Back to Journals » International Journal of General Medicine » Volume 16
Insight into the Progress in CAR-T Cell Therapy and Combination with Other Therapies for Glioblastoma
Authors Liang T, Song Y , Gu L, Wang Y, Ma W
Received 26 April 2023
Accepted for publication 2 August 2023
Published 11 September 2023 Volume 2023:16 Pages 4121—4141
DOI https://doi.org/10.2147/IJGM.S418837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tingyu Liang,* Yixuan Song,* Lingui Gu, Yu Wang, Wenbin Ma
Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenbin Ma; Yu Wang, Email [email protected]; [email protected]
Abstract: Glioblastoma (GBM) is the most common malignant primary brain cancer in adults. It is always resistant to existing treatments, including surgical resection, postoperative radiotherapy, and chemotherapy, which leads to a dismal prognosis and a high relapse rate. Therefore, novel curative therapies are urgently needed for GBM. Chimeric antigen receptor T (CAR-T) cell therapy has significantly improved life expectancy for hematological malignancies patients, and thus it increases the interest in applying CAR-T cell therapy for solid tumors. In the recently published research, it is indicated that there are numerous obstacles to achieve clinical benefits for solid tumors, especially for GBM, because of GBM anatomical characteristics (the blood–brain barrier and suppressive tumor microenvironment) and the tumor heterogeneity. CAR-T cells are difficult to penetrate blood–brain barrier, and immunosuppressive tumor microenvironment (TME), which induces CAR-T cell exhaustion, impairs CAR-T cell therapy response. Moreover, under the pressure of CAR-T cell therapy, the tumor heterogeneity and tumor plasticity drive tumor evolution and therapy resistance, such as antigen escape. Nonetheless, scientists strive for strategies to overcome these hurdles, including novel CAR-T cell designs and regional delivery. For instance, the structure of multi-antigen-targeted CAR-T cells can enrich CAR-T accumulation in tumor TME and eliminate abundant tumor cells to avoid tumor antigen heterogeneity. Additionally, paired with an immune modifier and one or more stimulating domains, different generation of innovations in the structure and manufacturing of CAR-T cells have improved efficacy and persistence. While single CAR-T cell therapy receives limited clinical survival benefit. Compared with single CAR-T cell therapy, the combination therapies have supplemented the treatment paradigm. Combinatorial treatment methods consolidate the CAR-T cells efficacy by regulating the tumor microenvironment, optimizing the CAR structure, targeting the CAR-T cells to the tumor cells, reversing the tumor-immune escape mechanisms, and represent a promising avenue against GBM, based on multiple impressive research. Moreover, exciting results are also reported to be realized through combining effective therapies with CAR-T cells in preclinical and clinical trials samples, have aroused inspiration to explore the antitumor function of combination therapies. In summary, this study aims to summarize the limitation of CAR-T cell therapies and introduces novel strategies to enhance CAR-T cell function as well as prospect the potential of the therapeutic combination.
Keywords: CAR-T, GBM, novel strategies, therapeutic combination
Introduction
Glioblastoma (GBM) is a highly aggressive and incurable brain tumor and is one of the most lethal human cancers.1,2 Compared with other solid tumor, therapeutic improvements for GBM have been minimal over the past 2 decades.3 Tumor-treating fields (TTFields) is the only treatment that has been shown to improve clinical outcome.4 Current Food and Drug Administration (FDA)-approved treatments for GBM are limited, which are majorly comprised of surgical resection, radiotherapy, chemotherapy, and TTFields, with a median survival following diagnosis of approximately 20.5 months.4,5
In recent years, immunotherapy strategies have revolutionized the treatment of multiple tumors, increasing the hope for GBM therapy.6 Unfortunately, GBM poorly responses to current immunotherapies. The extrinsic components that are native to the brain, such as the blood–brain barrier (BBB) and suppressive tumor microenvironment (TME), and intrinsic mechanisms, such as molecular heterogeneity which aid in immune evasion, are the main contributors to GBM resistance to various immunotherapies. Functional and tumor-specific cytotoxic T lymphocytes drive the adaptive immune response to the malignant tumor. Thus, the induction of their antitumor activities is the ultimate goal of all immunotherapies.7,8 Compared with the tumors, sensitive to immunotherapy, such as non-small-cell lung cancer and renal cell carcinoma, there are scarcity of T lymphocytes, whereas abundant tumor-associated macrophage (TAMs) in GBM microenvironment, because of the low immunogenicity and physical barriers. That is the reason that the efficacy of immune checkpoint inhibitors (ICIs) is limited in GBM. There are rare T cells that can be activated by ICIs in GBM TME. Furthermore, the immunosuppression TME impairs a beneficial response. Moreover, GBM is with a highly immunosuppressive TME, and the immunosuppressive myeloid cells/microglia account for the most abundant cellular constituents, while CD8+ T cells are the most cell subtype in immunotherapy-sensitive lung adenocarcinoma.9,10 The immunosuppressive cytokines, immune factors and myeloid cells/microglia can alter infiltrative T cells into exhaustion forms.11,12 Additionally, targeted and immunotherapy for GBM largely fail due to the complexities arising from intratumoral molecular heterogeneity.13 Glioma stem cells (GSCs), which are characterized by self-renewal, multi-lineage differentiation potential, play critical role in resistance to treatment and tumor heterogeneity.14,15 GSCs will evolute to other forms to adapt to the various treatment stress, and drive tumor treatment resistance. These features are the GBM specific factors that promote the failure of novel GBM treatments.
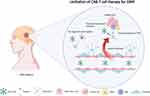
For decades, scientists invent chimeric antigen receptors (CARs) for expression on immune cells, among which T cells are the most common.16 The advantage of CAR-T cells is to reprogram abundant T cells, which aim to overcome the spare infiltration in GBM TME, against specific antigens, initiating a new era of personalized cancer therapy.17 While CAR-T cells have also been limited by a lack of sufficient delivery to the brain.18 For example, Maus et al report that intravenous delivery of CAR-T cells does not significantly prolong survival in GBM mice models, and intraventricular infusion with CAR-T cells is efficacious against GBM.19 Currently, regional delivery of CAR-T cells, radiotherapy and utilization of low-intensity pulsed ultrasound to open BBB are novel strategies to enhance CAR-T in situ delivery. Besides, the design of multi-targets or targeting GSCs CAR-T cells and combination of other therapies could eliminate adequate tumors cells and regulate immunosuppressive TME to avoid tumor recurrence and CAR-T inactivate. In the structure of CAR-T, there are four major components, including an extracellular target antigen-binding domain, a hinge region, a transmembrane domain, and one or more intracellular signaling domains.17 Currently, based on the various CAR-T cell structures, CAR-T cell has developed to the fifth generation, such as introduce of pro-inflammatory cytokines and signaling binding motif to sustain both long-term CAR-T cell proliferation and reverse suppressive TME.20,21
This review aims to describe the immune and molecular characteristics of GBM, which cause resistance to CAR-T cell therapy. Additionally, to overcome the current limitation, the development of genetically modified CAR-T cells and the combination of CAR-T cell therapy with other antitumor therapies are summarized.
