Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Integrating Chinese Herbal Medicine into Conventional Care Was Related to Lower Risk of Sarcopenia Among Rheumatid Arthritis Patients: A Retrospective, Population-Based Study
Authors Li HH, Livneh H , Huang HL, Wang YH, Lu MC , Chen WJ , Tsai TY
Received 4 July 2023
Accepted for publication 28 September 2023
Published 24 October 2023 Volume 2023:16 Pages 3117—3127
DOI https://doi.org/10.2147/JMDH.S428948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hsin-Hua Li,1,* Hanoch Livneh,2,* Hua-Lung Huang,3 Yu-Han Wang,4,* Ming-Chi Lu,5,6 Wei-Jen Chen,1,4,7,8 Tzung-Yi Tsai9– 11
1Department of Chinese Medicine, Dalin Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, Chiayi, 62247, Taiwan; 2Rehabilitation Counseling Program, Portland State University, Portland, OR, 97207-0751, USA; 3Department of Rehabilitation, Dalin Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan; 4Center of Sports Medicine, Dalin Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, Chiayi, 62247, Taiwan; 5Division of Allergy, Immunology and Rheumatology, Dalin Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, Chiayi, 62247, Taiwan; 6School of Medicine, Tzu Chi University, Hualien, 97004, Taiwan; 7School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, 97004, Taiwan; 8Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan, 33301, Taiwan; 9Department of Medical Research, Dalin Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, Chiayi, 62247, Taiwan; 10Department of Nursing, Tzu Chi University of Science and Technology, Hualien, 97004, Taiwan; 11Department of Environmental and Occupational Health, College of Medicine, National Cheng Kung University, Tainan, 70428, Taiwan
*These authors contributed equally to this work
Correspondence: Wei-Jen Chen; Tzung-Yi Tsai, Tel +886-5-2648000-5003 ; +886-5-2648000-3209, Fax +886-5-2648006, Email [email protected]; [email protected]
Objective: Sarcopenia is a frequently observed comorbidity of rheumatoid arthritis (RA) due to the chronic activation of the innate immune system. Accumulating evidence has indicated that Chinese herbal medicine (CHM) safely suppresses proinflammatory pathways and controls inflammation-associated disease, but its effect in reducing the risk of developing sarcopenia among RA subjects has not been established. We conducted a population-level cohort study to compare the sarcopenia risk in patients with RA who use or do not use CHM.
Methods: Using claims from a nationwide insurance database, we recruited patients with newly diagnosed RA and without sarcopenia between 2002 and 2010. Propensity score matching was applied to randomly select sets of CHM users and non-CHM users to compare the sarcopenia risk until the end of 2013. The risk of new-onset sarcopenia was assessed using the Cox proportional hazards model.
Results: As compared to non-CHM users, those receiving CHM treatment had a lower incidence of sarcopenia (7.69 vs 9.83 per 1000 person-years). CHM was correlated with a decreased chance of sarcopenia after controlling for potential covariates. Notably, use of CHM for more than two years may diminish the risk of getting sarcopenia by about 47% when taken as prescribed. Prescriptions of several herbal formulae may benefit the reduction of sarcopenia risk, such as Yan-Hu-Suo, Bei-Mu, Da-Huang, Huang Qin, Ping-Wei-San (PWS), Shu-Jing-Huo-Xue-Tang (SJHXT) and Chuan-Xiong-Cha-Tiao-San (CXCTS).
Conclusion: This study produced new evidence as it is the first to show that the longer duration of CHM use was correlated to reduced risk of sarcopenia in a dose-dependent manner, implying that CHM treatment could be embraced as a routine care strategy for preventing sarcopenia.
