Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Integrated Disease Management for Chronic Obstructive Pulmonary Disease in Primary Care, from the Controlled Trial to Clinical Program: A Cohort Study
Authors Hussey AJ, Wing K, Ferrone M, Licskai CJ
Received 16 September 2021
Accepted for publication 6 December 2021
Published 22 December 2021 Volume 2021:16 Pages 3449—3464
DOI https://doi.org/10.2147/COPD.S338851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Anna J Hussey,1 Kevin Wing,2 Madonna Ferrone,1,3 Christopher J Licskai1,4– 6
1Asthma Research Group Windsor-Essex County Inc., Windsor, ON, Canada; 2London School of Hygiene and Tropical Medicine, London, UK; 3Hotel-Dieu Grace Healthcare, Windsor, ON, Canada; 4London Health Sciences Centre, London, ON, Canada; 5Lawson Health Research Institute, London, ON, Canada; 6Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
Correspondence: Christopher J Licskai
Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
Email [email protected]
Purpose: Integrated disease management (IDM) for COPD in primary care has been primarily investigated under clinical trial conditions. We previously published a randomized controlled trial (RCT) where the IDM intervention improved quality of life (QoL) and exacerbation-related outcomes. In this study, we assess the same IDM intervention in a real-world evaluation and identify patient characteristics associated with improved outcomes.
Methods: This historical cohort study included patients enrolled for 12 (± 3 months) in the Best Care COPD IDM program. The main outcome was a ≥ 3 point improvement in COPD assessment test (CAT). Secondary outcomes were COPD exacerbations requiring antibiotics and/or prednisone, unscheduled physician visits, emergency department visits and hospitalizations.
Results: Data for 571 patients (all patients) were included, 158 met the reference RCT eligibility (RCT matched). Improved QoL was observed in 43% (95% CI:38.9,47.2) of all patients, 47% (95% CI:39.5,55.6) of RCT matched vs 92% (95% CI:79.2,95.1) in the reference RCT intervention arm (n=72). Reductions (12 months IDM vs prior year) were observed in the proportion of patients experiencing exacerbation-related events (all patients): antibiotics/prednisone (− 9.0%,95% CI:-13.9,-3.9); unscheduled physician (− 33.1%,95% CI:-38.2,-27.9); emergency department (− 9.6%,95% CI:-13.5,-5); and hospitalizations (− 6.8%,95% CI:-10.0,-3.7). For the RCT matched group all reductions were comparable to the reference RCT intervention arm. The strongest predictors of improved QoL were baseline CAT, CAT≥ 20 vs CAT< 10 (OR 15.6,95% CI:7.91,30.83), GOLD group B (OR 6.4,95% CI:3.42,11.85) and D (OR 5.64,95% CI:2.80,11.37) vs GOLD group A. Patients with prior antibiotic/prednisone use, FEV1 < 30% predicted and GOLD group D were less likely to have no urgent health service utilization (OR 0.5,95% CI:0.30,0.68), (OR 0.2,95% CI:0.07,0.78) and (OR 0.3,95% CI:0.14,0.51), respectively.
Conclusion: Best Care COPD improved QoL and reduced exacerbation-related outcomes in a manner directionally similar to the RCT from which it emanated. Baseline QoL, exacerbation history, and GOLD category were identified as possible predictors of IDM impact and will inform future program development and resource allocation.
Keywords: chronic disease management, COPD assessment test, health service utilization, health status, quality of life
Introduction
Chronic Obstructive pulmonary disease (COPD) is a chronic, progressive, lung disease with acute exacerbations and chronic symptoms that contribute to high use of urgent care facilities, hospitalizations, a decreased quality of life (QoL) and associated health system costs.1–6 When effectively implemented, guideline-based best practices can reduce the personal and health system disease burden.1,2 Globally, it is estimated that there are over 350 million adults with COPD with almost 1 million individuals residing in the Canadian province of Ontario where this study was conducted.7,8 COPD is a complex condition that is principally managed in primary care where there are significant care gaps that can limit guideline implementation.3–6,9–11 Existing knowledge-to-practice gaps include underdiagnosis, suboptimal disease assessment, poor therapy adherence, incorrect medication delivery and inadequate exacerbation management.5,10,12,13 It is becoming increasingly recognized that achieving optimal guideline adherent primary care necessitates a shift from physician-based management to a team collaborative.1,14–17 One such strategy is integrated disease management (IDM), a model of care well suited to delivering best practices.
IDM is a patient centered, multi-disciplinary approach to chronic disease with a focus on education and self-management, addressing all aspects of disease presentation and progression.18–20 Although a distinct strategy, it encompasses a wide array of components and rarely, if ever, is the same IDM intervention implemented in different study populations making intervention comparison complex. This point is well illustrated in a recently updated meta-analysis that evaluated the impact of IDM from 52 randomized controlled trials (RCTs).21 The programs ranged from, involving 2 to 7 health professionals, delivering 2 to 8 IDM components, with program durations of 3 to 48 months. Furthermore, they were conducted in all types of healthcare setting (primary, secondary and tertiary) and spanned five continents where there is probably a large variation in usual care. Dominant IDM subtypes identified by the studies included exercise predominant, self-management with exacerbation action-plans, structured follow-up and case management, and telemonitoring.21 Acknowledging this heterogeneity, meta-analyses of randomized controlled trials (RCTs) examining IDM have confirmed a favorable impact on health-related QoL, emergency department (ED) visits, and hospital admissions.15–17,21
The impact of IDM implemented in primary care is less certain with both positive and negative studies.21–27 In community COPD programs, outside of the rigid controls of an RCT, there is an even greater potential that implementation heterogeneity will affect program outcomes. Similarly, there are limited and conflicting studies investigating the impact of IDM on primary care COPD populations in real-world clinical programs.28,29
Innovative primary care COPD IDM programs are needed that address the substantial disease burden, identify, and address current gaps in primary care health systems, manage implementation heterogeneity and conduct robust program evaluations. We previously published the results of an RCT comparing COPD IDM in primary care to usual care which demonstrated differential improvements in QoL and a reduction in urgent health service utilization (HSU).24 The COPD IDM intervention from the RCT has since transitioned into the Best Care COPD program. Building on the foundation of a positive RCT, the objectives of this study were to, (i) assess the impact of the same IDM intervention delivered in a real-world program in primary care (RCT comparison) and (ii) identify patient characteristics associated with greater program benefits (Predictor analyses).