The Characteristics of TME and Molecular Features in GBM
The suppressive TME around tumor cells is an insurmountable barrier attenuating the efficacy of CAR-T cell therapies in solid tumors. Besides, compared to other solid tumors, GBM patients rarely benefit from current immunotherapies, due to the special suppressive TME, and there is no FDA-approved immunotherapy in GBM.22 First of all, as a highly selective physiologic barrier, the BBB can dampen immune cells’ infiltration into GBM, thus resulting in a relative “cold tumor”.23 Similarly, the unique anatomical boundary is also seen to be a huge break for CAR-T cell delivery. Secondly, complex GBM TME, including abundant endothelial cells, fibroblasts, macrophage, and suppressive cytokines, together with sparse T lymphocytes,24,25 is quite distinct from other cancer types. The hostile GBM TME can educate infiltrative CAR-T cells into exhaustive ones. Thirdly, given the theory of “seeds” (cancer cells) and “soil” (the organ that cancer cells grow), it is reported that the various tumor histology (seeds) rather than intracranial location (soil) can induce T cells exhaustion and that T cells exhaustion is significantly severe among malignant glioma, in comparison with melanoma, breast, and lung.26 This interesting finding highlights that GBM can strongly impair T cells’ function. In summary, key challenges restricting efficacy and/or preventing the broad application of CAR-T cell therapies for GBM, are the restricted trafficking to, infiltration into, and activation of GBM TME.27 Through using advanced CAR-T cell technologies, these limitations may be better solved. Integration of molecular studies has led to more refined diagnoses and allows further clarification of the pathophysiologic processes that contribute to malignant phenotype and immunosuppressive TME.28 The most recent update of the World Health Organization (WHO) classification of brain tumors in 2021 introduces single nucleotide substitute (isocitrate dehydrogenase 1/2 and telomerase reverse transcriptase mutation), chromosomal abnormalities (cyclin-dependent kinase inhibitor 2A/B homozygous deletion, 1p/19q deletions, chromosome 7 gain/10 loss, and epidermal growth factor receptor amplifications), and promoter methylations (O6 methylguanine DNA methyltransferase) into the diagnosis of glioma.29,30 In addition, according to bulk expression profiles, research reveals that GBM samples could be classified into one of three states-proneural, classical and mesenchymal, with distinct genetic events, such as platelet derived growth factor receptor alpha alterations in proneural, epidermal growth factor receptor (EGFR) amplification in classical, and neurofibromatosis type 1 (NF1) mutation in mesenchymal.31–33 These cellular state is plasticity, under treatment stress. These novel markers are reported to induce the immunosuppressive TME. For instant, the activated EGFR pathway (about 50% in GBM) induces the PD-L1 expression to enhance the immune escape,34 and T cells are depleted in EGFR-amplified GBM samples.35 Additionally, alpha-thalassemia/mental retardation syndrome X-linked (ATRX) inactivation contributes to oncogenesis and knock-down ATRX could induce PD-L1 and an immunosuppressive cytokine/chemokine profile, such as interleukin 6 (IL6), interleukin 8 (IL8), and interleukin 33 (IL33), in vitro and vivo.36 Moreover, T cells enrich in mesenchymal subtype, the most malignant GBM subtype, while the immunosuppressive cells subpopulation, such as regulatory T cells and myeloid-derived suppressor cells, and anti-inflammatory cytokines, such as interleukin -10, transforming growth factor, beta 1 (TGFB1), are also abundant, altering infiltrative T cells into exhaustion forms. Overall, in GBM, molecular alterations are related with infiltration of T cells, T cells are spare in GBM, compared with another solid tumor, and although in some malignant samples, T cells enrich, the immunosuppressive factors can lead T cells exhaustion. The specific TME and molecular features of GBM provide huge barriers for CAR-T cell therapy.
CAR Structure
Despite various novel CAR designs, four main components exist, which are an antigen-binding domain, a hinge, a transmembrane domain, and an intracellular signaling domain. In every domain, there is a unique function, and an optimal design is needed to perfect CAR efficacy.37 In tradition, antigen-binding domains of CARs include the variable heavy (VH) and variable light (VL) chains of monoclonal antibodies, which are connected by a flexible linker for forming a single-chain variable fragment (scFv).38 The scFv design, such as the relative position of the complementarity-determining regions, is important to recognize and bind the target antigen.39 Apart from scFv, other molecules, such as cytokines and ligands, can also be taken as the alternative antigen-binding domains for CARs.40,41 In addition to working as a bridge to connect extracellular antigen-binding domains to intracellular signaling domains, the hinge and transmembrane domains provide adequate length for facilitating access to the target antigen and enough flexibility to overcome the steric hindrance.27 The characteristics of the hinge and transmembrane domains could affect antigen binding, signaling transduction, and cytokine production.42 On the whole, scientists begin to focus on the design of this region to perfect CAR-T cell therapeutic effect.43–45 Additionally, involving an activation domain and one or more co-stimulatory domains, intracellular signaling domains play a key role in CAR-T cell activation and persistence. The cytokines production and CAR-T cell proliferation are ensured by combining co-stimulatory domains and activation domains.46 Recently, among GBM, scientists improve CAR-T cells in various aspects innovatively, following the four components of CARs above and the summary of study success and failures on CAR-T cells. This is to improve the efficacy of CAR-T cell therapies for GBM patients while maintaining safety.
The Therapeutic Effects and Limitations of CAR-T in Published Clinical Trials
Up to date, there are published results from clinical trials of CAR-T therapy for interleukin 13 receptor a2 (IL13Ra2), epidermal growth factor variant III (EGFR vIII), human epidermal growth factor receptor 2 (HER2), disialoganglioside (GD-2), and erythropoietin-producing hepatocellular carcinoma A 2 (EphA2) targets (Table 1). Despite the safety of CAR-T for GBM, there are no successful CAR-T Phase III clinical trials for GBM. In some Phase I CAR-T clinical trials, some patients receive prognostic improvement, but the sample size is small. In addition to the small sample size, these clinical trials show much limitation, such as antigen escape and activation of immunosuppressive molecules. For instance, in the clinical phase I trial (NCT02209376) by O’Rourke et al47 conducted a first-in-human study of intravenous delivery of a single dose of autologous T cells redirected to the EGFRvIII mutation by a CAR on 10 recurrent GBM (rGBM). The authors demonstrate intravenous delivery CAR-T cells is safe, with median overall survival (OS) of about 8 months in the 10 rGBM. Dependent on 7 samples receiving post-CAR-T surgical resection, CAR-T could traffic to the tumor TME with active GBM, while 5 of the 7 patients undergo EGFRvIII loss after CAR-T infusion. Moreover, after CAR-T infusion, in situ analysis of the TME discovers significant increase in inhibitory molecules, such as PD-L1, indoleamine 2,3-dioxygenase 1 (IDO1) and infiltration of Tregs. Besides, in another phase I trial performed by Stephanie L. Goff et al48 18 rGBM patients receive EGFRvIII targeted CAR-T cells after lymphodepleting chemotherapy and intravenous IL2 delivery to support post-transfer. The median progression-free-survival (PFS) is 1.3 months, and the median OS is 6.9 months, the results do not demonstrate clinically meaningful impact in included patients. To sum up, antigen escape, enrichment of immunosuppressive immune cells and cytokines, and the infiltration and persistence of CAR-T affect the efficacy of CAR-T. Next, the limitations of CAR-T cell therapies for GBM are summarized.
 |
Table 1 Published Clinical Trials of CAR T Therapy in GBM |
Limitations of CAR-T Cell Therapies for GBM
Treatment-Associated Toxicities
Although adverse effects are reported to be relatively uncommon in GBM compared with those in hematological malignancies,40 the CAR-T cell treatment-related toxicities cannot be lost sight, majorly involving cytokine release syndrome (CRS) and central nervous system (CNS)-specific complications. Through summarizing 8 studies, including 63 recurrent GBM (rGBM) patients receiving CAR-T therapies, a published systemic review and meta-analysis finds that 6 (9.5%) patients suffered from CRS (4 grade ≤2 and 2 grade 4).53 CRS is characterized by the systemic elevation of numerous cytokines like IL-6 and interferon-γ (IFN-γ).54 The clinical manifestations of CRS occur rapidly after receiving CAR-T cell therapies, and intensive care unit (ICU) management plays a necessary role in some cases because of respiratory distress and renal dysfunction. What’s more, some patients will leave evidence of organ damage, even though the cytokines return to normal.55 Patients with larger tumor burden and higher doses of infusion of CAR-T cells, which can induce robust immune activation, are more likely to suffer from CRS.55,56 Taking into account the central role of IL-6 in CRS, anti-IL-6 antibody tocilizumab and dexamethasone are usually used to alleviate inflammation,47,54 although these drugs may offset the antitumor effects of CAR-T cells.57 Apart from CRS, it can be found that complications in CNS, called neurotoxicities, such as seizure, intracerebral edema, and language dysfunction.58,59 In 2022, Majzner et al define “tumor inflammation-associated neurotoxicity” (TIAN) as that unique and specific toxicity profile in patients with primary brain tumors and neurological symptoms associated CAR-T mediated inflammation in CNS tumors. The first classification is related to an increase in intracranial pressure, while the second classification is related to a primary dysfunction of spinal or brain cord structures.51 In the year of 2017, O’ Rourke et al publish a pivotal phase I clinical trial about EGFRvIII targeting CAR-T and reveal that 3 of 10 rGBM experience grade 3 seizure, grade 4 cerebral edema, altered mental status, as well as grade 3 intracerebral hemorrhage.47 In addition, Ahmed et al report the safety and feasibility of HER2-directed CAR-T therapy in 17 patients (who are 7 pediatric patients and 10 adult patients), and the results indicate that only 3 grade 2 CNS complications events caused by CAR-T infusion, including 2 grade 2 seizures and 1 grade 2 headache.50 In summary, the treatment-associated toxicities in GBM cannot be ignored, although it is uncommon. The lower rate of adverse events in GBM may be linked with limited efficacy. Although we are committed to improving the efficacy of CAR-T, there is growing importance on the optimal management of CAR-T cell therapy-related toxicities.55
Antigen Escape
The proposed challenge in CAR-T cell immunotherapy of GBM is choosing tumor antigens widely expressed on the cell surface, and due to safety reasons, they are not highly expressed in the normal brain or some other life-sustaining tissues.60 Based on the above criteria, several antigens, such as EGFR vIII, HER2, IL13Ra2, and EphA2, are the most suitable CAR-T cell targets for GBM.57 According to expression features of antigens, these preferred antigens could be classified into tumor-restricted antigens expressed merely in tumor cells, and tumor-associated antigens highly expressed in tumor cells and sparsely expressed in normal cells. Unfortunately, under the stress of a single antigen that targets CAR-T cell therapies, GBM tumor cells will evolve to partial/complete loss of target antigen expression, leading to therapeutical resistance, which is known as antigen escape. For example, in 2017, the first-in-human clinical trial (NCT02209376) of CART-EGFRvIII (positive in around 30% of the newly diagnosed GBM samples) outcomes in 10 rGBM patients with EGFRvIII-positive is published. Among them, 5 of 7 patients receiving post-CART-EGFRvIII surgical intervention is with antigen decrease.47 In the same way, the utilization of CAR-T cells targeting IL13Ra2 induces the downregulation of IL13Ra2 expression in GBM. Unfortunately, antigen escape is inevitable in current ongoing CAR-T cell clinical trials, and this mechanism contributes to the primary and secondary resistance for CAR-T cell therapies (Figure 1).