Keywords: sarcopenia, rheumatoid arthritis, Chinese herbal medicine, cohort study
Introduction
Rheumatoid arthritis (RA) is a well-known progressive form of joint destruction that affects millions of people worldwide, particularly women. It is estimated that by 2022, the global population affected by RA have reach approximately 18 million individuals.1 Notably, the report showed that within 10 years of symptom onset, up to 70% of the affected patients became unable to work, leading to high economic costs as well.2 In the United States, for example, the annual healthcare cost due to RA was $19.3 billion, and the total societal costs exceed approximately $40 billion after accounting for indirect expenses.3
Not only did RA cause enormous economic losses, but it also triggered a higher risk of comorbidities for the affected persons, including sarcopenia. One recent meta-analysis of 17 reports mentioned that the sarcopenia prevalence in RA persons ranged from 11% to 46%, with a pooled mean estimate of 29.1%.4 Unfortunately, sarcopenia is a silent illness in which the affected patients may be unaware of their condition. Despite the etiological ambiguity of RA so far, the dysregulated expression of cytokines from T helper cells is presumed to play a definitive role in the pathogenesis of RA.5 Underlying a dysfunctional immune system, there may exist a link between pro-inflammatory cytokines and degradation of skeletal muscles. For example, the scholars found that the release of cytokines, such as (IL)-6, tumor necrosis factor (TNF)-alpha and interferon-γ (IFN-γ), might cause aberrant muscle homeostasis through the mediation of phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) signaling pathway.6 This pathway has been found to be capable of activating the nuclear factor kappa beta (NF-κB), consequently leading to an increased matrix metalloproteinase gene expression and migration, which in turn leads to degradation of specific muscle proteins that provokes the development of sarcopenia.7 As such, skeletal muscle wasting, incited by sarcopenia, may insidiously affect human health. One earlier meta-analysis indicated that patients with sarcopenia may face up to twice the risk of fracture than those without sarcopenia.8 Of note, once individuals experienced concomitant sarcopenia, their risk of earlier mortality more than doubled.9 Faced with this severe impact, initiating therapy to prevent sarcopenia among RA cohorts is highly important.10
At present, Chinese herbal medicine (CHM) has been widely employed for the treatment of a variety of chronic diseases.11,12 Several studies have addressed how the ingredients in CHM products may lessen bone disease progression and aid in the prevention of joint deformities. For instance, via the regulation of NF-κB signaling, Corydalis is believed to regulate serum pro-inflammatory cytokines, including IL-6, TNF-alpha and nitric oxide (NO),13 all of which may progressively cause muscle wasting and apoptosis.10 Moreover, the extract from Yan-Hu-Suo was found to suppress the activation of the receptor activator NF-κB ligand and the MAPK pathway,14 both of which have been demonstrated to be involved in the pathogenesis of sarcopenia.7,15 Based on these molecular mechanisms, the application of CHM might be beneficial in instituting a novel strategy for preventing, or delaying, sarcopenia among RA patients.
A detailed literature search revealed that no studies reported data on the relationship between CHM use and the sequential chance of having sarcopenia amongst RA persons. A closer look at this issue was therefore required. This cohort study sought to address this issue by using a nationwide database to compare the risk of having sarcopenia in RA persons with and without receiving adjunctive CHM treatment in addition to the routine care.
Methods
Data Source and RA Cohort
This cohort study was implemented using patient records from a national claim data handled by the Bureau of National Health Insurance (NHI) in Taiwan. As participation in social insurance is compulsory, more than 99% of the healthcare providers have contracted with the NHI so far. The national insurance program would enable the inhabitants to access the cost-effective and quality health care. In this work, all analytical data were obtained from the Longitudinal Health Insurance Database (LHID), which included the original claims data of 1 million insurants randomly extracted from all beneficiaries under the NHI program.16 This database holds the information on demographics, diagnoses, prescriptions, referrals, and hospitalization for these subjects covered by the NHI program. The diagnostic codes shown in this database are in the format of the International Classification of Diseases, Revision 9, Clinical Modification (ICD-9-CM).
