Back to Journals » Journal of Pain Research » Volume 16
Instrumented Posterior Arthrodesis of the Lumbar Spine: Prospective Study Evaluating Fusion Outcomes in Patients Receiving an Interspinous Fixation Device for the Treatment of Degenerative Spine Diseases
Authors Skoblar M, Hedman T , Rogers AJ, Jasper GP, Beall DP
Received 15 April 2023
Accepted for publication 17 August 2023
Published 24 August 2023 Volume 2023:16 Pages 2909—2918
DOI https://doi.org/10.2147/JPR.S417319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Krishnan Chakravarthy
Matthew Skoblar,1 Thomas Hedman,2,3 Adam J Rogers,3 Gabriel P Jasper,1 Douglas P Beall4
1The Jasper Spine Institute, Brick Township, NJ, USA; 2University of Kentucky, Lexington, KY, USA; 3Spinal Simplicity LLC, Overland Park, KS, USA; 4Comprehensive Specialty Care, Edmond, OK, USA
Correspondence: Douglas P Beall, Interventional Radiology, Comprehensive Specialty Care, 1023 Waterwood Pkwy, Edmond, OK, 73034, USA, Tel +1 (405) 601-2325 ; +1 (405) 213-9639, Email [email protected]
Purpose: Prospective evaluation of radiographic fusion outcomes in patients receiving instrumented posterior arthrodesis of the lumbar spine using a minimally invasive interspinous fixation device.
Patients and Methods: All patients (n = 110) from a single US physician’s practice who received instrumented posterior arthrodesis of the lumbar spine with a minimally invasive interspinous fixation device in the calendar year 2020 were invited to return for a follow-up CT scan to radiographically assess fusion. Forty-three patients, representing 69 total treated levels, consented to participate and received a lumbar CT scan at a mean of 459 days post-surgery (177 to 652). The interspinous/interlaminar fusion was assessed by 3 independent radiologists using a novel grading scale. Spinous process fractures were also assessed.
Results: 92.8% of the assessed levels were considered fused. There were no intraoperative spinous process fractures. There were 4 spinous process fractures (5.8%) identified on CT imaging, all of which were asymptomatic and healed without subsequent intervention. There were no instances of device mechanical failure or device-related reoperation.
Conclusion: Instrumented posterior arthrodesis of the lumbar spine using a minimally invasive interspinous fixation device provides clinically meaningful fusion rates with no reoperations and a low risk of spinous process fracture or other device-related complications.
Keywords: interspinous fusion, fusion grading scale, ligament sparing, spinal stenosis
Introduction
Lumbar spinal fusion has been an evolving surgical option for spine pain and radiculopathy associated with a number of degenerative spinal disorders including scoliosis, spondylolisthesis, and degenerative disc disease, for over a century. While the risk-benefit computation tilted away from conventional fusions in the 1980s,1 the advent of new surgical devices and minimally invasive approaches along with improved outcomes in clinical studies have led to a lumbar spine fusion resurgence. In the United States (US) from 2004 to 2015 the number of elective lumbar fusions increased by 62.3%.2 Lumbar spinal fusion has demonstrated superiority over non-surgical care for all degenerative spinal disorders.3 Decompression surgeries combined with fusions have demonstrated superiority in clinical outcomes compared to decompression surgery alone, but not for all surgical indications and in all studies.3
Lumbar spinal stenosis (LSS) is a common condition resulting from many years of intervertebral disc and facet joint degeneration,4 with approximately 1.4 million new cases diagnosed annually and an estimated prevalence of between 1.7% and 13.1% of the US population.5 The initial treatment following failure of conservative measures is typically a lumbar epidural steroid injection, which is only expected to provide temporary relief. Traditional surgical intervention performed on LSS patients is a decompression laminectomy with or without an accompanying spinal fusion. Laminectomy has been shown to offer superior clinical results compared to continuation of non-surgical care.6,7 Decompression surgery for spinal stenosis is the leading reason for spine surgery in the elderly and is one of the fastest growing reasons for spine surgery in the US.6,7