Materials and Methods
The Reference RCT
In 2011, a primary care IDM program for COPD was introduced experimentally as the intervention for an RCT at four Family Health Teams (FHTs) in Southwestern Ontario.24 Positive results from this study justified the commitment of funding for the ongoing and scalable spread of the Best Care COPD program. The inclusion criteria of the reference RCT specifically enriched the study with patients that had reduced lung function (forced expiratory volume in one second (FEV1) ≤70% predicted) and a history of COPD exacerbations.
Study Design and Data Collection
This historical cohort study was conducted at seven FHT’s from January 2012 to July 2019 and included the same geographic catchment area as the reference RCT. FHTs are community-based multidisciplinary healthcare teams that include family physicians, nurses, social workers, dietitians, and other professionals who provide primary health care to the community they serve. Patients in the Best Care COPD program were referred by their primary care provider (family physician or nurse practitioner) if they had a confirmed or suspected diagnosis of COPD. All patients in the program with complete data on the primary outcome (QoL) and a 12 (±3) month follow-up interval were included. The COPD diagnosis was confirmed by post-bronchodilator lung function testing. RCT participants were excluded.
The Best Care COPD Program
The IDM intervention from the reference RCT was implemented as the Best Care COPD program (Figure 1). In brief, the IDM intervention consists of case management, education, and skills training provided on-site in primary care by a certified respiratory educator (CRE) in collaboration with the primary care provider.24 The Best Care program targets the implementation of high impact best practices, as per the Global Initiative for Obstructive Lung Disease (GOLD) guidelines, including spirometry for diagnosis and management, medication review, immunization, self-management action plans, inhaler device instruction, referral to other health professionals as required and smoking cessation support.1
Study data were captured in a program specific, electronic data management system with a point of service user interface, used by the CREs at every patient encounter. This Best Care e-tool is an important component of the program as COPD guideline-based intervention standards are imbedded to standardize all encounters and capture performance metrics, patient clinical and demographic characteristics and patient outcomes. All Best Care patient records are collected and stored in this central database, inclusive of participant records from the reference RCT.24
Outcomes
The primary outcome evaluated in the reference RCT and in this study was health-related QoL measured by the COPD assessment test (CAT) score. The CAT consists of 8 questions on a 6-point (0 to 5) scale summed to a score of 0 to 40 where higher scores represent worse QoL. Reported minimum clinically important differences (MCIDs) range from 1.8 to 4.0 points. The reference RCT considered a change in CAT score >3 clinically meaningful; however, as this study considered a broader spectrum of COPD severity a slightly reduced MCID ≥3 was chosen.30–32 CAT score, was patient-completed and recorded in the Best Care e-tool by the CRE at most IDM appointments. Patients missing an initial and/or follow-up CAT score were excluded as we were unable to determine the primary outcome. Furthermore, patients with a CAT score <3 at initial appointment were unable to achieve a clinically relevant improvement in their CAT score and were also excluded.
Secondary outcomes evaluated in the reference RCT and that are included in this study were exacerbation-related outcomes measured by the number of COPD related, courses of antibiotics and/or prednisone taken, unscheduled physician visits, ED visits, and hospitalizations during 12 months of IDM follow-up. An exacerbation has been defined as a worsening of respiratory symptoms that are treated with a short acting bronchodilator (mild), plus antibiotics and/or oral corticosteroids (moderate) or requiring a visit to the ED or a hospitalization (severe).1 ED visits and hospitalizations for COPD were mutually exclusive, that is if an ED visit led to a hospitalization this was counted as one hospitalization event. The exacerbation-related outcome antibiotic and/or prednisone use gives a measure of the number of moderate exacerbations regardless of whether they lead to urgent health service utilization. Exacerbation-related outcomes are mandatory fields in the Best Care e-tool and are collected at every IDM encounter. Mild exacerbations treated with only a short acting bronchodilator are not captured.
Objective (I): RCT Comparison
Two predefined real-world program groups were selected and compared to the reference RCT intervention arm.
All patients (Group 1): This first comparative group seeks to investigate if IDM is impactful to a real-world primary care COPD cohort when it is delivered without the eligibility restrictions on program access and without the rigid controls when delivered under trial conditions. For example, follow-up intensity in the Best Care COPD program is determined by the CRE, primary care provider, and patient based on COPD severity and individual patient need, however in the reference RCT this was predetermined. We hypothesized that improvements would be observed, but that the effect size would be smaller than observed in the reference RCT. All Best Care program patients that had undergone 12 (±3) months of IDM were included in this group. Allowing the flexibility of 3 months either side of the RCT 12-month follow-up reflected the less controlled nature of the real-world program.