Between 2002 and 2010, a total of 6483 RA persons aged 20–70 years were identified from inpatient and outpatient claims, and all of them were further validated in the catastrophic illness registry, with ICD-9-CM code of 714.0. In Taiwan, RA is one of the statutory major diseases, such as malignancies, end-stage renal disease or autoimmune disorders, and only the beneficiaries who fulfill the related diagnostic criteria are issued a catastrophic card to exempt from the required cost under insurance program. By using this verification process, the diagnostic accuracy of RA herein is reliable. Thereafter, we assumed the date of approval for catastrophic illness registration due to RA as the index date. Cases who had exhibited sarcopenia prior to RA onset and those with incomplete demographic information were excluded. The remaining subjects were observed for the occurrence of sarcopenia, withdrawal from the insurance system or the end of 2013, whichever date came first. This study was performed under the guidelines of the Helsinki Declaration. Consultation with the Institutional Review Board of Buddhist Dalin Tzu Chi Hospital also confirmed that this study was exempt from full review by the Institutional as the de-identified secondary data used (No. B10803015).
Definition of Sarcopenia Incident
Based on former report,17 the major outcome of interest was regarded to be first-time sarcopenia onset, which was identified as one diagnostic appearance in a single inpatient, or at least two outpatient, claim records of ICD-9-CM codes 733.1 and 805–829, or the following conditions: fracture-related surgery with the following ICD-9-CM codes of 78.1 (application of external fixation device), 78.4 (other repair or plastic operation on bone), 78.5 (internal fixation of bone without fracture reduction), 78.9 (insertion of bone growth stimulator), 79 (reduction of fracture and dislocation), or 81 (repair and plastic operations on joint structures). As shown in Figure 1, after excluding 207 patients with pre-existing sarcopenia prior to RA onset and 41 persons who had missing demographic information or were followed for less than 1 year, a total of 6235 RA patients were recruited and followed to learn their CHM use pattern. All eligible RA patients were then randomly divided into a routine care group and a routine care plus CHM treatment group, based on individual use patterns of CHM therapy.
 |
Figure 1 Flow chart for population inclusion criteria. |
Measurement of CHM Use
In this investigation, CHM users were clarified as having ever visited a practitioner and having application of Chinese herbs during the entire time frame analyzed in the study. In accordance with the a priori rule11,12 the enrollees who used CHM for >30 days were defined as CHM users, and those who utilized CHM treatment for fewer than 30 days were considered non-CHM users. To reduce selection bias arising from participation or non-participation in CHM treatment, we randomly selected a comparison using 1:1 propensity score matching. The predicted probability of participating in treatment by CHM was calculated using a logistic regression model based on the enrollee’s baseline characteristics (Table 1). At the same time, to clarify the dose–response relation on the basis of CHM use duration, all subjects were further classified into four groups: non-CHM use (≤30 days), low intensity (CHM use for 31–365 days), medium intensity (CHM use for 366–730 days), and high intensity (CHM use for more than 730 days) based on their cumulative days of CHM prescriptions. Additionally, to correct for immortal time for patients who received CHM treatment,18 the index date of follow-up period for RA patients who never used CHM was assigned to the date of RA diagnosis, whereas those for CHM users were assigned to the first prescription date of CHM prescription. All of them were followed up to December 31, 2013. The follow-up time, shown in person-years (PYs), was calculated as the time interval from the index date until the study’s endpoint or study end.
 |
Table 1 Patient Demographic Data and Comorbidities |
Covariate Assessment
Covariates in the statistical analysis included age, gender, monthly income, urbanization and prior comorbidities, as listed in Table 1. As to income, we utilized the premium payment category as a proxy and transformed this indicator to a 3-level ordinal variable as follows: US Dollar (US) ≤623; 624–1394; and ≥1395. Regarding the urbanization degree, it was classified into three types of settlements based on former rule, which comprised cities, towns and semi-dense areas, and rural areas.19 Baseline comorbidities were assessed using the Charlson–Deyo comorbidity index.20 It has been well established to accurately assess the overall burden of illness with specific assessment of multiple medical comorbidities and severity to generate 17 specific medical category ratings, where higher scores correlated with a higher severity of comorbidities.