There are several degrees of surgical invasiveness and complexity for physicians to choose from with regard to decompression surgeries, ranging from minimally invasive surgery (MIS) techniques which preserve spinous processes, the midline ligaments and other soft tissues, to non-MIS techniques that disrupt these midline structures, to progressively more invasive and complex surgical decompression with spinal fusion. The intent of MIS procedures is to minimize iatrogenic injury to spinal and paraspinal tissues to decrease postoperative pain, recovery time, and muscular atrophy. MIS strategies also intend to reduce the potential for post-surgical segmental instability due to damage or removal of intrinsic support structures. MIS fixation devices are intended to add to these MIS objectives by providing near-term and long-term decompression and joint stability.8
Decompression surgery for spinal stenosis without an accompanying fusion, whether through traditional or minimally invasive tissue-sparing methods, has been found to significantly increase lumbar segmental range of motion and joint destabilization.9 Destabilization of the joint is exacerbated by disruption of the supraspinous-interspinous ligament complex which, as one biomechanical study suggests, contributes more than a third of the resistance to flexion.10 These studies suggest that it may be preferable for decompression surgery to be accompanied by fusion and combined with preservation of the posterior ligamentous complex in order to minimize instability, especially in the pre-fusion period. Currently, decompression is often supplemented with pedicle screw fixation and interbody fusion to mitigate instability. However, the use of pedicle screws and interbody fusion presents risks of pseudoarthrosis, neural injury, and adjacent segment disease. Aside from instability, laminectomy can result in significant complications such as epidural fibrosis, epidural hematoma, and epidural infection which can result in significant morbidity. Decompression accompanied with complex fusion surgeries have twice the rate of complications than decompression surgery alone.6 Together, these data suggest that a preferable solution for decompression of spinal stenosis would minimize disruption of musculoskeletal structures and provide long-term joint stabilization without the high complication rates associated with complex fusion surgeries.
Interspinous process devices (IPDs) are minimally invasive implants that have been used for patients with lumbar spinal stenosis (LSS) since 2005 and have been shown to provide significant symptom relief.11,12 The IPDs are comprised of two groups: those not providing rigid fixation – the interspinous distraction (spacers) and dynamic stabilization (ISD) devices, and interspinous fixation devices (IFD). Both types of devices are implanted between the spinous processes at the affected level to produce indirect decompression of the spinal canal, lateral recess and neural foramina. Given the more posterior placement of IPDs relative to pedicle screw systems, there is less risk of neural injury. Interspinous spacers and interspinous fixation devices are both intended to block extension motions while the interspinous spacers allow for free or constrained flexion motions.11 The permitted joint mobility may contribute to the high reoperation rate associated with interspinous process spacers.11,12 In addition to osseous distraction at the level of insertion, the interspinous fixation devices act to prevent all posterior process motions and typically contain a graft component to aid formation of bony fusion between the two spinous processes.
To date, there is minimal literature showing IFD fusion results, and an appropriate grading system has not been developed to define fusion success for IFDs. The purpose of the present study was to define an evaluation metric for IFD fusion and to evaluate the fusion outcomes by a single physician for an interspinous fixation device that is minimally invasive and preserves the posterior ligamentous complex. Complication and reoperation rates for this single physician analysis are also reported.