RCT matched (Group 2): The second comparative group was “matched” to the intervention arm of the reference RCT by applying the trial eligibility criteria to Group 1 to create a Best Care program subset that were better matched to the RCT participants. The aim of this objective was to explore if the same COPD IDM intervention would have a comparative impact when delivered as a real-world clinical program in a cohort of primary care COPD patients who were, to all intent and purposes, equivalent to the reference RCT intervention arm. It has been demonstrated that data from trial participation may lack external validity (specific patient characteristics may influence the decision to participate in a trial) and be susceptible to measurement bias (trial participants are aware they are being evaluated and this may influence behavior, eg, increase medication adherence).33,34
Reference RCT inclusion and exclusion criteria were applied to Group 1. Exacerbation history was matched based on the prior one year. We were not able to exclude program patients with two of the RCT exclusion criteria, those with a terminal illness or that had a “comorbid condition that would interfere with study participation”, however it is unlikely that patients fitting these criteria would have been enrolled. The RCT matched group was restricted to the 4 FHTs within which the reference RCT was conducted, to account for possible FHT provider or patient differences.
Statistical Analyses: RCT Comparison
Baseline data were presented, mean and standard deviation (SD) for continuous variables and/or count and percentage for categorical variables, for all patients (Group 1), RCT matched (Group 2) and the reference RCT intervention. Continuous variables were inspected graphically and for normally distributed variables unpaired two sample t-tests were used to identify any baseline differences between the all patients group vs RCT intervention arm and the RCT matched group vs RCT intervention arm (two-tailed p<0.05 considered significant). If the data were not normally distributed a Wilcoxon signed rank test was used. Chi-squared tests were used for categorical variables.
Pre- and post-point estimates and differences were presented for outcome variables, analysed in the reference RCT (CAT score, COPD exacerbation outcomes measured by antibiotic and prednisone use, unscheduled physician visits, ED visits and hospitalizations) and were tabulated alongside the all patients and RCT matched groups. Pre and post differences and confidence intervals (CIs) for repeated measures were calculated using t-tests for mean values and McNemar’s test for repeated proportions. Confidence intervals (95%) were used to compare absolute effects between each of the groups versus the trial intervention.
Objective (II): Predictor Analyses
All patients (Group 1) were used in the predictive analysis to assess two outcome variables. The first outcome variable evaluated was improvement in QoL, a binary variable capturing the proportion of patients achieving a decrease of ≥3 points in CAT score (or the proportion of patients experiencing a clinically relevant improvement in QoL). The second outcome assessed was the absence of urgent COPD-related HSU including unscheduled physician, walk-in clinic, urgent care, emergency department visits and hospital admissions. A binary variable was constructed grouping patients with no HSU events and those with one or more HSU events during 12 (±3 months) of IDM.
For this study, we included predictors commonly associated with COPD progression and prognosis and developed models to investigate their effect on positive outcomes (improvement in CAT score and an absence of HSU). Choice of predictor was based on clinical relevance and previous literature. Most prior literature, using predictive models to investigate COPD progression, examine variables associated with COPD exacerbation-related outcomes.35–37 Identified predictors include age, sex, body mass index (BMI), smoking status, smoking pack years, lung function measurements and prior exacerbations.35–37 Modelling predictors of change in QoL has not been widely developed but there are reported results linking baseline modified British medical research council (mMRC) score for dyspnea, age, FEV1 and prior exacerbations to change in QoL.38,39 Finally, although not included as a variable in the forementioned studies low socio-economic status has also been identified as a risk factor for adverse COPD related outcomes.1,40
Postal code linkage to the Ontario Marginalization Index was used as a proxy for individual measurement of the socio-demographic factors of material deprivation and residential instability.41 These socio-demographic measures are categorized into 5 levels of poverty whereby a score of 1 denotes the least level of poverty and 5 is the highest.
Statistical Analyses: Predictor Analyses
Univariable logistic regression was used to assess association between each variable and the two binary outcomes of improved QoL (CAT score reduction ≥3) and absence of urgent HSU. Where applicable potential predictor variables were assessed using likelihood ratio tests (continuous versus categorical) to assess their best fit in the model. Collinearity testing was performed for all potential predictor variables to assess the logistic regression assumption of little or no multicollinearity among the independent variables. Box-Tidwell tests for non-linearity were used to assess the logistic regression assumption of linearity between the log odds of the dependent variable and the continuous independent variables. Continuous variables meeting this assumption were modelled as such to prevent loss of power through categorization; however, they were presented categorically to ease interpretation.
Mixed effects multivariable logistic regression models were constructed including all potential prespecified predictor variables with FHT included as a random effect.42 Potential predictor variables included, identified for their clinical relevance, were age, sex, material deprivation, residential instability, smoking status, pack years of smoking, BMI, prior year COPD-related HSU and prior year COPD related antibiotic and prednisone use, baseline CAT score, baseline mMRC, and baseline FEV1% of predicted. Variables in the model were assessed, using 95% confidence intervals and p values (two-tailed p < 0.05 considered significant), as predictors of outcome.
Change in CAT score was modelled using logistic regression as a binary outcome to improve the clinical interpretation of results. Identification of patients who experienced a clinically relevant change gives a measure of the distribution of improved QoL.38,43 However, sensitivity analyses were performed that modelled change in CAT score as a continuous variable using linear regression for univariable and mixed effects linear regression for multivariable analysis.