Analysis
The Statistical Analysis System (SAS), version 9.3 (SAS Institute, Cary, NC) computer software program was applied to perform all statistical analyses. First, the descriptive analysis was done and reported as mean and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. Additionally, considering the statistic for assessing the balance between two groups should not be affected by the sample size, the approach of standardized differences was applied herein to compare the baseline balance between treated and untreated groups.21 A standardized difference of 0.1 or more was considered indicative of imbalance. The incidence rate of sarcopenia was presented as the number of cases per 1000 PYs. The Cox proportion hazards model was then applied to estimate the hazard ratio (HR) at 95% confidence interval (CI) of sarcopenia for those receiving CHM treatment, using non-CHM users as the reference group. The proportional hazards assumption was checked using the Schoenfeld residuals and constructing a log–log plot as well.
Additionally, two sensitivity analyses were employed to assess whether the individual a priori treatment patterns received would prejudice the findings herein. First, we merely selected RA subjects who reported no comorbidities. Second, we further considered the prescriptions of biological agents, used for six months or longer, as a surrogate indicator for RA severity in the regression model. These drugs included adalimumab, etanercept, infliximab, rituximab and tocilizumab. All statistical tests were performed at the two-tailed significance level of 0.05.
Results
Of the enrolled subjects, the matched pairs of subjects with and without CHM use provided data for 2746 cases. The mean age of the recruited subjects was 51.1 years (SD = 11.6). The sample consisted mostly of female (73.7%), and the majority of enrollees were reported to have a median monthly income (54.3%) and live in urbanized areas (57.9%). The baseline features of demographic data and comorbidities in CHM users were similar to those of non-CHM users (Table 1).
During the study timeframe, 395 first episodes of sarcopenia were detected, which consisted of 220 in non-CHM users and 175 in CHM users. CHM users indeed experienced a lower incidence rate of sarcopenia than non-CHM users (7.69 vs 9.83, respectively, per 1000 PYs), with an adjusted HR of 0.78 (95% CI: 0.65–0.94) (Table 2). Amongst those who ever used CHM, a longer period of CHM use was related to the greater decrease in sarcopenia chance, and this trend was more obvious for those using CHM for more than 730 days (adjusted HR: 0.53; 95% CI: 0.30–0.92).
 |
Table 2 Sarcopenia Incidence (per 1000 PY) and Risk in RA Patients with and without CHM Use |
At the same time, we performed a stratified analysis by age and sex to clarify the protective effects of CHM in offsetting sarcopenia onset. Multivariable analysis depicted that the benefit of CHM treatment was greater in females (adjusted HR: 0.69; 95% CI: 0.57–0.88) and younger patients (adjusted HR: 0.70; 95% CI: 0.51–0.95), respectively (Table 3). The ten most commonly prescribed herbal formulae among participants are displayed in Table 4. Among them, prescriptions of Huang Qin, Bei-Mu, Yan Hu Suo, Da-Huang, Ping-Wei-San (PWS), Chuan-Xiong-Cha-Tiao-San (CXCTS), and Shu-Jing-Huo-Xue-Tang (SJHXT) were strongly related to lower odds of sarcopenia (Figure 2).
 |
Table 3 Sarcopenia Incidence (per 1000 PY) and Risk in RA Patients with and without CHM Use, Stratified by Sex and Age |
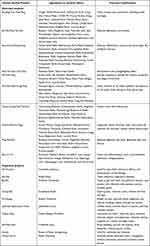 |
Table 4 Herbs Contained in the Most-Used Single-Herb and Multi-Herb Products Among Study Participants |
 |
Figure 2 Sarcopenia risk regarding uses of top ten most-used single-herb and multi-herb products among subjects. |
In the first sensitivity analysis that focused on RA subjects with no comorbidities, we ascertained that CHM use was still related to a lower risk of sarcopenia, with an adjusted HR of 0.80 (95% CI: 0.65–0.95). Furthermore, there were 1375 CHM users and 1343 non-CHM users who ever took these biological drugs. This variable was then treated in the multivariate Cox proportional hazards model. The reanalysis yielded that adding CHM to conventional care still correlated with subsequent risk of sarcopenia among RA subjects (adjusted HR: 0.79; 95% CI: 0.63–0.94).
