Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Insulin Stimulates IL-23 Expression in Human Adipocytes: A Possible Explanation for the Higher Prevalence of Psoriasis in Obesity
Authors Di Vincenzo A, Granzotto M, Crescenzi M, Costa C, Piaserico S, Vindigni V , Vettor R, Rossato M
Received 19 January 2023
Accepted for publication 19 May 2023
Published 23 June 2023 Volume 2023:16 Pages 1885—1893
DOI https://doi.org/10.2147/DMSO.S405374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Gian Paolo Fadini
Angelo Di Vincenzo,1 Marnie Granzotto,1 Marika Crescenzi,1 Camilla Costa,1 Stefano Piaserico,2 Vincenzo Vindigni,3 Roberto Vettor,1 Marco Rossato1
1Department of Medicine – DIMED, Clinica Medica 3, University - Hospital of Padova, Padova, Italy; 2Department of Medicine - DIMED, Section of Dermatology, University - Hospital of Padova, Padova, Italy; 3Department of Neurosciences, Clinic of Plastic Reconstructive and Aesthetic Surgery, University – Hospital of Padova, Padova, Italy
Correspondence: Marco Rossato, Department of Medicine – DIMED, Clinica Medica 3, University Hospital of Padova, Via Giustiniani 2, Padova, 35128, Italy, Tel +39 49 8218746, Fax +39 49 8213332, Email [email protected]
Purpose: Psoriasis is a chronic systemic inflammatory disease involving the production of many pro-inflammatory cytokines derived from immune cells and interacting with different tissues leading to the typical skin lesions. Psoriasis shows a higher prevalence and a worse progression in obese than in lean subjects. The IL-23/IL-17 immune axis has a pivotal role in the pathogenesis of psoriasis and anti-IL-23 monoclonal antibodies are highly effective in its treatment. Since obesity in frequently associated with elevated insulin plasma levels, we have investigated the ability of in vitro differentiated human adipocytes to produce IL-23 at basal conditions and after insulin stimulation.
Material and Methods: In vitro differentiated human adipocytes were incubated in the absence and presence of different insulin concentrations and the expression of IL-23 was analyzed by real-time PCR and Western blotting.
Results: The results of this study show that in vitro differentiated human adipocytes spontaneously express IL-23 mRNA and protein being stimulated by insulin in a dose-dependent manner. The stimulatory effects of insulin on IL-23 expression were specific since it did not stimulate the expression of other well-known cytokines involved in psoriasis pathogenesis such as Il-22 nor LL-37. Furthermore, lipopolysaccharide did not stimulate IL-23 expression in human adipocytes, thus highlightening the specific effects of insulin in the stimulation of IL-23 expression in human adipocytes.
Conclusion: Here we show that human adipocytes spontaneously express IL-23 and that insulin stimulates IL-23 production by these cells in a specific manner as other stimuli, known to be involved in psoriasis pathophysiology, are ineffective. These observations could explain the association between psoriasis and obesity, a condition frequently characterized by a state of insulin hypersecretion.