Variables with missing data were investigated and if assumed to be missing at random, multiple imputation with chained equations was used to avoid excluding any patients from the models.44,45 Results from complete case analyses were compared to the imputed results. Additionally, because the GOLD categorization is such a clinically important composite variable, GOLD group was included as a predictor in place of the variables from which it is derived (CAT score, hospitalizations and exacerbations) in a separate model.1 Loss to follow-up was assessed for selection bias through a descriptive comparison of baseline characteristics. Data analyses were performed using Stata v17.0.
Results
Patients
From November 2011 to July 2019, 2962 patients were enrolled in the Best Care COPD program. Study flow is presented in Figure 2. After excluding patients who did not meet pre-specified study criteria and those with missing outcome data, there were 571 patients who were included in the all patients group and 158 patients in the RCT matched group (Figure 2).
Baseline Characteristics
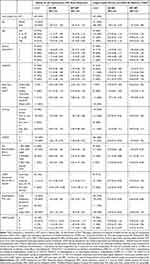
For the all patients group, mean age was 68 years (SD 9.6), 98% Caucasian, 75% were retired, mean 40 pack-year smoking history, CAT 15.9 (SD 7.6) and 51% experienced a moderate or severe exacerbation requiring antibiotics and/or prednisone in the prior year. The demographic characteristics were similar across all three groups except for female sex with 44% in the RCT matched versus 60% in the reference RCT (p = 0.030) (Table 1). There was a gradient of severity in lung health determinants across the three groups with all patients the least severe and the reference RCT the most severe. The RCT matched group was more similar to the reference RCT group comparatively, reference RCT vs RCT matched, baseline mean CAT score (22.6 vs 18.0. p < 0.0001), mMRC (1.9 vs.1.8, p = 0.4146), proportion of GOLD group D (54% vs 42%, p=0.0280), and mean HSU visits per person (3.3 vs 2.3, p = 0.258) (Table 1).
 |
Table 1 Baseline Demographical and Clinical Characteristics for Patients Completing 12 (±3) Months of Integrated Disease Management (IDM) |
RCT Comparison
Intervention intensity appeared to be similar between the three comparator groups. In the 12-month follow-up period, there was a mean of 3.8 appointments per patient for all patients, 3.7 for the RCT matched group and 4.0 for the reference RCT.
Similar to the RCT, there were significant improvements in the CAT score and significant reductions in all exacerbation-related outcomes comparing baseline to follow-up in the all patients and in the RCT matched groups (Table 2). Mean change in CAT score was directionally similar in all groups; however, confidence intervals of the all patients and the RCT matched groups did not overlap with the reference RCT. In the reference RCT, the mean change from baseline CAT score was −7.8 (95% CI; −9.0, −6.6), for RCT matched, −2.8 (95% CI, −3.9, −1.7) and for all patients −2.3 (95% CI, −2.8, −1.8). The percentage of patients experiencing an improved QoL (reduced CAT score ≥3) were 92%, 47% and 43% respectively. A post-hoc inspection revealed patients from the all patients with a poor baseline QoL (CAT score ≥20, n=171) showed a much greater improvement in mean CAT score (−6.4, 95% CI, −7.6, −5.3).
 |
Table 2 Comparison of Measured Outcomes for Group 1 (All Patients), Group 2 (RCT Matched), Reference RCT Intervention and Control Arms |
The changes in the number of patients who experienced exacerbation-related outcomes, comparing the year prior to the 12 months of IDM, for the RCT matched versus the reference RCT intervention group were comparable both in magnitude and directionality, demonstrated by 95% confidence intervals which overlapped for all exacerbation-related outcomes (Table 2, Figure 3A-D). Relative reduction for the three HSU outcomes ranged from 48% to 71% across the two real-world groups and from 39% to 68% in the reference RCT group.
The reference RCT control values are also presented in Table 2 and Figure 3, as a reference, giving an indication of outcomes under physician-based usual care under experimental conditions in this study population. Importantly, in this control group there was no significant improvement in CAT score or reduction in any of the exacerbation-related outcomes.
Predictor Analyses
Missing Data
The only predictor variable with missing data was FEV1% of predicted (76/571 missing, 13%) which is recorded during spirometry testing. Investigation revealed no one systematic reason for the missing data, but missingness was predominantly assessed as missing at random.44,45 Multiple imputations with chained equations were used by creating 20 imputed datasets. All predictor variables, FHT, and outcome variables were included in the imputation procedure. Complete case analyses did not reveal any substantial differences as compared to imputed model results [see Supplementary Table 1A-C].
Improved QoL
A clinically relevant improvement in QoL was experienced by 245/571 (43%) of patients. Multivariable analyses, Table 3, revealed the strongest predictor of improved QoL was baseline CAT score, patients with CAT scores ≥20 had over 15 times higher odds of achieving a ≥3-point improvement in QoL than patients with a baseline <10 (OR 15.75, 95% CI 7.97, 31.13). A baseline CAT score of 10 to 19 was associated with over 4 times higher odds (OR 4.21, 95% CI 2.36, 7.51). Other predictors of improved QoL were non-smokers compared to current smokers (adjusted OR 1.58, 95% CI 1.00, 2.48), and an underweight BMI category (adjusted OR 4.63, 95% CI 1.45, 14.81). A higher mMRC≥2 (moderate to high effect of breathlessness) was associated with a lower likelihood of improved CAT score (adjusted OR 0.58 (95% CI 0.36, 0.92) as was a lower FEV1 ≥ 30 to <50% of predicted (adjusted OR 0.45 (95% CI 0.24, 0.86)). GOLD groups B and D were more likely to experience improved QoL compared to GOLD A (adjusted OR 6.39, 95% CI 3.43, 11.91 and OR 5.71, 95% CI 2.83, 11.50 respectively).
