of cell clusters with a resolution from 0.05 to 0.5, and the appropriate resolution of cell clusters was selected according to the results. Finally, the method of umap is used for dimensionality reduction clustering.
Cell Identification
The Findallmarker function of seurat software package is used to calculate the differential genes of each cell cluster. The threshold value is set to 0.25, the minimum cell ratio is set to 0.25, and other parameters are the default parameters of the function. Analyze the differential genes of each cluster, especially the top 10 genes with the largest difference in expression, and identify each population in combination with the Cellmarker website (http://yikedaxue.slwshop.cn/index.php).
Functional Enrichment Analysis
The bitr function of the clusterProfile software package (version 4.4.4) was used to convert the screened differential gene symbols into ENTREZID, and then the enrichGO function and enrichKEGG function were used, and the org.Hs.eg.db software package was loaded for GO and KEGG enrichment.25 The enrichment content includes biological processes, cell components and molecular functions. The items with statistical significance are grouped by Dotplot function to display the enrichment results.
Gene Set Scoring
In order to evaluate the difference of immune response of immune cells, the gene set scoring method was used to analyze the gene set scoring of each immune cell population. A total of 6 gene sets were selected for this study, and the gene data of the gene set were downloaded on the GSEA official website (http://www.gseamsigdb.org/gsea/msigdb/index.jsp). The six gene sets are inflammation response gene set (200 genes in total), IL2-STAT5 signal pathway gene set (199 genes in total), IL6-JAKSTAT3 signal pathway (87 genes in total), NOTCH signal pathway (32 genes in total) and TNFA signal pathway (200 genes in total). All the original gene sets and the genes detected by the transcriptome were collected and analyzed. All the data were scored using the AddModuleScore function of the seurat software package, and the results were plotted using ggplot2 (version 3.4.0).
Cell Communication Analysis
Cell communication analysis uses cellchat software (version 1.5.0) to extract the objects to be analyzed from the seurat object, establish the cellchat object, and select the CellChatDB.human database according to the standard process of cellchat to predict the communication of all objects.26 Then, the mergeCellchat function is used to merge all single objects, and the inter-group differences of the merged objects are analyzed. The content of analysis includes the number of cell communication signals and the strength of cell communication.
Results
Cell Clusters and General Difference
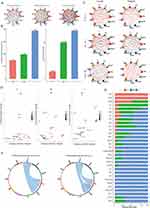
In this study, 13 samples from 6 data sets were integrated and analyzed (Table 1). After strict quality control and removal of batch effect, a total of 70,189 cells were included in the analysis. By using Harmony’s method, the batch effect is well removed, and the LISI scores for evaluating the batch effect are also greater than 2 (close to 1 is the obvious batch effect, and greater than 2 is the small batch effect) (Figure 1A and Figures S1-10). In addition, according to the elbow chart (Figure S3), the Principal Component Analysis (PCA) dimension of this study is selected as 30. The dimensionality reduction cluster diagram shows that there are 11 cell groups in all samples, including 1 cluster of fibroblasts (FIB), 2 clusters of basal cells (Basel1 and Basel2), 1 cluster of Myofibroblast (SMC), 1 cluster of vascular endothelial cells (EC), 1 cluster of lymphatic endothelial cells (Ly-Ec), 1 cluster of melanocytes (Melan), 1 cluster of T cells (T), 1 cluster of macrophages (Mac), 1 cluster of megakaryocytes (Mast) and 1 cluster of Schwann cells (Swan) (Figure 1B). The expression of specific markers of all cell populations is obvious (Figure 1C). In terms of cell distribution ratio, T cells and macrophages in the lesion area (L) of acne patients are significantly higher than those in healthy skin (H) and normal skin non-lesion (NL) of acne patients; Interestingly, the proportion of Basel2 cells in normal skin of acne patients is relatively high (Figure 1D). According to the ratio of skin cells in acne patients and healthy people, macrophages and Basel2 cells in acne patients increased significantly (Figure 1E). We also further extracted the information of the lesion samples of acne patients for separate analysis. From the perspective of gender ratio, the proportion of differences between macrophages and basal cells is very large (Figure 1F), which shows the significant gender difference in the regulation of cell proportion of acne patients.