Materials and Methods
This study is a prospective radiographic assessment of patients initially receiving an MIS interspinous fixation device (Minuteman®, Spinal Simplicity, Kansas City, KS, USA) with demineralized bone matrix (DBM, Vivex Biologics Inc.) from a single physician in 2020. De-identified individual participant data that support the findings of this study are available from the third author upon reasonable request within three years of publication of this article. Available data include participant demographic and baseline data, scans and fusion scoring. Other study documents, including the study protocol and the patient informed consent form, are available upon reasonable request. All patients (n = 110) from a single US physician’s practice who received instrumented posterior arthrodesis of the lumbar spine with an interspinous fixation device in the calendar year 2020 were invited to return for a follow-up CT scan to radiographically assess fusion. There were no alternative surgical procedures performed by the physician on any lumbar spinal stenosis patients. This clinical investigation was conducted in compliance with the principles that have their origin in the latest version of the Declaration of Helsinki, the clinical investigation plan, requirements of the approving ethics committee and local regulatory authorities, ICH GCP and ISO 14155. Institutional review board (IRB) approval was obtained from Solutions IRB (FWA: IRB00008523, Yarnell, AZ, IRB Registration Number: IORG0007116) for the analysis and follow-up CT imaging. In addition, a post-treatment chart review for participating patients was approved by the IRB prior to initiation of the study.
Inclusion criteria: All patients that received the IFD had been diagnosed with degenerative disc disease and lumbar spinal stenosis along with various other lumbar spinal disorders including grades 1 and 2 (mild to moderate) spondylolisthesis, lumbar radiculitis, and spondylosis (facet or ligamentum flavum hypertrophy and corresponding symptoms). Treatment levels were determined by the physician based on a combination of pre-treatment imaging, physical examination, and back pain history. The presence of radiological or clinical indicators of segmental instability was a determining factor in the indications for IFD fusion in this study.
Exclusion criteria: With regard to patients receiving the IFD surgery, there were no exclusion criteria related to other medical comorbidities such as osteoporosis, diabetes, obesity or tobacco abuse. None of the IFD treated patients were excluded from follow-up scan requests.
The patients provided informed consent and were scheduled for CT scans according to their availability. The elapsed time between the surgical procedure and the follow-up scan varied from 177 to 652 days (approximately 6 to 22 months). A total of 43 patients, representing 69 total treated levels, consented to participate and received lumbar CT scans a mean of 459 days after surgery. Demographic and baseline data including surgical indications and operative levels were reviewed and instances of adverse events, spinous process fractures, device-related mechanical failure and device-related reoperation were evaluated. Interspinous fusion from deidentified scans was assessed by 3 independent radiologists using a novel-hybrid grading scale. Median fusion grades for each fusion construct were determined from the three scores. All fusion grades were reported using descriptive statistics. Fleiss’ Kappa Statistic (κ) was used to assess the interobserver variability in grading the interspinous fusions.
The MIS interspinous-interlaminar fixation device is capable of being placed via an MIS lateral surgical approach with the patient in either the lateral decubitus or prone position. The MIS insertion technique is muscle sparing and preserves most of the posterior osteoligamentous structures including the supraspinous ligament and a portion of the interspinous ligament. This IFD is intended to offer a minimally invasive alternative to traditional posterior instrumentation and interbody fusion while providing similar biomechanical support. The spinous processes at the target level are decorticated using a dedicated instrument, and the hollow core of the device is filled with graft material to promote fusion between the posterior elements. Due to its minimally invasive design and associated surgical technique, the MIS interspinous fixation device has the potential to reduce operative time, morbidity and risk to the patient when compared to traditional posterior fusion techniques.
MIS Interspinous Fixation Device Operative Technique
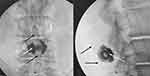
The level of interest is identified and a local anesthetic is applied to the skin and soft tissues. After making a skin incision just large enough to accommodate the soft tissue dilator a Steinmann pin is targeted toward the anterior interspinous space (Figure 1). Sequential soft tissue dilators are placed and a graduated tap is placed over the pin to decorticate the spinous processes and determine the size of the implant. The interspinous fixation device is packed with bone graft and placed into the interspinous space created by the tap. The extension plate wings are deployed and the proximal and distal portions are tightened against the spinous processes. The final placement is documented by anteroposterior and lateral fluoroscopic views (Figure 2).