Likelihood ratio tests did not identify any continuous variables that would be better suited to categorical modelling. Collinearity testing revealed no substantial collinear variables and Box-Tidwell tests for non-linearity did not identify any continuous variables that violated logistic regression assumptions. Linear regression modelling identified all the same predictor variables, and in addition an association (of borderline significance) was observed between prior antibiotic and prednisone use and improved QoL [Supplementary Table 2].
Absence of Urgent HSU
An absence of urgent HSU for COPD during the follow-up period was experienced by 339/571 (59%) of patients. Multivariable analyses, Table 3, showed absence of urgent HSU was less likely to occur in patients with antibiotics and/or prednisone use in the prior 12 months (adjusted OR 0.46, 95% CI 0.30, 0.68), patients with a FEV1% of predicted <30% (adjusted OR 0.23, 95% CI 0.07, 0.78) and patients meeting GOLD D criteria (adjusted OR 0.27, 95% CI 0.14,0.51). Restated in the obverse, this indicates that patients without a history of prednisone and antibiotic use in the prior year, those with a FEV1% of predicted ≥30, and those categorized as GOLD A-C were more likely to have no urgent health services use.
Loss to Follow-Up
We completed a descriptive sensitivity analysis to investigate whether the cohort lost to follow-up (n = 636) (see Figure 2) exhibited the same clinical and demographic baseline characteristics as patients completing 12 (±3 months) of IDM irrespective of missing CAT score (n = 868). There were a higher proportion of smokers (52%), ≤60-years (37%) and less retired patients (57%) in the loss to follow-up group compared to the group completing 12 (±3) months of IDM (32% smokers, 22% ≤60 years and 73% retired) [Supplementary Table 3].
Discussion
We evaluated Best Care COPD, an established primary care integrated disease management program, for its real-world impact on QoL and urgent health services use. We then compared those outcomes to the reference RCT from which the program emanated and identified independent predictors of response in a real-world setting.
The efficacy of the Best Care IDM intervention was confirmed in an RCT where the primary outcome, QoL, was dramatically improved and there were also substantial improvements in multiple secondary outcomes.24 In this study, we confirmed that the Best Care COPD program achieved directionally similar results when implemented in a real-world setting. The Best Care program all patients cohort included less severe patients than the RCT (which was specifically enriched with high-risk COPD patients) and expectedly the magnitude of improvement in outcomes was smaller. In this study, the RCT matched cohort that was constructed from the Best Care program population using the RCT inclusion and exclusion criteria demonstrated a similar magnitude of reduction in exacerbations and HSU. Improvements in QoL in the Best Care program were also substantial. Collectively, these findings suggest that program fidelity was maintained in the translation of knowledge from RCT into the scalable real-world Best Care community program.
Although the rate of chronological progression of QoL in the COPD population is unclear, COPD is a progressive disease and it is considered that there is a general decline in overall QoL over time.38,46,47 One prospective cohort study in the UK measured QoL in 567 patients with COPD, at baseline and one-year, by postal questionnaire and found that there was no significant change from baseline.48 In both real-world groups in this study, there was a small numerical decline (QoL improvement) in mean CAT score over a one-year timeframe. We found a clinically relevant QoL improvement in 43% of all patients and 47% in RCT matched patients after one-year of IDM. An improvement in QoL over a one-year period is independently important and has been associated with a lower likelihood of exacerbation, hospitalization, or mortality in the following 2 years.49 We identified a significant variance in the proportion of patients experiencing an improvement in QoL comparing the RCT matched cohort (47%) and the intervention group in the reference RCT (92%). The reason for this variation is unknown. QoL tends to be measured in experimental or prospective observational studies but is not generally collected or reported in routine real-world COPD data. We considered that program data may have been collected less rigorously and that the unblinded nature of the RCT may have introduced a bias to partially account for the difference. Alternatively, participant suitability for the RCT was determined by family physician, potentially introducing a subjective influence that was not regulated by eligibility criteria. Baseline CAT score was not an eligibility criterion and was considerably higher in the reference RCT than for the real-world group with the RCT eligibility applied (RCT matched). Future evaluation with a real-world cohort closely matched by baseline CAT may yield similar QoL improvements. Indeed, a post-hoc investigation including only patients with a poor baseline quality of life (CAT ≥20) from these data demonstrated the change in CAT score was comparable in magnitude to the reference RCT intervention arm.
The strongest predictors of improved QoL were baseline CAT score and GOLD category. Other predictive characteristics were not smoking, low mMRC score, a lower FEV1% of predicted and low BMI. CAT score as a predictor has been previously reported, and studies that included a COPD population with a good to moderate baseline QoL detected little or no impact of IDM on QoL.22,23,49 In this study, only 16% of patients with a CAT score <10 experienced a clinically relevant improvement, which is not unexpected as there is less scope for improvement.22,24 Non-smokers were more likely to experience an improved QoL at one year than smokers, supporting research findings that smoking cessation is known to be a key factor in slowing disease progression.1–3 This may have implications for the importance of smoking cessation support in future program improvements; however, this association was of borderline significance and the role of unobserved confounding should not be overlooked.