CAR-T Cell Trafficking and GBM Infiltration
First, GBM is tumor in the CNS, and peripheral blood administration is the traditional CAR-T delivery method. The blood circulation to the CNS should pass through the highly selective BBB, which consists mainly of monolayers of endothelial cells, ependymal cells and tanycytic cells connected by restrictive tight junctions.61 In addition to the physical blockade, a tumor endothelial barrier is commonly present in the tumor vasculature of solid tumors to inhibit immune cell infiltration.62 In prior research, it is found tumor vasculature can reduce immune cell extravasation and infiltration into tumors by downregulating intracellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1).62 Also, characteristics, such as slow and irregular blood flow in tumor vessels, help to create a hypoxic tumor microenvironment, which affects immune cell function.63 Apart from this, for human and mouse GBM metabolomic and transcriptomic studies, it is found phosphoglycerate dehydrogenase (PHGDH)-mediated endothelial cell (EC) metabolism can promote the formation of a hypoxic and immunodeficient vascular microenvironment driving GBM resistance to CAR-T cell immunotherapy.64 In addition to the obstruction of the BBB, the mismatch between tumor-expressed chemokines and T-cell surface chemokine receptors contributes to the limited infiltration of T cells in solid tumors.65 Chemokines which play an important role in T cell recruitment include CXC ligands (CXCL) 9, 10, 11, 16, CCL5, CCL21, and CCL27,66 and enhanced expression of these chemokines in solid tumors has helped to treat metastatic melanoma, lung cancer, and colorectal cancer.67–69 In 2019, Jin et al significantly enhance CAR-T cell infiltration and persistence in tumors by both increasing IL-8 expression in glioma and constructing CAR-T cells with IL-8 receptors (CXCR1 or CXCR2) in a preclinical model of glioma.70 In summary, there is a “cold tumor” in GBM because of the lack of infiltration of T cells,71 thus leading to CAR-T cells insufficiently encountering tumor cells (Figure 1).
Immunosuppressive Microenvironment
Although regional CAR-T cell therapies are used, the limitation of CAR-T cell trafficking and GBM infiltration still exists (see below). Another tricky barrier is the immunosuppressive tumor microenvironment, which is an obvious difference between hematological tumors and solid tumors. Among GBM, TME is a complicated milieu that includes factors that can regulate tumor proliferation, chemokines, nutrients, and other non-cancerous cell types like fibroblasts and immune cells.72 GBM-intrinsic and local adaptive mechanisms contribute to immunosuppressive TME.73 In numerous research, GBM-infiltrating lymphocytes are defined by both an increased prevalence of PD-1 marker expression and a decreased cytotoxic function. This phenomenon indicated that the GBM-specific microenvironment could convert antitumor T cells into exhaustion status.26,74 Similarly, Shi et al publish that an immunosuppressive GBM environment limits the antitumor ability of CAR-T cells, and it is found in teamwork EGFRvIII-CAR-T cells with PD-1 knockout improved the antitumor activity.75 Taken together, the immunosuppressive microenvironment may result from the oncogenic, genetic, and epigenetic deregulation of GBM cells, various tumor-supportive immune cells, including TAMs and Tregs, and soluble factor inhibitors, including IL-10 and TGF-β to limit CAR-T function (Figure 1).
Strategies to Enhance CAR-T Cell Function in GBM
Development of Novel CAR Antigens
Novel Target Antigens in GBM
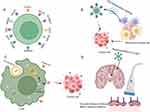
The ideal targets for GBM CAR-T cell therapy are those highly expressed in GBM cells but not within the healthy brain cells. Meanwhile, these targets could significantly induce malignant phenotypes.76,77 Till now, in the ongoing clinical trials, EGFR vIII, HER2, and IL-13 Rα2 are the most popular target antigens in GBM CAR-T cell therapies, while there are still some limitations to GBM treatment. Recently, advanced CAR-T cells with novel targets, such as B7-H3, CLTX, NKG2DLs, and CD133, have been designed (Figure 2A).
CD133 CAR-T Cells
Cancer stem cells (CSCs) play an important role in chemo- and radio-resistance in various malignant cancers, like GBM, because the self-renewing characteristic of CSCs may induce tumoral heterogeneity.78 It is reported that the first-line therapies of GBM including TMZ and radiotherapy could drive CSCs accumulation in GBM TME, hence indicating the potential therapeutic effectiveness of CSCs target therapies.79 CD133 is an important marker of CSCs.80 In pan-cancers, CD133 is a star molecule that can be applied to design CAR-T cells. In cholangiocarcinoma, it is found that the fourth-generation CAR-T cells targeting CD133 received an effective antitumor response, with higher secretion of IFN-γ and TNF-α.81 Additionally, promising antitumor activity and a manageable safety profile are proposed by the single-arm, open-label, Phase II trial of CD133-targeting CAR-T cell treatment of hepatocellular carcinoma.82 Nowadays, multiple CD133-targeting immunotherapies for GBM have received promising clinical outcomes in preclinical models.78 Among GBM, Zhu et al found that CD133-specific CAR-T cells significantly kill patient-derived GBM stem cells.83 Besides, Vora et al developed an advanced structure of CAR-T cells targeting CD133 (which is CART133). Through replacing the prior scFv domain with the anti-CD133 immunoglobulin G (RW03-IgG) scFv binding to human CD133, the robust and antigen-specific CD133 CAR-T cells respond upon contact with CD133+ tumor cells.78 Moreover, a pilot clinical research that can be carried out to evaluate the safety and efficacy of CD133 CAR-T cells is in the recruitment phase for patients with recurrent malignant gliomas (NCT03423992) at present.
B7-H3 CAR-T Cells
As an immune checkpoint member belonging to the B7-CD28 families, B7-H3 contributes much to inhibiting the T-cell function.84 There is a wide expression of B7-H3 in GBM cells.76 These characteristics make B7-H3 an attractive target to design CAR-T cells.85 Considering the in vitro and preclinical mice models, B7-H3 positive tumor cells can be significantly eliminated by B7-H3-specific CAR-T cells.85 In addition, in the diffuse intrinsic pontine glioma, it is pointed out by the primary first-in-human bioactivity and safety study that intracranial delivery may induce local immune activation and tumor repression, and that there is feasibility in the repeated intracranial B7-H3-specific CAR-T cell dosing.86 Importantly, B7-H3 is also an attractive target to design CAR-NK because of its homogenous expression on GBM. Moreover, the cytolytic activity of B7-H3 CAR-NK cells against B7-H3+ target cells is reported to show a significant increase while co-transducing B7-H3 CAR-NK cells with TGF-β dominant negative receptor can preserve cytolytic function against GBM in the presence of the exogenous TGF-β.87 Recently, multiple clinical trials (NCT04077866, NCT04185038, NCT04385173) are performed to assess the B7-H3-specific CAR-T cell therapy for malignant gliomas.