Discussion
The systemic inflammation observed in RA is directly related to sarcopenia, and both share mutual chemical mediators related to their respective pathophysiologies.4 As there are no specific therapies for the prevention of sarcopenia after RA onset, the assessment of complementary and alternative medicine may offer a promising direction for future research and clinical practice.
Using records from a population-based health claims database, findings from the present study suggested that if persons with RA could receive CHM in addition to conventional treatment, they may experience a decreased chance of sarcopenia. Additionally, the longer the duration of taking CHM herbs, the greater the benefit of reduction in incidence of sarcopenia found in this work. The largest reduction was observed for those who had CHM treatment for more than two years, who experienced a 50% lower risk of sarcopenia compared to the controls. Despite a lack of comparable studies, the result added to the body of the literature suggesting the clinical efficacy of CHM for individuals with chronic diseases.22,23
After stratifying patients by age and gender, we noticed that females benefited more from CHM treatment than males. Numerous surveys focusing on gender differences in health-related knowledge and healthy behaviors found that females are more aware of the importance of self-care than males.24,25 In this case, females may be more attuned to adhering to the prescribed medical regimen to minimize the likelihood of sarcopenia. Additionally, the release of sex hormones, especially estrogen, may account for this phenomenon. There is accumulating evidence that estrogen may stimulate the activation and proliferation of satellite cells, required for the growth, maintenance, and regeneration of skeletal muscle.26,27 Consequently, the decline of estrogen in elderly women may dilute the therapeutic effects of CHM.
To date, few assessments towards the long-term effects of CHM in RA patients have been done, let alone on the prevention of sarcopenia. CHM is a promising potential adjunct therapy for sarcopenia due to its antioxidant and anti-inflammatory activities. For example, use of SYGCT was associated with a lower risk of sarcopenia. In clinical practice, this remedy is commonly employed in treating muscle cramps as well as crampy pain.28 Some recent studies using both animal models and human patients observed that SYGCT was beneficial in attenuating the plasma levels of pro-inflammatory cytokines by modulating the activation of NF-κB signaling pathway.29,30 Not only being a key marker of inflammatory response, NF-κB is often considered one of the most critical muscle transcription factors, since its activation provokes muscle loss and muscular dystrophy.10 Consequently, NF-κB has been recognized to be an important molecular target for the prevention of skeletal muscle loss while instituting the novel therapy to mitigate musculoskeletal disorders.7
Those who received CXCTS experienced a deceased risk of sarcopenia when compared to controls. Modern pharmacological evidence shows that Angelica dahurica, a major compound of CXCTS, can notably lower the expression of inflammatory mediators, such as IL-6, TNF-alpha, IL-1β and IFN-γ, in ligature-induced periodontitis rats and lipopolysaccharide-induced RAW 264.7 cells.31 Extreme muscle loss often results from a combination of diminishing hormonal anabolic signals mediated through pro-inflammatory parameters.10 The reason for this is that elevated inflammatory cytokine values may abate the expression and activity of insulin-like growth factor hormone (IGF-I).27 This indicator often acts as a key myokine that is correlated with skeletal muscle mass and strength.7,10
Another multi-herb product shown to be effective in reducing sarcopenia risk is PWS. Based on a previously published animal study, it was found that the magnolia-bark, a major element purified from PWS, could significantly suppress the responses of inflammatory mediators by inhibiting the NF-κB pathway.32 The activation of the inflammatory process was also believed to cause a decrease in satellite cells and, accordingly, inciting the predisposition to develop sarcopenia.