GOLD group calculation can be made using mMRC or CAT score.1 In this study, we used CAT score to determine GOLD category. Higher CAT score and higher GOLD category were therefore linked and were both predictors of improved QoL. In contrast, lower mMRC meaning fewer symptoms of breathlessness equated to a greater likelihood of improved QoL in the adjusted analysis. Restated in the obverse, a higher mMRC was associated with a lower likelihood of an improved QoL. This was unexpected and may be related to a lack of consistency previously detailed between GOLD categorization when using CAT score vs mMRC. It has been demonstrated that using CAT score and mMRC for GOLD group classification are not interchangeable metrics.50 Another plausible explanation is that patients with a high mMRC score are more debilitated by their COPD symptoms and therefore even with appropriate treatment this group achieves less improvement in QoL. Interestingly, in the univariable analysis and in agreement with a previous study, high mMRC was associated with an improved QoL; however, unlike our study, this direction of association persisted in their adjusted analysis.38 One difference to consider is baseline QoL was not accounted for in the reported models, which we found to have one of the strongest associations with improved QoL.
The mMRC finding was directionally supported by FEV1% of predicted. FEV1% of predicted has been closely related to the occurrence of respiratory symptoms.51 As with mMRC, where worse respiratory symptoms showed lower odds of improved QoL, so too did a lower FEV1% of predicted or worse lung function. However, using logistic regression, only one category of FEV1% of predicted (≥30% and <50% FEV1) was found to be significant, possibly due to a small sample size in the lowest FEV1% of predicted category (<30% FEV1, 11/20 with outcome).
Patients in the Best Care program experienced an approximately 50% relative reduction in unscheduled physician visits, ED visits and hospitalizations. These program results were consistent with the reference RCT intervention arm and this accordance strengthened when the RCT eligibility restrictions were applied to Best Care program patients (RCT matched). The strongest predictors of an absence of urgent HSU were no antibiotic or prednisone requiring exacerbations in the prior year. Other predictors were low-risk GOLD category (GOLD A), and an FEV1% of predicted value greater than 30%. However, the group size for FEV1% of predicted <30% with an outcome was small (n=5/20) and the association should be interpreted cautiously.
Integrated disease management is associated with a reduction in HSU.15–17,21 A history of COPD exacerbation is highly correlated with an increased risk of future exacerbations and an associated acceleration of disease progression.36 In part, this may explain the relationship observed in this study between prior exacerbation (measured by antibiotic and prednisone use) and urgent HSU in the year of follow-up. Patients with antibiotic and prednisone requirements for COPD in the year prior were 55% more likely to experience urgent HSU over one year of follow-up. As GOLD group is comprised of exacerbation data, it is likely that prior year exacerbations are driving the observed association between GOLD D classification and a lower likelihood of avoiding urgent HSU.1
Identification of these predictive variables spotlights a group of patients with one year of IDM who still required urgent health services for COPD despite the extended support and self-management strategies. This supports an additional focus on patients with a GOLD D classification, frequent exacerbations, and low FEV1% predicted measurements.
This study has several limitations. Firstly, as this study was conducted on data from an established primary care COPD program, there was no random assignment to intervention and control, we do not know what the outcomes would be in a real-world usual care cohort and we cannot exclude that confounding variables influenced the QoL, exacerbation and HSU outcomes reported. To partially mitigate this limitation and as a point of reference for comparability, we present outcome measures from the control arm of the reference RCT. The reference RCT immediately preceded the Best Care program and was completed in the same general patient population. The patients in the RCT control arm receiving usual care showed no clinical or statistically significant improvement in any of the measured outcomes consistent with the published literature.22–24,27 In addition, data reported from observational studies in real-world primary care COPD populations and no specified IDM does not generally show improving trends in QoL and other outcomes.46,52,53
One component of Best Care COPD is to develop self-management strategies in the event of a COPD exacerbation episode. The exacerbation outcome data collected by the Best Care program measures moderate and severe exacerbation episodes. However, one limitation of this study is that mild exacerbations are not captured. A reduction in antibiotic, prednisone and urgent health service use is an indication of a reduction in moderate-to-severe COPD exacerbation episodes, but whether this is due to an absolute reduction in exacerbation episodes or alternatively, rapid activation of self-management strategies to treat mild exacerbations preventing a worsening in exacerbation severity is unknown.
An investigation of patients lost to follow-up indicated possible attrition bias with higher proportions of current smokers, and ≤60-year-olds lost to follow-up than for patients who completed the study. Based on our predictive analysis, the age difference is not expected to impact outcomes. However, a population with more smokers would be expected to have fewer patients that achieve a significant improvement in QoL. In future, a more in-depth analysis of program attrition would be of value to improve program accessibility.
Finally, in the predictive analysis, low power may have influenced null results for some variables. For example, odds ratios increased through age categories in univariable analysis (Table 3) but no significance was detected. Future studies with a larger cohort can overcome this limitation.
Ongoing program evaluation will build from this study, for example, investigations examining the durability of CAT score over a 3-year follow-up are underway; more robust evaluation of health service use and IDM is planned by linking Best Care data with administrative data; and finally, although adherence to medication is not measured using a formally validated tool, the CREs do document self-reported adherence and an exploration not only of medication adherence but also the achievement of guideline recommended therapy based on COPD severity will be investigated.