CLTX CAR-T Cells
Chlorotoxin (CLTX), which is a 36-amino acid peptide isolated from deathstalker scorpion Leiurus quinquestriatus venom,88 and CLTX GBM-binding potential is first identified through the conjugation with radioisotope 131I.89 Recently, based on the potential of binding to a great proportion of GBM cells, CLTX-CAR-T cell has been designed for achieving wider and more efficient GBM targeting. What’s more, there is no observable off-target effector activity and antigen escape against the normal cell in the CLTX-CAR-T cells, and meanwhile, tumor regression in orthotopic xenograft GBM tumor models is brought about by the treatment with CLTX-CAR T cells.90 Since CLTX is not expressed by GBM cells, CLTX possesses targeting properties toward GBM cells. As a result, it is enough for CLTX-CAR-T cells to avoid GBM antigen escape and on-target off-tumor effects.91 What’s more. CLTX-CAR-T cells recognize and kill GBM tumors independent of the other GBM-associated antigens.90
Natural Killer Group 2 Member D (NKG2D) CAR-T Cells
Increasing evidence demonstrated that NKG2D ligands (NKG2DLs), which are the ligands of NKG2D, are upregulated in GBM tumor cells and stem cells, while NKG2DLs slight expression is shown by just a few normal tissues.92 Nowadays, NKG2D-expressing CARs are designed to regress NKG2DLs-expressing GBM cells, utilizing the ability of ligands to bind to receptors. The published results are amazing, and NKG2D-CAR-T cells generated high levels of cytokines, perforin, and granzyme B and effectively lysed GBM cells and cancer stem cells in vitro. In vivo, CAR-T cells showed no obvious treatment-related toxicity in treated mice and markedly eliminated xenograft tumors.93 In addition, clinical trials to explore the clinical efficiency of NKG2D-based CAR-T cells in relapsed or refractory GBM patients (NCT04717999).
Multi-Antigen-Targeted CAR-T Cells
Up to now, despite ongoing numerous approved CAR-T cell-related clinical trials, there is the existence of lots of obstacles that prevent expanding CAR-T cell immunotherapy in GBM, including tumor heterogeneity and antigen escape. Therefore, novel CAR-T cell modalities are urgently needed for overcoming these problems. Recently, investigators have focused on the exploit of multi-antigen-targeted CAR-T cells. Bispecific CAR (BiCAR), and Tandem CAR (Tan-CAR) are defined as bivalent CAR-T cells co-expressing two CARs targeting various antigens on tumor cells.94 Through the establishment of the Tan-CAR T cells targeting HER2 and IL-13 Rα2, Hegde et al achieved promising results in preclinical models of GBM, which exhibit advanced Tan-CAR T cells offset antigen escape and enhance the antitumor function. Moreover, the authors presented TanCARs engaged both HER2 and IL13Rα2 at the same time through the induction of HER2-IL13Rα2 heterodimers, therefore giving impetus to superadditive T cell activation in case of the concurrent encountering of the two antigens.95 In addition, bispecific T cell engagers (BiTEs) refer to the bispecific antibodies redirecting T cells to target antigen-expressing tumors, and BiTE-secreting T cells are a useful therapy against GBM.96 Nowadays, the research published by Bryan D. Choi combines BiTE and CAR-T cell technologies. A bicistronic construct has been cultivated to induce the expression of a CAR-T cell that is specific for EGFRvIII and CAR-T cells could secret EGFR-targeted BiTEs against EGFR-positive GBM cells, which could make up insufficient tumor-killing function of EGFRvIII-specific CAR-T cell because of antigen escape.97 Although the appliance of bispecific CAR-T cells could enhance T-cell effector functions and offset antigen escape, the clinical impact of bispecific CAR-T cells is limited by the interpatient heterogeneity in surface antigen expression between patients. To solve this problem, a trivalent T-cell product armed with 3 CAR molecules specific for HER2, IL-13 Rα2, and EphA2 encoded by a single universal (U) tricistronic transgene (UCAR-T cells) is produced for the GBM patients.98 They observed that almost 100% of GBM patients harbored aberrant expression of the above antigens and that UCAR-T cells targeting these 3 antigens could improve the survival time of autologous GBM patient-derived xenografts (PDXs).98 Besides, the technology of logic gates CAR-T cells, recognizing both priming and killing CAR antigen, is considered another excellent model of multiple antigens CAR-T cells that can achieve equilibrium between antigens specificity and heterogeneity.99 Nowadays, investigators perfect the specificity and persistent antitumor activity of therapeutic T cells by using the synthetic Notch (synNotch) CAR circuits, which is the synNotch receptor recognizing a specific priming antigen, and next is primed to induce expression of a CAR-directed against killing antigens, such as IL-13Rα2 or CD133, therefore avoiding off-tumor activity and causing improvement of specificity and persistence of T cells directed against GBM.99,100
Taking Advantage of Bystander Immune Cells
Recently, the inability of CAR-T cells to sustain effector function and the activation of bystander immune cells in suppressive GBM TME is the main obstacle to CAR-T cell therapy success within GBM patients. Besides, cytokines, produced by various cell populations, are important immunoregulatory elements of TME contributing to the exhaustion of CAR-T cells.101 In recent years, research in CAR-T cell development, utilizing cytokines to enhance the function of CAR-T cells and bystander immune cells, is underway. Also known as the fourth-generation CAR-T cells, cytokine-secreting CAR-T cells are also a successful strategy.102 Technically, CAR-T cells are also engineered via the nuclear factor of activated T cell (NFAT)-responsive expression cassette for the inducible expression of the transgenic cytokine, like IL-12, which is a pro-inflammatory cytokine.102 Additionally, IL-12 could activate T cells, and recruit bystander immune cells against cancer cells without CAR-T cells targeting antigens. Among GBM, Burkhard Becher et al promoted the combination of CAR-T cells and local delivery of IL-12. In the meantime, IL-12 could support the persistent cytotoxic activity of CAR-T cells and reprogram endogenous T cells and myeloid cells against tumor cells, with only mild systemic effects.103 Besides, to overcome tumor antigen heterogeneity, investigator-engineered T cells secrete dendritic cell (DC) growth factor Fms-like tyrosine kinase 3 ligands (Flt3L) to stimulate the endogenous DCs, and engineered T cells overcome the clinical problem of antigen-negative tumor escape after the adoptive cell therapy104 (Figure 2B).
Multifunctional mRNA-Based CAR-T Cells
Up to now, major methods of genetically modified CAR-T cells to regress GBM have been through the retroviral vectors or the nonviral transposon-transposase systems to stably integrate transgenes that encode CAR.105 Despite reaching expression during the long term, they still possess some limitations and safety concerns, including genomic alterations risk bringing about the malignant transformation of T-cell clones.106 While mRNA-based CAR-T cells possess multiple advantages. Firstly, because of the production efficiency, there can be more easy testing of the iterative changes in the CAR binding site or structure.107 Secondly, mRNA is easier for clinical transformation and is less expensive compared to viral vectors. Last but not the least, the mRNA transient expression may guarantee safety, especially within the brain where significant morbidity and mortality may be caused by the on-tumor and on-target/off-tumor toxicity.108 In addition, the transient nature may be a barrier for mRNA-based CAR-T cells to provide persistent tumor elimination,107 and the repetitive infusion of CAR-T cells may solve this problem. Recently, mRNA-based treatments gradually become attractive means to counter various intractable diseases, such as SARS-CoV-2109,110 and malignant tumor.111,112 In numerous research, a therapeutic approach that can produce transient antifibrotic chimeric antigen receptor (CAR) T cells in vivo is developed through the delivery of the modified messenger RNA (mRNA) in T cell–targeted lipid nanoparticles (LNPs), which is called mRNA-based CAR-T cells.113,114 While mRNA-based CAR-T cells are characterized by rapid, safe, transient, and cost-effective T-cell modification. Meister et al created the multifunctional mRNA-based CAR-T cells which coexpressed NKG2D, IL12, and IFNα2. Importantly, the mRNA-based CAR-T cells decreased T-cell exhaustion, exhibited promising antitumor function, and induced a proinflammatory tumor microenvironment.115 In addition, mRNA GPC2-redirected CAR-T cells could elevate survival in pediatric brain tumor xenograft models and enhance tumor regression, with no clinical toxicity evidence.116 To sum up, dependent on mRNA transfection technologies, investigators could create CAR-T cells in vivo with efficient antitumor function and may be the potential to alter the GBM treatment landscape (Figure 2C).