Among other commonly used single-herb products, both Huang-Qin and Da-Huang were correlated with a decreased risk of sarcopenia. This trend may reflect the positive impact of corresponding compounds, such as emodin in Da-Huang and baicalin in Huang-Qin. They had been indicated to mediate the brain-derived neurotrophic factor (BDNF) expression.33,34 Besides the potential therapeutic benefits of cognitive function, the plasma level of BDNF has been implicated in the metabolic processes of peripheral systems, like muscle fibers.27 A murine model demonstrated that rodent muscle could lose its ability to synthesize proteins and reduce myogenic regulatory factors in the absence of BDNF signaling, thus provoking muscular dysfunction.35
Uses of Yan-Hu-Sou and Bei-Mu were also found to decrease the risk of sarcopenia. One recent study demonstrated that l-tetrahydropalmatine, a major compound purified by Yan-Hu-Sou, significantly suppressed the receptor activator of NF-κB ligand and the MAPK pathway.14 An earlier in vitro study noted that activation of the NF-κB and MAPK signaling pathways contributed to inflammatory responses throughout the body,15 which in turn increased the predisposition to develop sarcopenia.7 Our study demonstrated the benefits of Bei-Mu in preventing sarcopenia. In studies of both humans and animals,36,37 this herb was proven to exert anti-inflammatory and anti-oxidant effects by inhibiting the MAPK/NF-κB signaling pathways. These underlying mechanisms may, therefore, explain the benefits of Yan-Hu-Sou and Bei-Mu found in the present study.
Despite being a pioneering, albeit ex-post-facto, study providing strong evidence for the effects of CHM on reducing sarcopenia in RA patients, this work may face some noteworthy limitations. First and foremost, the data used are merely from a claims-based database; accordingly, the information regarding biochemical data, family history, lifestyle behaviors, or body weight was not obtained. Thus, it is inevitable that residual confounding by these factors may exist to partly bias the association herein. Caution, therefore, must be exercised while interpreting the present findings. Therefore, a large cohort of RA patients created by prospective randomized trials are suggested to further explore the potential mechanisms underlying the clinical benefits of CHM on the prevention of sarcopenia risk. Second, this study is on the basis of a retrospective cohort study design that applied ICD-9-CM codes. While misclassification of cases may arise in the administrative database research, we capitalized on the approach that patients must have had either one inpatient ICD-9-CM code or at least two outpatient ICD-9 codes for RA together with sarcopenia in a 12-month span, which allowed us to lessen the chance of misclassification to some extent. Furthermore, the probability of misclassification was the same for all enrollees, so any misclassification of exposure may tend to be nondifferential, and thus, if indeed present, would be biased toward the null relationship. Third, surveillance bias may lead to errors in reporting, possibly as the CHM users may tend to receive additional healthcare services than the comparison group. To confront this drawback, we added the frequency of medical visits for each participant into the multivariate regression model, and a significantly decreased risk of sarcopenia among the CHM users was still noted. Notwithstanding these limitations, this work is a sole population-based investigation to evaluate the association of CHM use with sarcopenia risk among RA persons via a nationwide health claims, which could leave little room for non-response or loss to follow-up. The second strength of this work is the long observation time used. The over 10-year follow-up period used provided ample time to observe outcome trajectories. The third strength is that we select the subjects with and without exposure to CHM treatment by the propensity score matching method, thus reducing the possibility of confounding effects.
Conclusion
In summary, this population-based nested cohort study found that, during conventional treatment for RA, adjunctive CHM use reduced the chance of developing sarcopenia by 22%. Furthermore, the duration of CHM use further influenced the inverse dose–response association with sarcopenia risk among enrollees. Findings from the current study indicated that a complementary therapy, specifically CHM, contributed to disease management of musculoskeletal pain. The results of our study further indicated that those commonly prescribed herbal products that are likely to lower sarcopenia risk, hence paving the way for further pharmacological research to cure and control other health maladies. As far as clinical practice is concerned, we recommend that clinicians and people diagnosed with RA become aware of the elevated likelihood of having sarcopenia and be vigilant in watching for early symptoms of this disease, so that appropriate diagnostic tests, along with provable treatments, could be administered when early symptoms of these conditions are experienced.