 |
Figure 1 Lateral fluoroscopic image shows the tip of the Steinmann pin (black arrow) targeting the anterior interspinous space between the L3 and L4 spinous processes. |
Novel Interspinous Fusion Grading Scale
Currently, there is no gold standard fusion grading metric for posterior interspinous fusions. As such, the authors have reviewed the existing literature assessing other lumbar fusion constructs to develop a reasonable, clinically relevant fusion grading scale that can be applied to all interspinous fixation devices. Bridwell et al13 defined a 4-point grading scale for lumbar spine fusions that has been widely cited in the literature. Bridwell’s team investigated anterior interbody and posterior pedicle-based instrumentation used to correct adult deformity conditions. The Bridwell scale for anterior and posterior fusion grading is presented in Table 1 along with the newly developed criteria to grade interspinous fusion. Their anterior grading scale conveys generally recognized degrees of successful or unsuccessful bony fusion, while the posterior scale is more specific to grading fusions around pedicle-based posterior constructs. Two other papers provide fusion characteristics scales specifically for interspinous fixation devices. Vokshoor et al14 graded interspinous fusions following implantation of the Zimmer Biomet Aspen interspinous fixation device according to a similar, but reversed, 4 grade scale, with the 4 grades distinguished by: small islands of bone, larger islands of coalescence with bridging to surrounding anatomy, some solid incorporation and bridging bone, and solid fusion with incorporation and obvious stability and maturity. They considered constructs exhibiting either Grade 3 or Grade 4 characteristics to be fused and reported a 94% interspinous fusion rate with the Aspen interspinous device in a 50 data point cohort assessed using CT imaging. Postacchini et al15 graded interspinous fusions after placement of the Nuvasive Affix interspinous device according to a more basic “Certain”, “Incomplete”, or “Absent” fusion definition scale. In their study, a total of 25 fusion assessments were made with CT imaging and 21 of 25 (84%) were reported as “Certain”, 1 of the 25 (4%) was “Incomplete”, and 3 of the 25 (12%) were reported as “Absent.”
 |
Table 1 Comparison of Bridwell’s Fusion Grading and the New Interspinous Fusion Grading System |
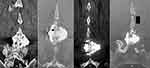
Since the Bridwell scale is specific to pedicle-based posterior fusion constructs, and not spinous process fixation devices like the device being evaluated in this study, the present study scoring system modified the Bridwell fusion grading scale taking into account the design intent and location for a posterior interspinous fusion with the device. The present study’s proposed scale also considers the 4-point fusion definition scale previously used by Vokshoor et al and Postacchini et al. The new fusion grading scale for interspinous fixation devices is presented in Table 1 and demonstrated in Figure 3.
Results
The distribution of primary spinal disorders in the patient cohort undergoing CT-imaging is listed in Table 2. Of the 43 patients, 21 had single level surgeries, 19 had 2-level surgeries, 2 patients had IFDs implanted at 3-levels and 1 had surgery at 4-levels. Typically, only 1 level and no more than 2 level surgeries were done on a single day for patients receiving implants at more than one level. There were no intraoperative spinous process fractures, device-related adverse events, mechanical failures, device dislocations, or device-related reoperations. Volumes of DBM used were from 1–2 cc per treated level depending on implant size. Follow-ups were based on the treating physician’s standard of care. Patients typically came back to the clinic 1–2 weeks post-procedure and around 6 weeks post-procedure. Other visits were patient initiated. Spinous process fractures were observed on the fusion assessment scans in 5.8% (4/69) of treated levels. All of the fractures were healed without subsequent intervention and had been asymptomatic (no patient with a spinous process fracture had sought treatment or registered any feedback indicating that the fracture was causing pain). There were no apparent relationships between surgical indications and occurrence of spinous fractures. Three of the four patients with spinous process fractures had IFDs implanted at two levels in the same procedure. The patients with spinous fractures had BMI above 30 (including the patient with the highest BMI in the study). The average BMI for all study participants was 30.0.