Regional Delivery of CAR-T Cells
Since GBM is a CNS tumor, the intravenous administration approach may have poor utility due to the presence of BBB, which is one of the reasons for the inefficiency of CAR-T therapy for GBM. Recent research about CLTX CAR-T cells treating GBM indicated tumor regression is enhanced by the regional delivery of CAR-T cells, while mice treated intravenously did not receive comparable tumor elimination.90 Besides, current CAR-T cell-related clinical trials gradually play an important role in regional delivery (intraventricular and/or intracavitary delivery).94 Meanwhile, a comprehensive review summarizes there is both feasible and safe regional delivery of CAR-T cells in patients with solid tumors, which could generate potent and long-lasting antitumor immunity.117 Similarly, in the preclinical studies targeting CNS tumors, various regional delivery approaches, including the ommaya device and catheter/reservoir system, show transient antitumor responses, with well-tolerated adverse events.118 Furthermore, the regional delivery devices enable the repetitive delivery of CAR-T cells to the lateral ventricle and/or tumor cavity and induce the spread of CAR-T cells to CSF circulation, therefore causing systemic immunity. Meanwhile, Brown et al have demonstrated that the peripheral blood in a patient with GBM treated with intraventricular regional therapy showed an absence of cytokines, escaping the cytokine-mediated organ damage. Additionally, using low-intensity pulsed ultrasound (LIPU), which is considered a safe therapeutic method, could temporarily open BBB, and then enhance CAR-T cells delivery to the tumor and surrounding brain parenchyma to treat GBM.18 In the EGFRvIII-U87 gliomas NSG mice model, CAR-T cell delivery with LIPU-induced BBB disruption led to a remarked increase in CAR-T cell delivery to the CNS and an increase in median survival by greater than 129%, in comparison with the CAR-T cells alone.18 Apart from the immune cell delivery, focused ultrasound could also influence immune cell activation and tumor antigens release119 (Figure 2D).
The Combination of CAR-T Cell Therapy with Other Therapies
To enhance the therapeutic effect of CAR-T cells and overcome the limitations of CAR-T cell therapy in GBM, modification of CAR-T cells is one idea, and another idea is to combine existing therapies with CAR-T cell therapy to achieve a synergistic treatment. Currently, there are certain combination therapies with CAR-T cells, which have achieved superior results in comparison with a single therapy, and there are also some trials that have demonstrated the promise of combination therapy in GBM (Table 2).
 |
Table 2 Summary of CAR-T Cell Combination Therapy Clinical Trials with Other Cancer Treatments in GBM |
Combination with Chemotherapy
Although temozolomide (TMZ) is seen to be a commonly used drug in treating glioblastoma, a combination of TMZ and immunotherapy is not promising. This is because TMZ treatment may induce hypermutation in glioblastoma, and hypermutation can adversely affect immunotherapy for GBM by creating resistance and increasing antigen escape.57,120 However, the inhibitory effect of temozolomide on immunotherapy may be neutralized by the positive therapeutic effect of temozolomide itself, and in preclinical experiments, temozolomide-induced lymphodepletion increased pro-inflammatory factor expression and antigen-specific T cell proliferation in mice, improving the effectiveness of adoptive treatment of brain tumors.121 Therefore, clinical trials comparing the effects of temozolomide alone and a combination of CAR-T cell therapy with temozolomide still exist (NCT04077866). Despite there being no published results yet, there are positive clinical implications. In patients with malignant lymphocytic leukemia, extensive use of lymphodepleting chemotherapy regimens (cyclophosphamide alone, fludarabine, and cyclophosphamide) before CAR-T cell therapy suppresses the patient’s autologous Tregs cell concentration, increases pro-inflammatory factors, and promotes proliferation and stabilization of CAR-T cells.122–124 As for lung tumors and neuroblastoma, good clinical efficacy has also been shown by lymphodepleting chemotherapy before CAR-T cell therapy.125,126 During treating glioblastoma, there are currently two clinical studies with published results via the combined therapy of CAR-T cells and lymphodepleting chemotherapy. In the clinical phase I trial of Goff et al, an effective antitumor effect has not been shown by the lymphodepleting chemotherapy and intravenous IL-2 adjuvant anti-EGFRvIII-CAR-T cells therapy.48 Through lymphodepletion chemotherapy combined with EphA2-redirected CAR-T cells, Lin et al explored the expansion of CAR-T cells in peripheral blood and cerebrospinal fluid for over 4 weeks.126 Although the experiment is still ongoing, this observation suggests a good clinical effect (Figure 3A).
Combination with Radiotherapy
By mutually reinforcing each other’s approach, radiation therapy, and CAR-T cell therapy may act synergistically in combination therapy. In mouse models, CART133 cells showed good efficacy in terms of targeting CD133+ CSCs in patient-derived GBM xenograft models, which are the chemo- and radiation resistance markers of glioblastoma.78 This implies that applying CART133 cells could optimize the clinical efficacy of standard GBM treatments. In addition, since CAR-T cells can mediate apoptosis of target cells through the Fas/FasL or TRAIL axis engagement,127 and DeSelm et al found that low-dose radiotherapy can sensitize antigen-negative tumor cells to TRAIL-mediated apoptosis, CAR-T cells activated by antigen-positive tumor cells expressing TRAIL can resist tumor heterogeneity through killing antigen-negative tumor cells (Figure 3B).128 In the treatment of brain tumors, it is found radiotherapy increases vascular permeability at the tumor site.129 In a preclinical study by Murty et al, it is found that radiotherapy allows rapid extravasation of CAR-T cells from the vascular system and expansion in the tumor microenvironment, which can cause a more durable and robust immune response.130 This feature of radiotherapy may imply its good adjuvant effect on CAR-T cell delivery. Weiss et al found that in preclinical models of glioma, by promoting CAR-T cell migration to the tumor site and enhancing effector function, subtherapeutic doses of local radiotherapy combined with NKG2D-CAR-T cells’ treatment generated synergistic activity in mouse glioma models.131 In addition, Sampson et al found that autologous lymphodepletion with total body irradiation before CAR-T cell treatment upregulated CAR-T cells’ pro-inflammatory cytokine levels and increased the CAR-T cell proliferation and survival.132,133 Overall, there is a good synergy between radiation therapy and CAR-T cell therapy, which can also be found in the former clinical models. In application, there should be more precise limits for the dose and site of radiation therapy. Generally, it is believed that low doses of 2 Gy of radiation favor the formation of an immunogenic tumor microenvironment,133 while high doses of 5–10 Gy may increase the hypoxia area and limit infiltration of T cells to the tumor, harming immunotherapy.134 Meanwhile, hypofractionated radiation therapy also favors the maintenance of the suppressive immune microenvironment.133,135 Thus, more exploration of radiation therapy dose and modality in combining radiotherapy and CAR-T cell therapy is worth to be carried out.