Gender Differences in Basal Cells of Acne Patients
The above results have shown significant differences in basal cells in acne patients’ samples. We further extracted basal cells (including Basel1 and Basel2) for detailed analysis. The number of Basel2 cell cluster is small, but the distribution proportion shows obvious specificity. Basel2 cell cluster is mainly distributed in the lesion area of female patients (Figure 2A). We have studied the differences between Basel2 cluster and other cell cluster in transcriptome level. From the volcanic map, it can be seen that this population is quite different from other cell populations, and there are many different genes (Figure 2B). We have enriched the functions of the genes that are up-regulated by other cell clusters, and found that the up-regulated genes are mainly functioned in cell adhesion and energy metabolism regulation (Figure 2C). However, down-regulated genes are mainly functioned in the processes of epithelial differentiation and keratinization (Figure 2D). We also focused on the detailed study of the difference between Basel2 and Basel1. From the perspective of the difference genes between the two, Basel2 shows more epithelial differentiation and inflammatory factor recruitment and regulation functions (Figure 2E), and the enrichment of KEGG pathway shows a strong oxidative phosphorylation characteristic (Figure 2F).
The Proportion of Macrophages and Inflammatory Reaction in Male Acne Patients are Higher Than That in Female Patients
We further extracted all immune cells, including macrophages, T cells and Mast cells. From the change of the proportion of each state (health, non-lesion and lesion), the proportion of macrophages in the lesion area of acne patients increased significantly, and the number of Mast cells decreased significantly (Figure 3A). In addition, according to the sex ratio of acne patients, the number of macrophages in male patients is significantly higher than that in female patients, and the number of T cells is significantly lower than that in female patients (Figure 3B). From the expression of various typical marker proteins, IL6 and CD3D show certain gender differences (Figure 3C–H). Six gene sets related to inflammatory response and energy metabolism were selected to score the expression differences of all inflammatory cells. From the results, it can be seen that in acne patients, all inflammation has a slight upward trend (Figure 3I). In addition, in terms of gender characteristics, the scores of inflammation and energy metabolism in male are slightly higher than those in female (Figure 3J). We further extracted all macrophages for in-depth analysis. After further dimensionality reduction clustering, macrophages were divided into three groups (Figure 4A), of which cluster 2 was mainly distributed in the lesion area of acne patients (Figure 4A and B). In addition, from the perspective of gender characteristics, cluster 2 is mainly distributed in male acne patients (Figure 4C). From the function of the three macrophage clusters, both cluster0 and cluster1 are related to the regulation of inflammation, and the top 10 of the difference genes are mainly inflammation-related genes, which are presumed to be M1-macrophages. The function of cluster 2 involves extracellular mechanism remodeling. The main difference genes are collagen-related genes, which are presumed to be M2-macrophages (Figure 4D and E). In male and female patients, there are also significant differences in the expression of genes related to inflammation and remodeling in different groups. In female patients, there are significant differences in the expression of genes related to inflammation such as CXCL8 and IL1B in cluster1 and cluster2, mainly distributed in male. However, COL1A1 and COL3A1 genes related to tissue remodeling are expressed in all groups and in male patients (Figure 4F).