 |
Table 2 Diagnosed Spinal Disorders for Every Patient in the CT-Scanned Cohort |
Table 3 summarizes the fusion scores for each of the 4 grades for each radiologist and lists the median score for each patient. Combining the first 2 grades, similar to the technique used by Vokshoor,14 resulted in a 92.3% fusion rate based on the individual observer scores, and 92.8% fusion rate based on the median of the 3 scores for each patient. Fleiss’ Kappa statistic for interobserver variability was 0.3542 representing fair to moderate agreement between observers. There were no apparent relationships between surgical indications (such as presence of pre-surgical mild spondylolysis) or high BMI or multiple levels treated and fusion grades.
 |
Table 3 Fusion Score Summary from the CT Evaluation of the Single Center Cohort Undergoing IFD Surgery |
Discussion
In the last three decades, there has been an exponential increase in the number of minimally invasive devices used for the treatment of various spine and pain disorders.16–18 This growth trend has been accentuated over the past decade by a corresponding rapid growth of spine surgeries occurring in ambulatory care centers.17,18 One example is the many interspinous devices that have emerged to provide indirect decompression of the central canal and neural foramina via distraction of the spinous processes.
Of these posterior interspinous distraction devices, the MIS interspinous fixation device investigated here was designed to produce interspinous distraction along with immediate and long-term posterior stabilization via an MIS lateral approach. Some of the advantages of the lateral MIS approach include: 1) access to the interspinous space positioned posterior to the facet line which minimizes risk of neural injury, 2) no disruption of the supraspinous ligament, and 3) no multifidus muscle retraction or disruption of the lumbodorsal fascia.
Iatrogenic injury to the multifidus may cause devascularization and denervation of the muscle with associated postoperative pain and muscle atrophy.19,20 Likewise, resection of the posterior ligamentous complex has been associated with an increase in compressive forces on the intervertebral discs,21 and with a loss of a third of the resistance to flexion.10 The absence of device migration preceding joint fusion in this study supports the expectation that the winged design of the IFD distal plate, threaded core, and compression generated with the proximal plate are able to produce sufficient multi-axial interspinous stability,22 assisted by an essentially intact posterior ligamentous complex. Aside from the device fixation capabilities and muscle and ligament sparing MIS lateral approach, the use of demineralized bone matrix and bone decortication can provide long-term stability if interspinous fusion is achieved.
The results of this study show comparable or slightly superior posterior fusion results compared to prior studies. A large meta-analysis by Formica et al23 showed lumbar interbody fusion rates from standard surgical techniques to be 93%. The present results show fusion rates of 92%. Importantly, there were no exclusions of patients with osteopenia, diabetes, tobacco abuse, or other vascular issues in the present study. Vokshoor et al14 (Aspen device) demonstrated a comparable fusion rate (94%); however, additional stabilization was used for 51.4% of their subjects including pedicle screw instrumentation, interbody cages or both. The Aspen device insertion approach involved muscle splitting and retraction, and resection of the supraspinous ligament. There was also a difference in the use of bone graft materials between the two studies. Vokshoor et al placed graft materials in the barrel of the Aspen fixation device, over the lamina when it was considered safe, in the facet joints, and in the interbody cage when one was used. In the present study, the only graft materials used were preloaded into the body of the fixation device by the physician. Table 4 shows a comparison of the MIS IFD fusion results presented in this study with the Aspen device, Affix device, and standard interbody fusion results.
 |
Table 4 Comparison of MIS IFD Fusion Rates to Other Interspinous Fixation Devices and Standard Lumbar Interbody Fusion Techniques |
The ability to achieve arthrodesis has a particular benefit in patients with grade 1 and 2 anterolisthesis and retrolisthesis leading to symptomatic lumbar neurogenic claudication. Anterior spondylolisthesis is fairly common among older and middle-aged adults. A recent study showed a prevalence of anterior spondylolisthesis of 7% among 4548 subjects aged from 50 to 64.24 Laminectomy has been traditionally used for decompression of symptomatic neurogenic claudication with anterior spondylolisthesis, and fusion was often considered for grade 1 or grade 2 anterior spondylolisthesis with or without instability. The ability to provide indirect decompression of the central and neural foramina with the added ability to stabilize mild (grades 1 or 2) spondylolisthesis is a capability offered by MIS interspinous fixation devices.