Combination with Other Immunotherapies
As previously mentioned, fourth-generation CAR-T cells secreting cytokines prolong the maintenance of CAR-T cells’ effector effects and overcome limitations of the suppressive immune microenvironment.16 Apart from modifying CAR-T cells themselves, there is a similar adjuvant effect in combination therapy with local delivery of cytokines. IL-15 is an immunostimulatory factor that targets tumor cells. Lanitis et al established a CAR-T cell model co-expressing IL-15 and found that IL-15 can promote CAR-T cells infiltration in tumors, enable enhanced CAR-T cell effector function, and promote NK cell activation and reduction of M2 macrophages in the tumor microenvironment.136 Besides, IL-15 can prevent T-cell exhaustion and reduced PD-1 expression on the cell surface.137,138 In addition, Alizadeh et al found the co-culture of IL-15 and CAR-T cells targeting human glioma cell lines could maintain a less-differentiated stem cell memory phenotype through mediating reduced mTORC1 activity, hence showing less differentiation, less depletion, and increased anti-apoptotic properties, and enhanced proliferation after antigen stimulation.139 The investigators optimized the dosing regimen in a preclinical trial of combined cytokine and CAR-T cell therapy. Tang et al found that protein nanogels (NGs) loaded with IL-15 superagonist (IL-15Sa) equipped with CAR-T cells proliferated with higher tumor specificity and better therapeutic efficacy compared to systemic administration of cytokines, improving survival of glioblastoma mouse model.140 Through a single intratumoral injection (300 ng) of IL-12 combining EGFRvIII-targeted CAR-T cells to treat a mouse glioma model, Agliardi et al found that IL-12 remodels TME with little systemic cytokine toxicity and enhances CAR-T cells cytotoxicity.103 Currently, there are two clinical trials in GBM documenting a combination of IL-2(Aldesleukin) and CAR-T cell therapy, and the intratumoral (NCT01082926) and intravenous drug delivery (NCT01454596) methods are used for each, in which the former is reported to be well tolerated for Intracranial administration of CAR-T cells (GRm13Z40-2) with aldesleukin.141 Due to the suppressive immune microenvironment of GBM, it is theoretically thought that the synergistic use of immune checkpoint inhibitors plays a synergistic role in the CAR-T cell therapy breakthrough in GBM.142 Traditionally, PD-1 is a marker of T-cell exhaustion, and PD-1 inhibition serves as a therapeutic tool to increase T-cell toxicity and keep the T-cell activation state.143 In GBM models, it is thought that PD-L1/PD-1 axis blockade can improve T-cell function by reducing Treg expansion.144 In the treatment of metastatic melanoma, PD-1-targeted combination therapy could have a synergistic effect on the antitumor effects of CAR constructs.145 On this basis, although published results are not yet available, there are clinical trials of CAR-T cell and immune checkpoint inhibitors combination therapies to treat GBM (NCT03726515, NCT04003649). However, there are positive voices for anti-PD-1/PD-L1 in GBM. Gargett et al analyzed samples from a recurrent GBM clinical trial (NCT02209376). The results showed that PD-1 expression in CAR-T cells may predict a better prognosis for GBM patients after receiving CAR-T cell therapy through a positive regulation of AUC (concentration in peripheral blood) of CAR-T cells in peripheral blood and PFS of patients.143 This result may be related to the complex mechanism of the PD1-PDL1 pathway in gliomas.143 In previous experiments, it has been confirmed that PD-1+ T cells have higher expression of activation and depletion markers within gliomas and that compared to its PD1 counterpart, there is higher T cell receptor diversity and IFN-γ production in this population.143,146 Therefore, further clinical controlled trials are still needed to validate the combination of immune checkpoint inhibitors and CAR-T cell therapy. Apart from this, combination therapy of immunomodulatory agents and CAR-T cells has been studied. Lenalidomide (Thalidomide analog) inhibits the inhibitory effects of CTLA4-Ig on T-cell proliferation and cytokine production, increases the number and activity of T cells and natural killer cells, and directly enhances antibody-dependent cell-mediated cytotoxicity (ADCC) by using the increase of the secretion of IL-2 and INF-γ.137,138 In 2015, Kuramitsu et al demonstrated that lenalidomide induced the proliferation of EGFRvIII-specific CAR-T cells target in a mouse glioma model and enhanced immune synapses between effector and target cells.147 In 2020, it was found by Wang et al that lenalidomide enhanced CD133-specific CAR-T cells cytotoxicity in glioma cell lines, possibly regulating CAR-T cell function by inducing degradation of transcription factors Ikaros and Aiolos.148 Therefore, the use of modest immunomodulatory elements could be a new direction in subsequent clinical trials of CAR-T cell combination therapy (Figure 3C).
Combination with Vaccines
The idea of cancer vaccine therapy, especially therapeutic vaccines, mainly causes T-cell immune response.149 When used with CAR-T cell therapy, its main role is promoting the proliferation and killing efficacy of CAR-T cells. Regarding treating GBM, it can be found that the related studies are still at the stage of preclinical trials. In the case of peptide vaccines, Ma et al designed an amphipathic CAR-T cell ligand (amph-ligand) as a vaccine, which is transported to lymph nodes after injection and modified antigen-presenting cell surface. Thus, this can initiate CAR-T cells in the natural lymph node microenvironment and trigger massive CAR-T cells expansion, which can demonstrate stronger antitumor efficacy compared to the single CAR-T cell treatment in an EGFRvIII mouse glioma model150 (Figure 3C). Additionally, in cellular vaccines, using radiation-induced immunogenic cell death of glioma stem cells as vaccines, Sun et al confirmed that enhanced recognition of tumor stem cells by CAR-T cells promotes proliferation and antitumor effects of CAR-T cells.151 Apart from this, in the immunotherapy approach for glioblastoma proposed by Altinoz et al, the authors proposed to make the GBM patients being injected with lysates of their tumor cells and lysates of the GBM cell line U251 before the CAR-T cell treatment. This allows transgenic T cells to show greater potency with and through intercellular interactions with native immunocytes.152 The therapeutic vaccines are mainly injected in peripheral blood, whereas as mentioned earlier, CAR-T cell therapy is administered locally in glioblastoma. In combining tumor vaccines and CAR-T cell therapy, attention should also be paid to the exploration of the dosing modality.
Combination with Oncolytic Adenovirus
Oncolytic adenoviruses (oAD) refer to viruses that can specifically infect tumor cells, directly lyse them, and induce endogenous antitumor immune responses. Because of their specificity, they can also be used as a platform for targeted tumor delivery of drugs.153 They can be genetically engineered to express pro-inflammatory cytokines,154 small molecule immune checkpoint inhibitory antibodies,155 BiTEs,156 and some other structures to act in concert with CAR-T cell therapy (Figure 3C). Through the establishment of an interleukin-7-loaded oncolytic adenovirus (oAD-IL7) and B7H3-targeted CAR-T cell, Huang et al pointed out that for in vitro and in vivo experiments in glioblastoma, oAD-IL7 inhibits CAR-T cell apoptosis and enhances CAR-T cell proliferation, with better-combined efficacy.157 Recently, Evgin et al demonstrated the therapeutic efficacy of CAR-T cells is significantly optimized in a glioma mouse model by the advance loading of CAR-T cells with reovirus and restimulating them with reovirus 8 days after treatment.158 There should also be clinical trials on combining oncolytic adenovirus and CAR-T cells, and further exploration of the mode of administration in mouse models is worthwhile.