Cell Communication in Acne Patients are Higher Than That in Healthy Skin
The number of cell communication reflects the active state of cell population. The active degree of cells in healthy skin of normal people is significantly lower than that of acne patients, and its number and weight show the high active state of cells in acne patients (Figure 5A and B). From the perspective of the regulation between each cell, it is mainly manifested in the regulation between fibroblasts and macrophages (Figure 5C), which is further confirmed in the weight diagram of the sending and receiving strength of signals between each cell (Figure 5D). In patients with acne, endothelial cells show strong signal receiving characteristics (Figure 5D). In addition, the proportion weight of the ligand receptor of each sample was ranked. From the results, it can be seen that the inflammatory regulation in normal healthy skin is dominated by TNF signal, while in acne patients, PARs regulation is dominated (Figure 5E). From the perspective of the regulation of PARs in nonlesion and lesion areas, it is mainly the regulation between Basel1 cells combine fibroblasts and giant cells. However, the regulation of basal cells and the regulation between basal cells and fibroblasts are also increased in the tissue of lesion areas (Figure 5F).
Significant Gender Difference in Cell Communication of Acne Patients
In order to further compare the differences in cell communication between acne tissue cells of different gender, the cells in the lesion area were extracted for gender-specific cell communication analysis. It can be seen from the results that the cell communication intensity and proportion of female acne patients were significantly higher than that of male patients (Figure 6A). The difference of cell communication between male and female patients is mainly manifested in the difference between endothelial cells and melanocytes, fibroblasts and myofibroblasts, endothelial cells and Schwann cells (Figure 6B–D). In addition, from the perspective of the proportion of ligand receptor regulation weight, the unique signal ligand receptor for male is SPP1, and the proportion of other ligand receptors is smaller than that of female (Figure 6E). The analysis of SPP1 signal in male patients found that signal regulation involves the mutual regulation of multiple cells, especially the mutual regulation of endothelial cells, macrophages and other cells (Figure 6F). In female patients, the regulation of inflammatory signal is relatively simple, and the regulation of representative IL-1 signal only involves macrophages (Figure 6G). Further, it can be seen from the regulation diagram of intercellular ligand receptor that the regulation intensity between macrophages and endothelial cells through VEGF signal in female patients is significantly greater than that in male patients, while the regulation of macrophages and other cells in male patients is mainly manifested in the regulation signal of SPP1 and CD44 (Figure 6H and I).
Discussion
Acne is a chronic inflammatory skin disease that occurs in hair follicles and sebaceous glands, with a global prevalence of 9.38%.10 At present, most studies have pointed out that acne is a multifactorial disease, and the increase of androgen secretion in adolescence has an important relationship with the onset of acne.6 Relevant studies have pointed out that the sebaceous glands of acne patients are swollen, the secretion of sebaceous glands is increased, the hair follicles and sebaceous ducts are keratinized and embolized.12 However, there are few reports about the specific regulatory mechanism at the whole cell level change. In this study, combining the recently published acne single cell transcriptome data and the normal health skin data, we analyzed the cell composition and changes of the skin in the lesion area and normal non-lesion area of acne patients and the normal skin of healthy people. At the same time, we analyzed the gender differences from the single cell transcriptome level in combination with the gender characteristics. The results showed that there were significant differences in skin cell composition between male and female patients with acne, and some basal cells were specific in patients with specific gender; The degree of cell communication also showed great differences, especially in the communication relationship between macrophages and endothelial cells. These results provide a good reference for the follow-up treatment of acne and the mechanism of pathological changes.
Androgen is the main hormone that regulates the activity of human sebaceous glands. In the traditional concept, androgen is secreted by the gonads and adrenal glands under the control of the pituitary gland, and acts on the corresponding receptors on the target organs through blood circulation, exercising biological functions or participating in the occurrence of related diseases.27,28 However, in recent years, an important discovery in the field of androgen research is that androgen target organs can convert androgen precursors into active androgens under the action of androgen metabolic enzymes, and carry out their biological functions through autocrine, paracrine or internal secretion pathways, or release them into the blood circulation as part of androgens in the blood circulation.29 In this way, local tissues can regulate the synthesis and metabolism of androgens as needed. Human skin is such a male hormone endocrine organ, and human sebaceous gland cells are the main sites for the synthesis and metabolism of male hormones in the skin. The active male hormone produced by skin is mainly converted from Dehydroepiandrosterone (DHEA). The level of DHEA in the blood circulation of adult men is 100–500 times higher than that of testosterone, which shows that the sebaceous glands of men are greatly affected by sex hormones.30 Our results also show that there are significant differences in cell composition in male acne patients, especially the basal cells that have important regulatory effects on male hormones.