Many investigators have presented positive patient reported outcomes and a low number of adverse events when using interspinous process devices for treatment of LSS.11,14,25 Shorter mean operative times, lower blood loss, and less incidence of dural tears compared to traditional lumbar spine surgery have all been consistently reported. Interspinous spacer devices have, however, been associated with a relatively high rate of spinous process fractures and device dislocations, and reoperation rates have been reported to be as high as 25%.11,12,26 This is in contrast to the 0% device migration seen in this study. This lack of device migration may suggest that the complications generally associated with non-fixated interspinous distraction devices (spacers) may be mitigated by the interspinous fixation and fusion capabilities of the MIS IFD. These low device migration (0%) and reoperation rates (0%) are comparable to those reported by Babu et al,27 0% and 6.9%, respectively, in a 144-device cohort evaluating the Aspen interspinous fixation device for the treatment of degenerative disc disease, spinal stenosis, spondylolisthesis, or prior hardware complications.
There were no intraoperative and 4 (5.8%) total spinous process fractures seen in this study. None of the spinous process fractures were symptomatic or resulted in a reoperation. This rate is substantially similar to the 4.2% asymptomatic and no reoperation required spinous process fracture rate reported previously for the Aspen fixation device.27 The absence of symptomatic fractures or fractures requiring reoperation is lower than the rates of spinous process fractures resulting in device removal reported by Vokshoor et al for the Zimmer Biomet Aspen interspinous device (2%)14 and by Chen et al25 who experienced intraoperative spinous process fractures for the Surgalign BacFuse interspinous device (3%).
Limitations of the Study
The primary limitation of this study was that clinical outcomes including patient reported pain and disability outcomes and analgesic use pre- and post-procedure were not included in this radiographic study. Future clinical studies are required to document clinical outcomes at regular post-surgery timepoints. The study is also limited by all the data being obtained from a single physician with no control group. Another limitation involves the range of months between the date of surgery and the date that the patients made themselves available for CT scans to assess fusion status. It would be preferable in future clinical studies to assess fusion quality at regular time periods such as 6-months, 1 year and 18 months post-surgery. There was a wide spectrum of patients in the current study, but with a high fusion rate there was insufficient numbers of patients with failed fusions to look at differences in fusion grades corresponding to specific spinal pathologies, amount of pre-surgical listhesis, and patient demographics. Despite the varied evidence and lack of conclusions or guidelines from a recent review article on the relationships between clinical outcomes and restoration of sagittal alignment, pelvic parameters and spinopelvic mismatch,28 spinal positioning in lumbar arthrodesis remains an area for concern. Therefore, the influence of the indirect, distractive decompression of interspinous fixation devices on clinical outcomes beyond initial relief of symptoms associated with lumbar stenosis should be investigated.
Conclusions
In this prospective study, an interspinous fixation device was implanted via a ligament-sparing lateral MIS approach in a total of 69 levels in 43 patients. The patients subsequently underwent CT scans which were assessed by 3 independent radiologists using a novel grading scale. 92.8% of the treated levels were considered fused. There were no instances of intraoperative spinous process fractures, device migration, mechanical failures, or device-related reoperations. Instrumented posterior arthrodesis of the lumbar spine using the MIS interspinous fixation device provides clinically meaningful and comparable fusion rates with a low risk of spinous process fracture or other device-related complications.