The Overview of Promising CAR-T Cells Therapies Strategies
Although most of the CAR-T studies in glioma are currently at the stage of preclinical studies or starting clinical studies but not publishing results, there are still some promising trials in CAR-T cell therapy where results have been reported. For systemic administration, in the phase I clinical trial of Ahmed et al50 on CAR-T cells targeting HER2, authors utilize autologous modified virus-specific T (VST) cells specific for cytomegalovirus, Epstein–Barr virus, or adenovirus to enhance the CAR-T persistence. This clinical trial involves 17 patients with progressive GBM. Patients receive 1 or more infusion of autologous HER2-CAR VST (1×106/m2 to 1×108/m2) without prior lymphodepletion, and CAR-T cells can be detected for 12 months in the peripheral blood, which is longer than another research.48,159 The median OS is 11.1 months from the first T-cell infusion and 24.5 months from diagnosis, indicating the clinical beneficial. Moreover, Ahmed’s team successfully constructed bispecific CAR-T cells targeting IL13Rα2 and HER2,95 and this multi-targeted CAR-T cells that can compensate for the defect of antigen escape may be a hope for future applications regarding CAR-T in glioma. The research in preclinical GBM mice models demonstrates that intravenous delivery of CAR-T cells does not significantly prolong survival, while intraventricular infusion with CAR-T is efficacious against GBM,19 indicating the value of regional delivery. In the phase I clinical trial of Brown et al on CAR-T cells targeting IL13Rα2, 3 rGBM patients receive 12 regional deliveries at a maximum dose of 108 CAR-T cells targeting IL13Rα2 via a catheter/reservoir system. Although the sample size is too small, 3 patients with relapsed GBM attain a median overall survival time of 10.9 months (NCT00730613).49 For preclinical studies, recently, addition of Nivolumab, an anti-PD-1 monoclonal antibody, can promote cytotoxicity of GD2 CAR-T in vitro, and in orthotopic NOD/SCID GBM animal model, combination of GD2 CAR-T and Nivolumab can expand the survival time.160
Summary and Discussion
Recently, although there is increasing attention on the appliance of CAR-T cell therapies for GBM, the clinical outcome is still dismal. The obstacle of BBB impedes the infiltration of CAR-T into GBM TME. Besides, extensive heterogeneity of tumor subpopulation can enhance the tumor recurrence under the stress of CAR-T therapies. For the characteristics of tumor TME, after CAR-T infusion, the permeation of suppressive immune cell subpopulations, such as Tregs and TAMs and the secretion of immunosuppressive cytokines, can limit efficacy of CAR-T therapy. These challenges restrain the appliance of CAR-T therapy among GBM patients. Up to date, for GBM, there is a lack of two arms large-scale clinical trials to compare the efficacy of CAR-T therapy. According to the data from complete clinical trials, the systemic and regional delivery of CAR-T cells is safe and feasible, with controlled complication, while antitumor function of single CAR-T therapy is limited. Frontier research is working on designing and combining novel antigens, applying regional CAR-T cell delivery, activating bystander immune cells and TME, developing multifunctional CAR-T cells via mRNA transfection technology, and combing promising methods to overcome the challenge in GBM. Encouragingly, there is preliminary efficiency in preclinical practice in the advanced strategies, and need further demonstration in clinical trials. Although multiple immunotherapies are demonstrated to be safe for GBM samples, the feasible for treatments combination remain unclear. The ongoing clinical trial (NCT04003649) aims to recruit 60 GBM patients to elevate the efficacy and safety of combination of CAR-T cells targeting IL13Rα2 with nivolumab and ipilimumab. Moreover, the individualized treatment may be the future trends in GBM CAR-T therapy. Haruhiko Kishima et al establish a library of monoclonal antibodies against tumor cell lines derived from GBM samples, and they find E61 and A13, two antibodies, which can bind with tumor cell lines from most GBM patients, but not with nonmalignant human brain cells. In the future, more antibodies against multiple GBM samples and novel target antigens will be found via this method.161 Accordingly, combination and multidisciplinary immunogenic regimens, and more precious technology may provide new avenue for CAR-T-related treatments and need to be verified in two-arm large-scale clinical trials.
Funding
This work was funded by Beijing Municipal Natural Science Foundation (7202150) for Yu Wang, and by the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-113), the Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (2019ZLH101) and the Beijing Municipal Natural Science Foundation (19JCZDJC64200[Z]) for Wenbin Ma.
Disclosure
The authors declare that there is no conflict of interest in this work. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
1. Shen SH, Woroniecka K, Barbour AB, Fecci PE, Sanchez-Perez L, Sampson JH. CAR T cells and checkpoint inhibition for the treatment of glioblastoma. Expert Opin Biol Ther. 2020;20(6):579–591. doi:10.1080/14712598.2020.1727436
2. Le N, Do DT, Chiu FY, Yapp E, Yeh HY, Chen CY. XGBoost improves classification of MGMT promoter methylation status in IDH1 wildtype glioblastoma. J Pers Med. 2020;10(3):128.
3. Bagley SJ, Kothari S, Rahman R, et al. Glioblastoma clinical trials: current landscape and opportunities for improvement. Clin Cancer Res. 2022;28(4):594–602. doi:10.1158/1078-0432.CCR-21-2750
4. Fisher JP, Adamson DC. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines. 2021;9(3):324. doi:10.3390/biomedicines9030324
5. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. doi:10.1001/jama.2017.18718
6. Bausart M, Préat V, Malfanti A. Immunotherapy for glioblastoma: the promise of combination strategies. J Exp Clin Cancer Res. 2022;41(1):35. doi:10.1186/s13046-022-02251-2
7. Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151(1):41–53. doi:10.1007/s11060-020-03448-1
8. Huang B, Li X, Li Y, Zhang J, Zong Z, Zhang H. Current immunotherapies for glioblastoma multiforme. Front Immunol. 2020;11:603911. doi:10.3389/fimmu.2020.603911
9. Bikfalvi A, da Costa CA, Avril T, et al. Challenges in glioblastoma research: focus on the tumor microenvironment. Trends Cancer. 2023;9(1):9–27. doi:10.1016/j.trecan.2022.09.005
10. Wang Z, Wang Y, Chang M, et al. Single-cell transcriptomic analyses provide insights into the cellular origins and drivers of brain metastasis from lung adenocarcinoma. Neuro Oncol. 2023;25(7):1262–1274. doi:10.1093/neuonc/noad017
11. Grabowski MM, Sankey EW, Ryan KJ, et al. Immune suppression in gliomas. J Neurooncol. 2021;151(1):3–12. doi:10.1007/s11060-020-03483-y
12. Nixon BG, Kuo F, Ji L, et al. Tumor-associated macrophages expressing the transcription factor IRF8 promote T cell exhaustion in cancer. Immunity. 2022;55(11):2044–2058.e5. doi:10.1016/j.immuni.2022.10.002
13. Lee E, Yong RL, Paddison P, Zhu J. Comparison of glioblastoma (GBM) molecular classification methods. Semin Cancer Biol. 2018;53:201–211. doi:10.1016/j.semcancer.2018.07.006
14. Ma Q, Long W, Xing C, et al. Cancer stem cells and immunosuppressive microenvironment in glioma. Front Immunol. 2018;9:2924. doi:10.3389/fimmu.2018.02924
15. Rodriguez S, Staicu GA, Sevastre AS, et al. Glioblastoma stem cells-useful tools in the battle against cancer. Int J Mol Sci. 2022;23(9):4602. doi:10.3390/ijms23094602
16. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257(1):107–126. doi:10.1111/imr.12131
17. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
18. Sabbagh A, Beccaria K, Ling X, et al. Opening of the blood-brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin Cancer Res. 2021;27(15):4325–4337. doi:10.1158/1078-0432.CCR-20-3760
19. Choi BD, Yu X, Castano AP, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7(1):304. doi:10.1186/s40425-019-0806-7
20. Asmamaw Dejenie T, Tiruneh G/Medhin M, Dessie Terefe G, Tadele Admasu F, Wale Tesega W, Chekol Abebe E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum Vaccin Immunother. 2022;18(6):2114254. doi:10.1080/21645515.2022.2114254
21. Chen L, Chen F, Li J, et al. CAR-T cell therapy for lung cancer: potential and perspective. Thorac Cancer. 2022;13(7):889–899. doi:10.1111/1759-7714.14375
22. Dunn GP, Cloughesy TF, Maus MV, Prins RM, Reardon DA, Sonabend AM. Emerging immunotherapies for malignant glioma: from immunogenomics to cell therapy. Neuro Oncol. 2020;22(10):1425–1438. doi:10.1093/neuonc/noaa154
23. Tudor T, Binder ZA, O’Rourke DM. CAR T Cells. Neurosurg Clin N Am. 2021;32(2):249–263. doi:10.1016/j.nec.2020.12.005
24. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97(5):498–518. doi:10.1038/labinvest.2017.19
25. Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi:10.1016/j.copbio.2016.02.007
26. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res. 2018;24(17):4175–4186. doi:10.1158/1078-0432.CCR-17-1846
27. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–167. doi:10.1038/s41571-019-0297-y