Keywords: adipocyte, human, IL-23, insulin, obesity, psoriasis
Introduction
Psoriasis is a chronic systemic immune-mediated inflammatory disease with a prevalence of 1–3% worldwide. Although the etiology of this disease is still not precisely known, its pathogenesis, albeit incompletely, has been clarified and in particular the role of many pro-inflammatory cytokines derived from immune cells and interacting with different tissues leading to the typical lesions affecting the skin. The interleukin-23/interleukin-17 (IL-23/IL-17) immune axis is considered to have a pivotal role in the pathogenesis of psoriasis and in line with this, IL-17- and IL-23 are highly effective targets of psoriasis therapeutic strategies.1
Psoriasis may be associated with several comorbidities such as psoriatic arthritis, mood disorders including depression, and a range of cardio-metabolic disorders that include myocardial infarction, hypertension, obesity, type 2 diabetes mellitus and non-alcoholic fatty liver disease (NAFLD).2
In particular, a significant number of cross-sectional studies have shown that psoriasis is independently associated with obesity.3–5 Recently, increasing evidence from prospective studies showed that obesity precedes psoriasis occurrence, with a clear dose–response association between body mass index (BMI), waist circumference (WC), and waist-to-hip ratio (WHR), and weight gain with psoriasis risk.3 Accordingly, recent Mendelian randomization studies (based on genome-wide association) identified a causal link between obesity and psoriasis in European and Japanese populations, showing that genetically higher BMI increases the odds of psoriasis occurrence.4,6
Several studies have also shown that weight loss in obese psoriatic patients improves psoriasis, with a linear dose–response association, with more weight loss being related to higher improvements in psoriasis severity.2,5,7–9 Moreover, weight loss following lifestyle interventions such as diet and/or physical exercise may also prevent de novo psoriasis in obese subjects.10
While the relationship between obesity and psoriasis is well ascertained, the precise pathophysiologic link between obesity and psoriasis is not established although different explanations have been hypothesized such as pro-inflammatory mediators stemming from inflamed adipose tissue leading to systemic inflammation,11 promotion of the development of Th17 cells induced by adipose tissue,12 and increased leptin production by adipose tissue leading to the modulation of the immune response.13 Most of these hypotheses are based on animal models or on indirect evidence derived from observational studies and compelling direct evidence of a mechanism explaining the obesity-psoriasis link in humans is missing. Since obesity is a well-known disease characterized by hyperinsulinemia,14 in the present study we aimed to investigate the ability of human adipocytes to produce IL-23, one of the main cytokines involved in psoriasis pathogenesis.
Given the fact that lipopolysaccharide (LPS), a complex molecule of gram-negative bacteria cellular wall, can stimulate an inflammatory response in adipocytes15 and keratinocytes,16 we have compared the effects of insulin on adipocyte expression of IL-23 with those of LPS.
Materials and Methods
Isolation of Human Pre-Adipocytes and in vitro Differentiation to Mature Adipocytes
Subcutaneous adipose tissue (SAT) samples were obtained from five subjects (3 females and 2 males) undergoing abdominoplasty after bariatric surgery and important weight reduction and who were otherwise healthy (in particular with no history of skin nor autoimmune diseases) and not taking any drug. At the time of abdominoplasty, the patients showed a BMI of 36.2 ± 7.3 kg/m2, with a percentage total body weight loss (TBWL) of 28.5 + 8.8%. One set of cell culture experiments was performed from each subject. The stromal vascular fraction (SVF) was isolated from SAT by collagenase type II digestion (1 mg/mL; Sigma-Aldrich, St. Louis, MO) in DMEM/F12 at 37 °C for 1 hour. Cell suspension was centrifuged (350 × g for 8 min), and pellet containing stromal cells was resuspended in erythrocyte-lysing buffer, washed, and seeded in DMEM/F12 supplemented with 10% fetal bovine serum (0.7 × 106 cells per well in 24-well plates). After 16–20 hours for cell attachment, cultures were refed with a serum-free adipogenic medium containing DMEM/F12 supplemented with 33 µM biotin, 17 µM pantothenate, 10 µg/mL human transferrin, 66 nM insulin, 100 nM dexamethasone, 1 nM T3, 0.25 mM 3-isobutyl-1-methylxanthine and 10 µM rosiglitazone were supplemented for 72 hours then the medium was changed three times per week. After 12 days of differentiation, adipocytes were stimulated for 24 hours with the low and high insulin concentration (0.1 and 5 µM insulin dissolved in saline, respectively) and lipopolysaccharide at a dose intended to mimic a low-grade inflammation (LPS, 1.0 ng/mL). Control experiments were performed adding vehicle only (saline). A complete set of five experiments (one for each patient) that were run in triplicate was performed from each patient.
RNA Isolation
Total RNA was extracted from adipocytes after acute treatment with LPS, different concentration of insulin and vehicle using an affinity column-based method (RNEasy Kit, Qiagen GmbH, Germany) following the manufacturer’s protocol. The amount of RNA recovered by eluting the column was evaluated with a spectrophotometer (DS-11 Denovix), while the quality of the same was evaluated by a Bioanalyzer (Agilent 2100). RNA samples after extraction were placed at −80° C for the subsequent analysis of gene expression.