Acknowledgments
The authors gratefully acknowledge the contributions of Dr. Douglas Beall, Dr. Reade De Leacy, and Dr. Edward Yoon in conducting the radiographic assessments of patients’ scans according to the new interspinous fusion rubric.
Disclosure
Dr Thomas Hedman reports personal fees from Spinal Simplicity LLC, during the conduct of the study. Mr Adam J Rogers is an employee of Spinal Simplicity LLC, outside the submitted work. In addition, Mr Adam J Rogers reports various patents issued to Spinal Simplicity with no royalties and other payments for these patents. Dr Gabriel P Jasper is part of the medical advisory of and owns stock options from Spinal Simplicity, outside the submitted work. Dr Douglas P Beall reports grants from Medtronic, Medical Metrics, Avanos, Relievant, Boston Scientific, Stryker, Sollis Pharmaceuticals, Simplify Medical, Lenoss Medical, Spine BioPharma, Eliem Therapeutics, Smart Soft, Tissue Tech, Vivex, Stratus Medical, Restorative Therapies, Kolon TissueGene, Companion Spine, DiscGenics; personal fees from Medtronic, Spineology, Merit Medical, Johnson & Johnson, IZI, Techlamed, Peterson Enterprises, Medical Metrics, Avanos, Boston Scientific, Sollis Pharmaceuticals, Simplify Medical, Stryker, Lenoss Medical, Spine BioPharma, Piramal, ReGelTec, Nanofuse, Spinal Simplicity, Pain Theory, Spark Biomedical, Micron Medical Corp, Bronx Medical, Smart Soft, Tissue Tech, RayShield, Stayble, Thermaquil, Vivex, Stratus Medical, Genesys, Abbott, Eliquence, SetBone Medical, Amber Implants, Cerapedics, Neurovasis, Varian Medical Systems, Companion Spine, DiscGenics, Discure, SpinaFX, PainTEQ, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Frymoyer JW, Hanley E, Howe J, Kuhlmann D, Matteri R. Disc excision and spine fusion in the management of lumbar disc disease – a minimum ten-year followup. Spine. 1978;3(1):1–6. doi:10.1097/00007632-197803000-00001
2. Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2018;44(5):369–376. doi:10.1097/BRS.0000000000002822
3. Yavin D, Casha S, Wiebe S, et al. Lumbar fusion for degenerative disease: a systematic review and meta-analysis. Neurosurg. 2017;80(5):701–714. doi:10.1093/neuros/nyw162
4. Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14(3):491–504. doi:10.1016/S0030-5898(20)31329-8
5. Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9(7):545–550. doi:10.1016/j.spinee.2009.03.005
6. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi:10.1001/jama.2010.338
7. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810. doi:10.1056/NEJMoa0707136
8. Mooney J, Michalopoulos GD, Alvi MA, et al. Minimally invasive versus open lumbar spinal fusion: a matched study investigating patient-reported and surgical outcomes. J Neurosurg Spine. 2021;14:1–14.
9. Smith ZA, Vastardis GA, Carandang G, et al. Biomechanical effects of a unilateral approach to minimally invasive lumbar decompression. PLoS One. 2014;9(3):e92611. doi:10.1371/journal.pone.0092611
10. Gillespie KA, Dickey JP. Biomechanical role of lumbar spine ligaments in flexion and extension: determination using a parallel linkage robot and a porcine model. Spine. 2004;29(11):1208–1216. doi:10.1097/00007632-200406010-00010
11. Onggo JR, Nambiar M, Maingard JT, et al. The use of minimally invasive interspinous process devices for the treatment of lumbar canal stenosis: a narrative literature review. J Spine Surg. 2021;7(3):394–412. doi:10.21037/jss-21-57
12. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Superion interspinous spacer treatment of moderate spinal stenosis: 4-year results. World Neurosurg. 2017;104:279–283. doi:10.1016/j.wneu.2017.04.163
13. Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine – do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20(12):1410–1418. doi:10.1097/00007632-199506020-00014
14. Vokshoor A, Khurana S, Wilson D, Filsinger P. Clinical and radiographic outcomes after spinous process fixation and posterior fusion in an elderly cohort. Surg Technol Int. 2014;25:271–276.