At the early stage of acne inflammation, neutrophils were found in the complete acne cavity. Since the early stage did not involve the rupture of the hair follicle wall, it was thought that there might be some water-soluble small molecular weight pro-inflammatory substances in the acne cavity, which attracted neutrophils, and small molecular weight pro-inflammatory substances could also diffuse into the dermis from acne, causing inflammation reaction in the dermis.16 Studies have found that there are pro-inflammatory factors with histamine-like activity, prostaglandin-like activity, IL-1-like activity and TNF-like activity molecules in acne.31 In addition, there is the expression of vascular endothelial cell adhesion molecules around the sebaceous duct of hair follicles, which causes the aggregation of lymphocytes and neutrophils in the peripheral blood, leading to inflammation around hair follicles.32,33 In our study, we also found that signal pathways and related molecules such as IL-1, IL-2 and IL-6 are highly expressed in inflammatory cells. In addition, we also found significant differences in macrophages in patients with acne, as well as gender-specific groups of macrophages. This also shows that macrophages play a very important role in the inflammatory regulation of acne.
TNF-a is produced by macrophages under the stimulation of viruses, bacteria and parasites. Studies have found that the expression of TNF-a in sebaceous glands is significantly higher than that in keratinocytes, hair follicles and sweat gland cells, which indicates that sebaceous glands may have non-specific defense function against invasive microorganisms.34 In addition, the expression of IL-1 was found in the acne of acne patients, in the unstimulated human sebaceous gland cells and in the funnels of human hair follicles and sebaceous glands, and this expression was not affected by the normal human flora, such as Propionibacterium acnes, Staphylococcus epidermidis, Lactobacillus furfur and non-specific bacterial product - lipopolysaccharide (LSP), suggesting that there might be some endogenous mechanism that led to the expression of IL-1 in human hair follicles and sebaceous glands, rather than bacterial antigen.
hair follicles and sebaceous glands, and this expression was not affected by the normal human flora, such as Propionibacterium acnes, Staphylococcus epidermidis, Lactobacillus furfur and non-specific bacterial product - lipopolysaccharide (LSP), suggesting that there might be some endogenous mechanism that led to the expression of IL-1 in human hair follicles and sebaceous glands, rather than bacterial antigen.35–37 In this study, we noticed that TNF-a was not enriched in acne skin, but PARs changed significantly. In vivo experimental studies show that activated PARs not only cause various symptoms related to inflammation such as edema, vasodilation, burning sensation and pain, but also mediate the contraction of human endothelial cells, the migration of immune cells to tissues, the production of prostaglandins, and the enhancement of the expression of platelet-derived growth factors.38–40 Early studies found that inflammatory mediators such as TNF-a, IL-1a and endotoxin caused the up-regulation of PARs expression.41 In addition, TNF-a can also reduce the production of IL-4 induced by trypsin and tryptase, and also increase the PARs induced by trypsin and tryptase.41 In this study, we found that fibroblasts and basal cells and mast cells play a regulatory role through PARs. In addition, they also regulate inflammation through PARs through basal cells and fibroblasts themselves.
Conclusion
In conclusion, by analyzing the single-cell transcriptome data in the public database, we found that there was a significant change in the cell composition of acne skin, especially in the proportion of basal cells and macrophages; In addition, from the perspective of cell changes, there are significant gender differences. The proportion of macrophages and the intensity of inflammatory reaction in male patients are significantly higher than that in female patients. Through cell communication analysis, it was found that this gender difference might have an important relationship with the specific inflammatory response of PARs in acne patients.