Quantitative RT-PCR
First-strand cDNAs were synthesized from 0.5 μg of total RNA using 150 ng random primers and 200 U M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA). cDNA was used to quantify gene expression levels of specific markers of inflammation, IL-23 and LL-37; each reaction was carried out in a final volume of 20 μL containing 10 ng cDNA, 10 μL of SYBR select master mix (Life-Technologies), 300 nM forward and reverse primers, specific for each gene (IL23: sense – AGAGAAGAGGGAGATGAAGAGACT, antisense – TCCTTTGCAAGCAGAACTGAC; LL37: sense – CCCAGGCCCACGATGGATG, antisense – CTGTCCCCATACACCGCTTC). Each reaction provided the following protocol: Taq polymerase activation (95° C for 2 minutes) and 45 × (30 s 95°C; 30s 60°C). The change in fluorescence at every cycle was monitored and a threshold cycle above background for each reaction was calculated. A melt curve analysis was performed following every run to ensure a single amplified product for every reaction. Each sample was run in duplicate, and the mean value of the duplicate was used to calculate the relative amount of individual mRNAs. Each mean value was normalized to that of the Small Subunit 18 ribosomal ribonucleic acid (18S rRNA) as housekeeping (internal consistent control gene) using the comparative the 2–∆∆Ct method.
Western Blotting Analysis for IL-23 in Human Adipocytes
After 24 hours of treatment, mature adipocytes were homogenized in RIPA Lysis Buffer with phosphatase and protease inhibitors cocktail (Sigma-Aldrich, Milan, Italy) for protein analysis. Thirty µg proteins were separated on precast 4–12% SDS-polyacrylamide gel (NuPage Bis Tris mini Gels, Life Tech) and then transferred to a Hybond ECL nitrocellulose membrane. This was blocked with 5% (w/v) dried non-fat milk in TBS buffer with 0.1% Tween 20 and then incubated with 1:500 diluted rabbit polyclonal anti-human IL-23 (#ab45420, Abcam, Cambridge, MA) and 1:1000 diluted mouse monoclonal anti-human β-actin (# SAB1305546, Sigma Aldrich, Milan, Italy). For IR detection of two proteins, blots were incubated by IRDye 680RD Goat anti-rabbit IgG (#926-68071, LI-COR Biosciences GmbH, Bad Homburg, Germany) and IRDye 800CW Goat anti-mouse IgG (#926-32210 LI-COR Biosciences GmbH, Bad Homburg, Germany) for IL-23 and β-actin, respectively. Blots were evidenced with the Odyssey Infrared System (LI-COR Biosciences GmbH, Bad Homburg, Germany) at both 700 and 800 nm channels in a scan at 169 μm resolution.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (version 9.5.1, GraphPad Software Inc., San Diego, CA, USA). Results from five separate experiments were considered and expressed as mean values ± standard deviation (SD). Data comparisons from two samples were analyzed with the Student’s “t-test”. Comparisons of means from multiple groups were conducted by ANOVA analysis. The variables were tested for normality using the Shapiro–Wilk test. Differences between groups were considered statistically significant at P value <0.05.
Results
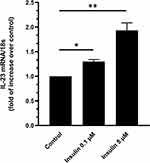
Insulin Stimulates IL-23 mRNA Expression in Human Adipocytes
As shown in Figure 1, insulin induced a dose-dependent increase in the expression of IL-23 mRNA in human adipocytes in vitro with a 1.3- and 1.9-fold increase of IL-23 mRNA expression at 0.1 (P<0.05) and 5 μM (P<0.01), respectively (Figure 1). Given the effects observed at the highest insulin concentration tested (5 µM), successive experiments were performed with this insulin concentration.
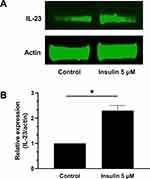
Insulin Stimulates IL-23 Protein Expression in Human Adipocytes
As shown in Figure 2, insulin stimulation of human adipocytes for 2 hours induced a significant increase in the expression of IL-23 also at protein level (P<0.01, Figure 2).