Conclusion
The heterogeneity of complex IDM interventions and the effectiveness of implementation contributes to the variability in published RCTs and is an important factor determining successful transition from study to program. Best Care COPD, a primary care COPD IDM program, improved QoL and reduced urgent health services similar to the reference RCT from which the program emanated. Nearly half the patients experienced a clinically relevant improvement in QoL and most commonly this improvement was observed in those with more severe COPD. In addition, an approximate 50% relative reduction in unscheduled physician visits, ED visits and hospitalizations was observed. These program results were directionally consistent with the reference RCT intervention arm, and this accordance strengthened when the RCT eligibility restrictions were applied to Best Care program patients. A further exploration of the variation in QoL outcomes between study and the real-world program may identify opportunities for program improvement. Furthermore, understanding the predictors of patients who are less likely to achieve these important outcomes can help identify who will benefit the most from program participation and which patients are the most vulnerable and may require more intensive intervention.
Abbreviations
CAT, COPD assessment test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; FEV1, forced expiratory volume in one second; FHT, family health team; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HSU, health service utilization; IDM, integrated disease management; mMRC, modified Medical Research Council; OR, odds ratio; RCT, randomized controlled trial; SES, socioeconomic status; QoL, quality of life.
Data Sharing Statement
Data availability requests can be made to the corresponding author.
Ethics Approval
Ethical approval for this study was obtained from the Western University Health Sciences Research Ethics Board, Ontario, Canada (Reference Number 114259). Waivers of informed consent were granted by the Western University Sciences Research Ethics Board in accordance with Tri-Council Policy Statement 2, article 5.5 A/B. All data were used in the study was secondary non-identifiable data. The study was conducted in accordance with the principles stated in the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all patients enrolled in the Best Care COPD program, all participating family health teams, and all members of the Best Care team for their hard work and dedication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No specific funding was acquired to support this work.
Disclosure
CL reports grants from Western University Professor of Health System Innovation; also reports personal fees for Advisory Board member for GlaxoSmithKline, AstraZeneca, Teva, Sanofi Genzyme and Valeo Pharma; and Research Grants from AstraZeneca; Honoraria or personal fees from AstraZeneca and GlaxoSmithKline, outside the submitted work. MF received honorarium from AstraZeneca outside the submitted work. The authors report no other conflicts of interests related to this study.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease. Report; 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.0-12Nov2021_WMV.pdf.
2. Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Can J Respir Crit Care Sleep Med. 2019;3(4):210–232. doi:10.1080/24745332.2019.1668652
3. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. The Lancet. 2019;379(9823):1341–1351. doi:10.1016/S0140-6736(11)60968-9
4. Yawn BP, Kim V. Treatment options for stable chronic obstructive pulmonary disease: current recommendations and unmet needs. Cleve Clin J Med. 2018;85(2 Suppl 1):S28–S37. doi:10.3949/ccjm.85.s1.05
5. Cho EE, Mecredy GC, Wong HH, Stanbrook MB, Gershon AS. Which physicians are taking care of people with COPD? Chest. 2019;155(4):771–777. doi:10.1016/j.chest.2018.12.018
6. Bellamy D, Bouchard J, Henrichsen S, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: management of chronic obstructive pulmonary disease (COPD). Prim Care Respir J. 2006;15(1):48–57. doi:10.1016/j.pcrj.2005.11.003
7. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health.. 2015;5(2)
8. Public Health Infobase: Canadian Chronic Disease Surveillance System (CCDSS). PHAC; 2017. Available from: https://health-infobase.canada.ca/ccdss/data-tool/.
9. Hernandez P, Balter MS, Bourbeau J, Chan CK, Marciniuk DD, Walker SL. Canadian practice assessment in chronic obstructive pulmonary disease: respiratory specialist physician perception versus patient reality. Can Respir J. 2013;20(2):97–105. doi:10.1155/2013/369019
10. Bourbeau J, Sebaldt RJ, Day A, et al. Practice Patterns in the management of chronic obstructive pulmonary disease in primary practice: the Cage study. Can Respir J. 2008;15(1):13–19. doi:10.1155/2008/173904
11. Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(3). doi:10.1186/1465-9921-11-122.
12. Philip K, Gaduzo S, Rogers J, Laffan M, Hopkinson NS. Patient experience of COPD care: outcomes from the British Lung Foundation Patient Passport. BMJ Open Respir Res. 2019;6(1):1–5.