15. Postacchini F, Postacchini R, Menchetti PP, Sessa P, Paulino M, Cinotti G. Lumbar interspinous process fixation and fusion with stand-alone interlaminar lumbar instrumented fusion implant in patients with degenerative spondylolisthesis undergoing decompression for spinal stenosis. Asian Spine J. 2016;10(1):27–37. doi:10.4184/asj.2016.10.1.27
16. Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus. 2009;27(3):E9. doi:10.3171/2009.7.FOCUS09121
17. Best MJ, Buller LT, Eismont FJ. National trends in ambulatory surgery for intervertebral disc disorders and spinal stenosis: a 12-year analysis of the national surveys of ambulatory surgery. Spine. 2015;40(21):1703–1711. doi:10.1097/BRS.0000000000001109
18. Basil GW, Yang MY. Trends in outpatient minimally invasive spine surgery. J Spine Surg. 2019;5(Suppl1):S108–S114. doi:10.21037/jss.2019.04.17
19. Kim CW. Scientific basis of minimally invasive spine surgery - prevention of multifidus muscle injury during posterior lumbar surgery. Spine. 2010;35(26S):S281–S286. doi:10.1097/BRS.0b013e3182022d32
20. Klinger N, Yilmaz E, Halalmeh DR, Tubbs RS, Moisi MD. Reattachment of the multifidus tendon in lumbar surgery to decrease postoperative back pain: a technical note. Cureus. 2019;11(12):e6366. doi:10.7759/cureus.6366
21. Merter A, Karaca MO, Yazar T. Biomechanical effects of sequential resection of the posterior ligamentous complex on intradiscal pressure and resistance to compression forces. Acta Orthop Traumatol Turc. 2019;53(6):502–506. doi:10.1016/j.aott.2019.08.016
22. Hedman TP, Ohnmeiss DD, Leasure J, Raji OR, Hochschuler SH. Interspinous-interbody fusion via a strictly lateral surgical approach: a biomechanical stabilization comparison to constructs requiring both lateral and posterior approaches. Cureus. 2023;15(7):e41918. doi:10.7759/cureus.41918
23. Formica M, Vallerga D, Zanirato A, et al. Fusion rate and influence of surgery‑related factors in lumbar interbody arthrodesis for degenerative spine diseases: a meta‑analysis and systematic review. Musculoskelet Surg. 2020;104(1):1–15. doi:10.1007/s12306-019-00634-x
24. He D, Li Z, Zhang T-Y, et al. Prevalence of lumbar spondylolisthesis in middle-aged people in Beijing Community. Orthop Surg. 2021;13(1):202–206. doi:10.1111/os.12871
25. Chen M, Tang H, Shan J, et al. A new interspinous process distraction device BacFuse in the treatment of lumbar spinal stenosis with 5 years follow-up study. Medicine. 2020;99:
26. Tram J, Srinivas S, Wali AR, Lewis CS, Pham MH. Decompression surgery versus interspinous devices for lumbar spinal stenosis: a systematic review of the literature. Asian Spine J. 2020;14(4):526–542. doi:10.31616/asj.2019.0105
27. Babu R, Gottfried ON, Stevenson JC. Outcomes and complications following spinous process fixation: a single-center analysis of 192 cases. Surg Technol Int. 2013;23:283–290.
28. Tartara F, Garbossa D, Armocida D, et al. Relationship between lumbar lordosis, pelvic parameters, PI-LL mismatch and outcome after short fusion surgery for lumbar degenerative disease. Literature review, rational and presentation of public study protocol: rELApSE study (registry for evaluation of lumbar arthrodesis sagittal alignEment). World Neurosurg. 2023;18:100162. doi:10.1016/j.wnsx.2023.100162
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.