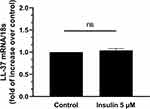
Insulin Induced Expression of IL-23 in Human Adipocytes are Specific
Many different cytokines have been involved in psoriasis pathogenesis. To verify that the effects of insulin were not aspecific, we evaluated the expression of another well-known cytokines involved in psoriasis such as IL-22 and Leucine–Leucine 37 (LL-37).17,18 As shown in Figure 3, insulin did not stimulate the expression of LL-37, a peptide involved in skin inflammation in psoriasis.17 Similarly, we did not detect any stimulatory effect of insulin on the expression of another cytokine involved in the development and pathogenesis of psoriasis such as IL-22,18 that was undetectable (under the limit of detection of the technique) (data not shown).
Lipopolysaccharide Does Not Stimulate IL-23 Expression in Human Adipocytes
Finally, lipopolysaccharide (LPS), a component of the gram-negative bacterial wall that is known to induce inflammatory cytokine secretion by adipocytes and involved in keratinocyte stimulation in psoriasis,15,16 did not stimulate any increase of IL-23 expression in human adipocytes in vitro (Figure 4). All these results further suggest that the stimulatory effect of insulin on IL-23 expression by human adipocytes is selective and specific.
Discussion
In this study we showed that insulin directly stimulates an increase of IL-23 expression in human adipocytes. IL-23, belonging to the IL-12 cytokine family, together with IL-12, IL-27 and IL-35, is a heterodimeric cytokine composed of the unique p19 subunit linked with the p40 subunit, which is in common with IL-12.19
IL-23 is produced by Langerhans cells,20 dendritic cells,21 and monocytes/macrophages (ie, antigen-presenting cells),22 in response to an inflammatory or biochemical insult, specifically at barrier sites such as the skin, gut and entheses. Moreover, keratinocytes are a skin-specific source of IL-23,23 and very recently it has been demonstrated that also ex-vivo human subcutaneous adipose tissue expresses IL-23.24
The present study provides the first evidence that insulin stimulates IL-23 in in vitro differentiated human adipocytes derived from subcutaneous adipose tissue. Intriguingly, these cells were fully differentiated adipocytes and did not require the presence of dendritic cells or other cell types to produce IL-23.
Previous studies reported that in obese women and in patients with metabolic syndrome IL-23 plasma levels were higher than in lean controls.25,26 Our finding could potentially help to explain the association between psoriasis and obesity, a condition characterized by quite elevated insulin plasma levels, with adipocytes directly producing IL-23, which could contribute to the higher plasma levels observed in obese subjects and then to the development of psoriatic plaques and possibly of other conditions such as psoriatic arthritis (PsoA). Noticeably, if subcutaneous adipose tissue is able to secrete IL-23, its local cutaneous concentrations in obese subjects could be several orders higher than those after dilution within blood. Indeed, obesity is a well-known risk factor for the development of psoriasis in the general population.3–5,27,28 Furthermore, several prospective studies showed that BMI is associated with an increased risk of PsoA development,29 and a higher PsoA severity.30 In particular, the development of enthesitis, which is arguably the primary lesion of PsoA and is pathogenetically related to IL-23, has been shown to be strongly associated with a higher BMI.31
The role of fat accumulation in psoriasis manifestations is further supported by different retrospective and prospective studies and by many meta-analyses showing that weight loss in patients suffering from psoriasis lowered the activity of the disease, even without an active treatment for psoriasis.2,5–9,32–34 Moreover, obesity in psoriatic patients may be a predictor of lower response to antitumor necrosis factorα (TNFα) agents and biologic treatment discontinuation.35,36 Accordingly, weight loss is associated with a better response to treatment with systemic therapies.37
Heretofore, several hypotheses have been proposed to explain the association between obesity and psoriasis,11 although not completely clear.