Data Sharing Statement
The data used in the present study was obtained via an online database. The GSE175817, GSE130973, GSE181316, GSE156326, GSE176415 datasets were collected from the GEO (https://www.ncbi.nlm.nih.gov/geo/). The Tabib2018 dataset was collected from the database in University of Pittsburgh (https://dom.pitt.edu/wpcontent/uploads/2018/10/Skin_6Control_rawUMI.zip and https://dom.pitt.edu/wpcontent/uploads/2018/10/Skin_6Control_Metadata.zip).
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki. All procedures performed in this study involving human participants was approved by the Ethics Committee of the Chongqing Hospital of Traditional Chinese Medicine (2018-ky-10).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Layton AM, Ravenscroft J. Adolescent acne vulgaris: current and emerging treatments. Lancet Child Adolesc Health. 2023;7:136–144. doi:10.1016/S2352-4642(22)00314-5
2. Bikash C, Sarkar R. Topical management of acne scars: the uncharted terrain. J Cosmet Dermatol. 2023;22:1191–1196. doi:10.1111/jocd.15584
3. Vempati A, Zhou C, Tam C, et al. Subcision for atrophic acne scarring: a comprehensive review of surgical instruments and combinatorial treatments. Clin Cosmet Investig Dermatol. 2023;16:125–134. doi:10.2147/CCID.S397888
4. Mohsin N, Hernandez LE, Martin MR, Does AV, Nouri K. Acne treatment review and future perspectives. Dermatol Ther. 2022;35:e15719. doi:10.1111/dth.15719
5. Kutlu O, Karadag AS, Wollina U. Adult acne versus adolescent acne: a narrative review with a focus on epidemiology to treatment. An Bras Dermatol. 2023;98:75–83. doi:10.1016/j.abd.2022.01.006
6. Gebauer K. Acne in adolescents. Aust Fam Physician. 2017;46:892–895.
7. Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician. 2012;86:734–740.
8. Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10(5754):10.1038/s41598-020-62715–3.
9. Dursun R, Daye M, Durmaz K. Acne and rosacea: what’s new for treatment? Dermatol Ther. 2019;32:e13020. doi:10.1111/dth.13020
10. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172:3–12. doi:10.1111/bjd.13462
11. Gieler U, Gieler T, Kupfer JP. Acne and quality of life - impact and management. J Eur Acad Dermatol Venereol. 2015;29(4):12–14. doi:10.1111/jdv.13191
12. Gonzalez-Mondragon EA, Ganoza-Granados LDC, Toledo-Bahena ME, et al. Acne and diet: a review of pathogenic mechanisms. Bol Med Hosp Infant Mex. 2022;79:83–90. doi:10.24875/BMHIM.21000088
13. Kanwar IL, Haider T, Kumari A, Dubey S, Jain P, Soni V. Models for acne: a comprehensive study. Drug Discov Ther. 2018;12:329–340. doi:10.5582/ddt.2018.01079
14. Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(5):8–12. doi:10.1111/jdv.14374
15. Rao A, Douglas SC, Hall JM. Endocrine disrupting chemicals, hormone receptors, and acne vulgaris: a connecting hypothesis. Cells. 2021;10:1439. doi:10.3390/cells10061439