Acknowledgments
This study uses data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes, Taiwan. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes. HHL, HL and YHW contributed equally to this work.
Funding
This work was supported by the Tzu Chi Medical Foundation (TCMF-CM2-111-07).
Disclosure
The authors declare that they have no conflicting interests in this work.
References
1. World Health Organization. Musculoskeletal health; 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
2. Allaire S, Wolfe F, Niu J, Lavalley MP. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Rheum. 2008;59(4):474–480. doi:10.1002/art.23538
3. Chen CI, Wang L, Wei W, Yuce H, Phillips K. Burden of rheumatoid arthritis among US medicare population: co-morbidities, health-care resource utilization and costs. Rheumatol Adv Pract. 2018;2(1):i1–i9.
4. An HJ, Tizaoui K, Terrazzino S, et al. Sarcopenia in autoimmune and rheumatic diseases: a comprehensive review. Int J Mol Sci. 2020;21(16):5678. doi:10.3390/ijms21165678
5. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372. doi:10.1001/jama.2018.13103
6. Bennett JL, Pratt AG, Dodds R, Sayer AA, Isaacs JD. Rheumatoid sarcopenia: loss of skeletal muscle strength and mass in rheumatoid arthritis. Nat Rev Rheumatol. 2023;19(4):239–251. doi:10.1038/s41584-023-00921-9
7. Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med. 2008;86(10):1113–1126. doi:10.1007/s00109-008-0373-8
8. Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. doi:10.1002/jcsm.12411
9. Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. doi:10.1371/journal.pone.0169548
10. Lu W, Xiao W, Xie W, et al. The role of osteokines in sarcopenia: therapeutic directions and application prospects. Front Cell Dev Biol. 2021;9:735374. doi:10.3389/fcell.2021.735374
11. Lai NS, Livneh H, Fan YH, Lu MC, Liao HH, Tsai TY. Use of Chinese herbal medicines by rheumatoid arthritis patients was associated with lower risk of stroke: a retrospective cohort study. Complement Ther Med. 2019;45:124–129. doi:10.1016/j.ctim.2019.05.029
12. Lin MC, Livneh H, Chen WJ, Lai NS, Lu MC, Tsai TY. Association of Chinese herbal medicines use with development of chronic obstructive pulmonary disease among patients with rheumatoid arthritis: a population-based cohort study. Int J Chron Obstruct Pulmon Dis. 2020;15:691–700. doi:10.2147/COPD.S233441
13. Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(Suppl 3):S2. doi:10.1186/ar4174
14. Zhi X, Wang L, Chen H, et al. l-tetrahydropalmatine suppresses osteoclastogenesis in vivo and in vitro via blocking RANK-TRAF6 interactions and inhibiting NF-êB and MAPK pathways. J Cell Mol Med. 2020;24(1):785–798. doi:10.1111/jcmm.14790
15. Xiao K, Liu C, Tu Z, et al. Activation of the NF-êB and MAPK signaling pathways contributes to the inflammatory responses, but not cell injury, in IPEC-1 cells challenged with hydrogen peroxide. Oxid Med Cell Longev. 2020;2020:5803639. doi:10.1155/2020/5803639
16. National Health Insurance Research Database. National health insurance annual report 2014–2015. Available from: https://nhird.nhri.edu.tw//en/index.html.
17. Lin MH, Chiu SY, Chang PH, Lai YL, Chen PC, Ho WC. Hyperlipidemia and statins use for the risk of new diagnosed sarcopenia in patients with chronic kidney: a population-based study. Int J Environ Res Public Health. 2020;17(5):1494. doi:10.3390/ijerph17051494
18. Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841–843. doi:10.1681/ASN.2007121354
19. Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4(1):1–22.
20. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
21. Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–2049. doi:10.1002/sim.3150
22. Li HH, Livneh H, Chen WJ, et al. Effect of Chinese herbal medicines on hearing loss risk in rheumatoid arthritis patients: retrospective claims analysis. Front Med. 2021;8:683211. doi:10.3389/fmed.2021.683211
23. Chen CJ, Livneh H, Chen WJ, et al. The prescription of Chinese herbal medicine and risk of endometriosis in women with rheumatoid arthritis: a population-based cohort study. Int J Womens Health. 2022;14:1603–1612. doi:10.2147/IJWH.S386134
24. Shih CC, Liao CC, Su YC, Tsai CC, Lin JG. Gender differences in traditional Chinese medicine use among adults in Taiwan. PLoS One. 2012;7(4):e32540. doi:10.1371/journal.pone.0032540
25. Aljefree NM, Almoraie NM, Althaiban MA, Hanbazaza MA, Wazzan HA, Shatwan IM. Gender differences in knowledge, attitudes, and practices with respect to type 1 diabetes among Saudi public-school teachers. BMC Public Health. 2023;23(1):118. doi:10.1186/s12889-023-15043-w
26. Ikeda K, Horie-Inoue K, Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J Steroid Biochem Mol Biol. 2019;191:105375. doi:10.1016/j.jsbmb.2019.105375
27. Minniti G, Pescinini-Salzedas LM, Minniti GA, et al. Organokines, sarcopenia, and metabolic repercussions: the vicious cycle and the interplay with exercise. Int J Mol Sci. 2022;23(21):13452. doi:10.3390/ijms232113452
28. Ota K, Fukui K, Nakamura E, et al. Effect of Shakuyaku‐kanzo‐to in patients with muscle cramps: a systematic literature review. J Gen Fam Med. 2020;21(3):56–62. doi:10.1002/jgf2.302
29. Chen IC, Lin T-H, Hsieh YH, et al. Formulated Chinese medicine shaoyao gancao tang reduces tau aggregation and exerts neuroprotection through anti-oxidation and anti-inflammation. Oxid Med Cell Longev. 2018;2018:9595741. doi:10.1155/2018/9595741
30. Chang ZP, Deng GF, Shao YY, et al. Shaoyao-gancao decoction ameliorates the inflammation state in polycystic ovary syndrome rats via remodeling gut microbiota and suppressing the TLR4/NF-êB pathway. Front Pharmacol. 2021;12:670054. doi:10.3389/fphar.2021.670054
31. Lee HJ, Lee H, Kim MH, et al. Angelica dahurica ameliorates the inflammation of gingival tissue via regulation of pro-inflammatory mediators in experimental model for periodontitis. J Ethnopharmacol. 2017;205:16–21. doi:10.1016/j.jep.2017.04.018
32. Zhang Z, Shen P, Xie W, et al. Pingwei San ameliorates dextran sulfate sodium-induced chronic colitis in mice. J Ethnopharmacol. 2019;236:91–99. doi:10.1016/j.jep.2019.01.043
33. Yu HY, Yin ZJ, Yang SJ, Ma SP. Baicalin reverse AMPA receptor expression and neuron apoptosis in chronic unpredictable mild stress rats. Biochem Biophys Res Commun. 2014;451(4):467–472. doi:10.1016/j.bbrc.2014.07.041
34. Gao LL, Wang ZH, Mu YH, Liu ZL, Pang L. Emodin promotes autophagy and prevents apoptosis in sepsis-associated encephalopathy through activating BDNF/TrkB signaling. Pathobiology. 2022;89(3):135–145. doi:10.1159/000520281
35. Delezie J, Weihrauch M, Maier G, et al. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc Natl Acad Sci USA. 2019;116(32):16111–16120. doi:10.1073/pnas.1900544116
36. Li H, Hung A, Li M, Yang AWH. Fritillariae thunbergii bulbus: traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. Int J Mol Sci. 2019;20(7):1667. doi:10.3390/ijms20071667
37. Kim JH, Kim M, Hong S, et al. Anti-inflammatory effects of Fritillaria thunbergii Miquel extracts in LPS-stimulated murine macrophage RAW 264.7 cells. Exp Ther Med. 2021;21(5):429. doi:10.3892/etm.2021.9846
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