The ability of subcutaneous adipocytes to directly produce IL-23 without the need of an interaction with immune cells may represent the molecular basis of the dangerous liaison between fat tissue and psoriasis. On the other hand, the role of locally produced IL-23 in promoting in situ proliferation of skin Th17 cells has been recently demonstrated, thus confirming the therapeutic efficacy of IL-23 blockade in the treatment of psoriasis.38
Intriguingly, IL-23 production was stimulated by insulin. Obesity is frequently characterized by elevated insulin plasma levels.39 Therefore, we can speculate that hyperinsulinemia in obese patients may be able to directly enhance the inflammatory pathway characterizing psoriasis via the secretion of IL-23 directly from adipocytes. To this respect, Wu et al reported that diabetic subjects are at risk to develop first-time psoriasis and that the frequent use of insulin is associated with a dose-dependent increase in the risk of psoriasis.40 On the contrary, the use of oral antidiabetic drugs, such as metformin and thiazolidinediones, synthetic ligands for the peroxisome proliferator-activated receptor-gamma (PPARγ) receptor is associated with a lower risk to develop psoriasis, in particular if those diabetic subjects had never used insulin.41,42 However, these studies do not exclude the fact that the insulin dose-dependent increase in psoriasis may be due to a common inflammatory pathway between diabetes and psoriasis instead of a direct action of insulin per se on the effector cells involved in immune response in psoriasis. In support of the latter hypothesis, it has been reported that insulin exacerbates psoriasis in diabetic patients,43 and induction of new psoriasis plaques at insulin injection site has been described.44 Furthermore, it has been reported that psoriasis may worsen after the development of insulinoma.45
The link between insulin and psoriasis induction or exacerbation has not been precisely clarified yet although it has been suggested an involvement of the well-known mitogenic effects of insulin via its receptor or via the activation of the insulin-like growth 1 factor receptor, thus promoting keratinocyte proliferation.46
The demonstration of direct effects of insulin on IL-23 secretion by human adipocytes might open new avenues to tailor and improve the treatment of obese subjects with psoriasis pointing primarily to body weight reduction as the first strategy to improve the clinical results of psoriasis treatment protocols. A limitation of the present study could be that this is an in vitro study which results should be transferred to the patients, thus to definitely demonstrate that hyperinsulinemia represents the link between psoriasis and obesity.
Conclusion
In conclusion, the demonstration that insulin stimulates IL-23 expression in human adipocytes might be potentially important to unveil the complex relationship between obesity and psoriasis. In fact, since adipose tissues is well represented within the skin dermis, our observations suggest that these dermal adipocytes might possibly participate in the pathogenesis of psoriatic plaques, particularly in subjects with obesity, a condition frequently characterized by elevated insulin plasma levels.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon justified request, without undue reservation.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University Hospital of Padova (approval n. RF-2016-02363566). Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors wish to thank Mrs Sonia Leandri for her technical support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present work was funded by PRIN – Research Projects of National Relevance – by the Italian Minister of University, project #20178YTNWC_004 to Marco Rossato and by the grant n. ROSS_PRIV20_01 from the University of Padova, Italy, to Marco Rossato.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605–1613.
2. Costa L, Ramonda R, Ortolan A, et al. Psoriatic arthritis and obesity: the role of anti-IL-12/IL-23 treatment. Clin Rheumatol. 2019;38:2355–2362.
3. Aune D, Snekvik I, Schlesinger S, et al. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2018;33:1163–1178.
4. Budu-Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 2019;16:e1002739.
5. Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165–178.
6. Ogawa K, Stuart PE, Tsoi LC, et al. A transethnic Mendelian randomization study identifies causality of obesity on risk of psoriasis. J Invest Dermatol. 2019;139:1397–1400.
7. Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
8. Jensen P, Christensen R, Zachariae C, et al. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: a prospective observational follow-up study. Am J Clin Nutr. 2016;104:259–265.
9. Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes. 2015;39:1197–1202.
10. Mahil SK, McSweeney SM, Kloczko E, et al. Does weight loss reduce the severity and incidence of psoriasis or psoriatic arthritis? A critically appraised topic. Br J Dermatol. 2019;181:946–953.
11. Wong Y, Nakamizo S, Tan KJ, Kabashima K. An Update on the role of adipose tissues in psoriasis. Front Immunol. 2019;10:1507.
12. Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635.
13. Su X, Cheng Y, Chang D. The important role of leptin in modulating the risk of dermatological diseases. Front Immunol. 2021;11:593564.
14. Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J. 2021;45:285–311.
15. Jung TW, Park HS, Choi GH. Kim D and Lee T: β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J Biomed Sci. 2018;25:27.