16. Harvey A, Huynh TT. Inflammation and acne: putting the pieces together. J Drugs Dermatol. 2014;13:459–463.
17. Hu T, Wei Z, Ju Q, Chen W. Sex hormones and acne: state of the art. J Dtsch Dermatol Ges. 2021;19:509–515. doi:10.1111/ddg.14426
18. Do TH, Ma F, Andrade PR, et al. TREM2 macrophages induced by human lipids drive inflammation in acne lesions. Sci Immunol. 2022;7:eabo2787. doi:10.1126/sciimmunol.abo2787
19. Sole-Boldo L, Raddatz G, Schutz S, et al. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun Biol. 2020;3(188). doi:10.1038/s42003-020-0922-4
20. Direder M, Weiss T, Copic D, et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022;108:55–76. doi:10.1016/j.matbio.2022.03.001
21. Vorstandlechner V, Laggner M, Copic D, et al. The serine proteases dipeptidyl-peptidase 4 and urokinase are key molecules in human and mouse scar formation. Nat Commun. 2021;12:6242. doi:10.1038/s41467-021-26495-2
22. Singh K, Rustagi Y, Abouhashem AS, et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J Clin Invest. 2022;132. doi:10.1172/JCI157279
23. Tabib T, Morse C, Wang T, Chen W, Lafyatis R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Invest Dermatol. 2018;138:802–810. doi:10.1016/j.jid.2017.09.045
24. Korsunsky I, Millard N, Fan J, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16:1289–1296. doi:10.1038/s41592-019-0619-0
25. Wu T, Hu E, Xu S, et al. ClusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation. 2021;2:100141. doi:10.1016/j.xinn.2021.100141
26. Jin S, Guerrero-Juarez CF, Zhang L, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12(1088):10.1038/s41467-021-21246–9.
27. Kircik LH. Androgens and acne: perspectives on clascoterone, the first topical androgen receptor antagonist. Expert Opin Pharmacother. 2021;22:1801–1806. doi:10.1080/14656566.2021.1918100
28. Carmina E, Dreno B, Lucky WA, et al. Female adult acne and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Endocr Soc. 2022;6:bvac003. doi:10.1210/jendso/bvac003
29. Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Dunkel L, Sankilampi U. Transient postnatal secretion of androgen hormones is associated with acne and sebaceous gland hypertrophy in early infancy. J Clin Endocrinol Metab. 2013;98:199–206. doi:10.1210/jc.2012-2680
30. Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)--a precursor steroid or an active hormone in human physiology. J Sex Med. 2011;8:2960–2982; quiz 2983. doi:10.1111/j.1743-6109.2011.02523.x
31. Tan JKL, Stein Gold LF, Alexis AF, Harper JC. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37:S60–S62. doi:10.12788/j.sder.2018.024
32. Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6:27–35.
33. Dreno B, Gollnick HP, Kang S, et al.; Global Alliance to Improve Outcomes in Acne. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol. 2015;29(4):3–11. doi:10.1111/jdv.13190
34. Younis S, Shamim S, Nisar K, et al. Association of TNF-alpha polymorphisms (−857, −863 and −1031), TNF-alpha serum level and lipid profile with acne vulgaris. Saudi J Biol Sci. 2021;28:6615–6620. doi:10.1016/j.sjbs.2021.07.042
35. Taylor M, Porter R, Gonzalez M. Intense pulsed light may improve inflammatory acne through TNF-α down-regulation. J Cosmet Laser Ther. 2014;16:96–103. doi:10.3109/14764172.2013.864198
36. Chen X, Min S, Chen C, Lin X, Wang D, Jiang G. Influence of RETN, IL-1, and IL-6 gene polymorphisms on the risk of acne vulgaris in the Chinese population. J Cosmet Dermatol. 2022;21:4965–4973. doi:10.1111/jocd.14911
37. Thiboutot DM. Inflammasome activation by Propionibacterium acnes: the story of IL-1 in acne continues to unfold. J Invest Dermatol. 2014;134:595–597. doi:10.1038/jid.2013.528
38. Hollenberg MD, Mihara K, Polley D, et al. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol. 2014;171:1180–1194. doi:10.1111/bph.12544
39. Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153 Suppl 1:S263–282. doi:10.1038/sj.bjp.0707507
40. Chandrabalan A, Ramachandran R. Molecular mechanisms regulating Proteinase-Activated Receptors (PARs). Febs j. 2021;288:2697–2726. doi:10.1111/febs.15829
41. Zhang H, Yang H, He S. TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol Biochem. 2010;26:327–336. doi:10.1159/000320556
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.