13. Gregoriano C, Dieterle T, Breitenstein AL, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res. 2018;19(1):1–15. doi:10.1186/s12931-018-0936-3
14. Wagner EH. The role of patient care teams in chronic disease management. Br Med J. 2000;320(7234):569–572. doi:10.1136/bmj.320.7234.569
15. Kruis AL, Smidt N, Assendelft WJ, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD009437. doi:10.1002/14651858.CD009437.pub2
16. Zwerink M, Brusse‐Keizer M, van der Valk P, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3. doi:10.1002/14651858.CD002990.pub3
17. Lenferink A, Brusse-Keizer M, van der Valk PD, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;8(8):CD011682. doi:10.1002/14651858.CD011682.pub2
18. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi:10.1001/jama.288.14.1775
19. Norris SL, Glasgow RE, Engelgau MM, et al. Chronic disease management. Dis Manage Health Outcomes. 2003;11:477–488. doi:10.2165/00115677-200311080-0000118
20. Schrijvers G. Disease management: a proposal for a new definition. Int J Integr Care. 2009;9(March):2008–2010. doi:10.5334/ijic.301
21. Poot CC, Meijer E, Kruis AL, Smidt N, Chavannes NHHP, Honkoop PJ. Integrated disease management interventions for patients with chronic obstructive pulmonary disease (Review). Cochrane Database Syst Rev. 2021;9(9). doi:10.1002/14651858.CD009437.pub3
22. Kruis AL, Boland MRS, Assendelft WJJ, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ. 2014;349:g5392. doi:10.1136/bmj.g5392
23. Bischoff EWMA, Akkermans R, Bourbeau J. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ. 2012;7642(November):1–12. doi:10.1136/bmj.e7642
24. Ferrone M, Masciantonio MG, Malus N, et al. The impact of integrated disease management in high-risk COPD patients in primary care. Npj Prim Care Respir Med. 2019;29(1):8. doi:10.1038/s41533-019-0119-9
25. Freund T, Peters-Klimm F, Boyd C, et al. Medical assistant-based care management for high-risk patients in small primary care practices: a cluster randomized clinical trial. Ann Intern Med. 2016;164(5):323–330. doi:10.7326/M14-2403
26. Lou P, Chen P, Mph PZ, Yu J, Wang Y. A COPD health management program in a community-based primary care setting: a randomized controlled trial. Respir Care. 2015;60(1):102–112. doi:10.4187/respcare.03420
27. Chavannes NH, Grijsen M, Van Den AM, et al. Integrated disease management improves one-year quality of life in primary care COPD patients: a controlled clinical trial. Primary Care Respiratory Journal: Journal of the General Practice Airways Group. 2009;18:171–176. doi:10.3132/pcrj.2009.00003
28. Lisspers K, Johansson G, Jansson C, et al. Improvement in COPD management by access to asthma/COPD clinics in primary care: data from the observational PATHOS study. Respir Med. 2014;108(9):1345–1354. doi:10.1016/j.rmed.2014.06.002
29. Monteagudo M, Rodríguez-Blanco T, Llagostera M, et al. Factors associated with changes in quality of life of COPD patients: a prospective study in primary care. Respir Med. 2013;107(10):1589–1597. doi:10.1016/j.rmed.2013.05.009
30. Smid DE, Franssen FME, Houben-Wilke S, et al. Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53–58. doi:10.1016/j.jamda.2016.08.002
31. Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi:10.1016/S2213-2600(14)70001-3
32. Alma H, De Jong C, Jelusic D, et al. Health status instruments for patients with COPD in pulmonary rehabilitation: defining a minimal clinically important difference. Prim Care Respir J. 2016;26(1). doi:10.1038/npjpcrm.2016.41
33. Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:1–2.
34. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. doi:10.1371/journal.pctr.0010009
35. Bertens LCM, Reitsma JB, Moons KGM, et al. Development and validation of a model to predict the risk of exacerbations in chronic obstructive pulmonary disease. Int J COPD. 2013;8:493–499. doi:10.2147/COPD.S49609
36. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
37. Adibi A, Sin DD, Safari A, et al. The Acute COPD Exacerbation Prediction Tool (ACCEPT): a modelling study. Lancet Respir Med. 2020;8(10):1013–1021. doi:10.1016/S2213-2600(19)30397-2
38. Meijer E, Van Eeden AE, Kruis AL, et al. Exploring characteristics of COPD patients with clinical improvement after integrated disease management or usual care: post-hoc analysis of the RECODE study. BMC Pulm Med. 2020;20(1):1–10. doi:10.1186/s12890-020-01213-838
39. Jones PW, Anderson JA, Calverley PMA, et al. Health status in the TORCH study of COPD: treatment efficacy and other determinants of change. Respir Res. 2011;12:1–8. doi:10.1186/1465-9921-12-71
40. Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race, and COPD health outcomes. J Epidemiol Community Heal. 2011;65(1):26–34. doi:10.1136/jech.2009.089722
41. Matheson FI, van Ingen T. Ontario Marginalization Index: User Guide. Toronto, ON: St. Michael’s Hospital; 2018. Joint publication with Public Health Ontario; 2016.
42. Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–58. doi:10.7326/M18-1376
43. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(20):2379–2390. doi:10.1016/j.jacc.2020.09.542
44. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;339(7713):157–160.
45. Bhaskaran K, Smeeth L. What is the difference between missing completely at random and missing at random? Int J Epidemiol. 2014;43(4):1336–1339. doi:10.1093/ije/dyu080
46. Habraken JM, Van Der Wal G, Riet T, Weersink EJM, Tobene F, Bindels PJE. Health-related quality of life and functional status in end-stage COPD: a longitudinal study. Eur Respir J. 2011;37(2):280–288. doi:10.1183/09031936.00149309
47. Nagai K, Makita H, Suzuki M, et al. Differential changes in quality of life components over 5 years in chronic obstructive pulmonary disease patients. Int J COPD. 2015;10:745–757. doi:10.2147/COPD.S77586
48. Peters M, Crocker H, Dummett S, et al. Change in health status in long-term conditions over a one year period: a cohort survey using patient-reported outcome measures. Health Qual Life Outcomes. 2014;12(1):1–10. doi:10.1186/s12955-014-0123-2
49. Wilke S, Jones PW, Müllerova H, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425. doi:10.1136/thoraxjnl-2014-205697
50. Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. doi:10.1183/09031936.00125612
51. Jakeways N, McKeever T, Lewis SA, Weiss ST, Britton J. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur Respir J. 2003;21(4):658–663. doi:10.1183/09031936.03.00069603
52. Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(3):295–306. doi:10.1164/rccm.201501-0081OC
53. Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respir Med. 2007;101(1):146–153. doi:10.1016/j.rmed.2006.04.001
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.