16. Wang X, Yao Y, Li Y, Guo S, Li Y, Zhang G. Experimental study on the effect of luteolin on the proliferation, apoptosis and expression of inflammation-related mediators in lipopolysaccharide-induced keratinocytes. Int J Immunopathol Pharmacol. 2023;37:3946320231169175.
17. Lao J, Xie Z, Qin Q, Qin R, Li S, Yuan Y. Serum LL-37 and inflammatory cytokines levels in psoriasis. Immun Inflamm Dis. 2023;11:e802.
18. Hao JQ. Targeting interleukin-22 in psoriasis. Inflammation. 2014;37:94–99. doi:10.1007/s10753-013-9715-y
19. Teng MW, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729.
20. Yoshiki R, Kabashima K, Honda T, et al. IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells. J Invest Dermatol. 2014;134:1912–1921.
21. Wohn C, Ober-Blöbaum JL, Haak S, et al. Langerin(neg) conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc Natl Acad Sci USA. 2013;110:10723–10728.
22. Arnold IC, Mathisen S, Schulthess J, et al. CD11c+ monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 2016;9:352–363.
23. Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In-vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915.
24. Kochumon S, Hasan A, Al-Rashed F, et al. Increased adipose tissue expression of IL-23 associates with inflammatory markers in people with high LDL cholesterol. Cells. 2022;11:3072.
25. Pirowska M, Obtułowicz A, Lipko-Godlewska S, et al. The level of proinflammatory cytokines: interleukins 12, 23, 17 and tumor necrosis factor α in patients with metabolic syndrome accompanying severe psoriasis and psoriatic arthritis. Postepy Dermatol Alergol. 2018;35:360–366.
26. Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes. 2009;33:151–156.
27. Kumar S, Han J, Li T, Qureshi AA. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293–1298.
28. Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: nurses’ Health Study II. Arch Intern Med. 2007;167:1670–1675.
29. Scher JU, Ogdie A, Merola JF, et al. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15:153–166.
30. Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther. 2020;7:447–456.
31. Polachek A, Li S, Chandran V, Gladman DD. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res. 2017;69:1685–1691.
32. Higa-Sansone G, Szomstein S, Soto F, et al. Psoriasis remission after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2004;14:1132–1134.
33. de Menezes Ettinger JE, Azaro E, de Souza CA, et al. Remission of psoriasis after open gastric bypass. Obes Surg. 2006;16:94–97.
34. Hossler EW, Wood GC, Still CD, et al. The effect of weight loss surgery on the severity of psoriasis. Br J Dermatol. 2013;168:660–661.
35. Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One. 2018;13:e0195123.
36. Mourad A, Straube S, Armijo-Olivo S, et al. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181:450–458.
37. Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634–642.
38. Whitley SK, Li M, Kashem SW, et al. Local IL-23 is required for proliferation and retention of skin-resident memory TH17 cells. Sci Immunol. 2022;7:eabq3254.
39. Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377.
40. Wu CY, Shieh JJ, Shen JL, et al. Association between antidiabetic drugs and psoriasis risk in diabetic patients: results from a nationwide nested case-control study in Taiwan. J Am Acad Dermatol. 2015;72:123–130.
41. Krentz AJ, Friedmann PS. Type 2 diabetes, psoriasis and thiazolidinediones. Int J Clin Pract. 2006;60:362–363.
42. Tsuji G, Hashimoto-Hachiya A, Yen VH, et al. Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: implications for preventing the development of psoriasis. Cell Death Discov. 2020;6:11.
43. Moore AF, Soper T, Jones N, et al. Psoriatic exacerbation associated with insulin therapy. Diabetes Care. 2008;31:e31.
44. Wang P, Ran Y. Subcutaneous injection of isophane protamine biosynthetic human insulin induced psoriasis at the injection site. Eur J Dermatol. 2011;21:807–808.
45. Field S, Kelly G, Tobin AM, et al. Severe deterioration of psoriasis due to an insulinoma. Clin Exp Dermatol. 2008;33:145–147.
46. Cao J, Yee D. Disrupting Insulin and IGF Receptor Function in Cancer. Int J Mol Sci. 2021;22:555.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.