28. Singer LS, Feldman AZ, Buerki RA, Horbinski CM, Lukas RV, Stupp R. The impact of the molecular classification of glioblastoma on the interpretation of therapeutic clinical trial results. Chin Clin Oncol. 2021;10(4):38. doi:10.21037/cco-21-33
29. Śledzińska P, Bebyn M, Szczerba E, et al. Glioma 2021 WHO classification: the superiority of NGS over IHC in routine diagnostics. Mol Diagn Ther. 2022;26(6):699–713. doi:10.1007/s40291-022-00612-3
30. Stichel D, Ebrahimi A, Reuss D, et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136(5):793–803. doi:10.1007/s00401-018-1905-0
31. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi:10.1016/j.ccr.2009.12.020
32. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32(1):42–56.e6. doi:10.1016/j.ccell.2017.06.003
33. Le N, Hung T, Do DT, Lam L, Dang LH, Huynh TT. Radiomics-based machine learning model for efficiently classifying transcriptome subtypes in glioblastoma patients from MRI. Comput Biol Med. 2021;132:104320. doi:10.1016/j.compbiomed.2021.104320
34. Du L, Lee JH, Jiang H, et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. 2020;217(11):e20191115. doi:10.1084/jem.20191115
35. Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19(18):4951–4960. doi:10.1158/1078-0432.CCR-13-0551
36. Hu C, Wang K, Damon C, et al. ATRX loss promotes immunosuppressive mechanisms in IDH1 mutant glioma. Neuro Oncol. 2022;24(6):888–900. doi:10.1093/neuonc/noab292
37. Choi SI, Yin J. Prospective approaches to enhancing CAR T cell therapy for glioblastoma. Front Immunol. 2022;13:1008751. doi:10.3389/fimmu.2022.1008751
38. Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37(12):1425–1434. doi:10.1038/s41587-019-0137-8
39. Chailyan A, Marcatili P, Tramontano A. The association of heavy and light chain variable domains in antibodies: implications for antigen specificity. FEBS J. 2011;278(16):2858–2866. doi:10.1111/j.1742-4658.2011.08207.x
40. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi:10.1056/NEJMoa1610497
41. Lee L, Draper B, Chaplin N, et al. An April-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma. Blood. 2018;131(7):746–758. doi:10.1182/blood-2017-05-781351
42. Alabanza L, Pegues M, Geldres C, et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther. 2017;25(11):2452–2465. doi:10.1016/j.ymthe.2017.07.013
43. James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180(10):7028–7038. doi:10.4049/jimmunol.180.10.7028
44. Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184(12):6938–6949. doi:10.4049/jimmunol.0901766
45. Guedan S, Posey AD Jr, Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3(1). doi:10.1172/jci.insight.96976
46. Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi:10.1038/nbt0102-70
47. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). doi:10.1126/scitranslmed.aaa0984
48. Goff SL, Morgan RA, Yang JC, et al. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother. 2019;42(4):126–135. doi:10.1097/CJI.0000000000000260
49. Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. doi:10.1158/1078-0432.CCR-15-0428
50. Ahmed N, Brawley V, Hegde M, et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a Phase 1 dose-escalation trial. JAMA Oncol. 2017;3(8):1094–1101. doi:10.1001/jamaoncol.2017.0184
51. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941. doi:10.1038/s41586-022-04489-4
52. Lin Q, Ba T, Ho J, et al. First-in-human trial of EphA2-redirected CAR T-cells in patients with recurrent glioblastoma: a preliminary report of three cases at the starting dose. Front Oncol. 2021;11:694941. doi:10.3389/fonc.2021.694941
53. Jang JK, Pyo J, Suh CH, Park HS, Chae YK, Kim KW. Safety and efficacy of chimeric antigen receptor T-cell therapy for glioblastoma: a systemic review and meta-analysis. Front Oncol. 2022;12:851877. doi:10.3389/fonc.2022.851877
54. Feldman L, Brown C, Badie B. Chimeric Antigen Receptor (CAR) T cell therapy for glioblastoma. Neuromolecular Med. 2022;24(1):35–40. doi:10.1007/s12017-021-08689-5
55. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729
56. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi:10.1038/mt.2010.24
57. Karschnia P, Teske N, Thon N, et al. Chimeric antigen receptor T cells for glioblastoma: current concepts, challenges, and future perspectives. Neurology. 2021;97(5):218–230. doi:10.1212/WNL.0000000000012193
58. Brammer JE, Braunstein Z, Katapadi A, et al. Early toxicity and clinical outcomes after chimeric antigen receptor T-cell (CAR-T) therapy for lymphoma. J Immunother Cancer. 2021;9(8):e002303. doi:10.1136/jitc-2020-002303
59. Rubin DB, Danish HH, Ali AB, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142(5):1334–1348. doi:10.1093/brain/awz053
60. Brown CE, Aguilar B, Starr R, et al. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26(1):31–44. doi:10.1016/j.ymthe.2017.10.002
61. Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N, Barker EL. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70(3):412–445. doi:10.1124/pr.117.014944
62. Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. 2015;33:55–63. doi:10.1016/j.coi.2015.01.011
63. Palazón A, Aragonés J, Morales-Kastresana A, de Landázuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18(5):1207–1213. doi:10.1158/1078-0432.CCR-11-1591
64. Zhang D, Li AM, Hu G, et al. PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy. Cell Metab. 2023;35(3):517–534.e8. doi:10.1016/j.cmet.2023.01.010
65. Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Invest. 2017;97(6):669–697. doi:10.1038/labinvest.2017.25
66. Wang G, Zhang Z, Zhong K, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134–153. doi:10.1016/j.ymthe.2022.08.021
67. Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–3085. doi:10.1158/0008-5472.CAN-08-2281
68. Andersson A, Yang SC, Huang M, et al. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol. 2009;182(11):6951–6958. doi:10.4049/jimmunol.0803340
69. Mlecnik B, Tosolini M, Charoentong P, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138(4):1429–1440. doi:10.1053/j.gastro.2009.10.057
70. Jin L, Tao H, Karachi A, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10(1):4016. doi:10.1038/s41467-019-11869-4
71. Salmon H, Remark R, Gnjatic S, Merad M. Host tissue determinants of tumour immunity. Nat Rev Cancer. 2019;19(4):215–227. doi:10.1038/s41568-019-0125-9
72. Zhao B, Xia Y, Yang F, et al. Molecular landscape of IDH-mutant astrocytoma and oligodendroglioma grade 2 indicate tumor purity as an underlying genomic factor. Mol Med. 2022;28(1):34. doi:10.1186/s10020-022-00454-z
73. Kato D, Yaguchi T, Iwata T, et al. Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):68–77. doi:10.2177/jsci.40.68
74. Mohme M, Schliffke S, Maire CL, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res. 2018;24(17):4187–4200. doi:10.1158/1078-0432.CCR-17-2617
75. Zhu H, You Y, Shen Z, Shi L. EGFRvIII-CAR-T cells with PD-1 knockout have improved anti-glioma activity. Pathol Oncol Res. 2020;26(4):2135–2141. doi:10.1007/s12253-019-00759-1
76. Wang Z, Wang Z, Zhang C, et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109(9):2697–2705. doi:10.1111/cas.13744
77. An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–1575. doi:10.1038/s41388-017-0045-7
78. Vora P, Venugopal C, Salim SK, et al. The rational development of CD133-targeting immunotherapies for glioblastoma. Cell Stem Cell. 2020;26(6):832–844.e6. doi:10.1016/j.stem.2020.04.008
79. Yang YN, Zhang XH, Wang YM, Zhang X, Gu Z. miR-204 reverses temozolomide resistance and inhibits cancer initiating cells phenotypes by degrading FAP-α in glioblastoma. Oncol Lett. 2018;15(5):7563–7570. doi:10.3892/ol.2018.8301
80. Liu K, Jiang L, Shi Y, et al. Hypoxia-induced GLT8D1 promotes glioma stem cell maintenance by inhibiting CD133 degradation through N-linked glycosylation. Cell Death Differ. 2022;29(9):1834–1849. doi:10.1038/s41418-022-00969-2
81. Sangsuwannukul T, Supimon K, Sujjitjoon J, et al. Anti-tumour effect of the fourth-generation chimeric antigen receptor T cells targeting CD133 against cholangiocarcinoma cells. Int Immunopharmacol. 2020;89(Pt B):107069. doi:10.1016/j.intimp.2020.107069
82. Dai H, Tong C, Shi D, et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology. 2020;9(1):1846926. doi:10.1080/2162402X.2020.1846926
83. Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget. 2015;6(1):171–184. doi:10.18632/oncotarget.2767
84. Zhang C, Zhang Z, Li F, et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology. 2018;7(11):e1461304. doi:10.1080/2162402X.2018.1461304
85. Tang X, Zhao S, Zhang Y, et al. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol Ther Oncolytics. 2019;14:279–287. doi:10.1016/j.omto.2019.07.002
86. Vitanza NA, Wilson AL, Huang W, et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2022;13(1):114–131.
87. Chaudhry K, Geiger A, Dowlati E, et al. Co-transducing B7H3 CAR-NK cells with the DNR preserves their cytolytic function against GBM in the presence of exogenous TGF-β. Mol Ther Methods Clin Dev. 2022;27:415–430. doi:10.1016/j.omtm.2022.10.010
88. DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol. 1993;264(2 Pt 1):C361–C369. doi:10.1152/ajpcell.1993.264.2.C361
89. Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58(21):4871–4879.
90. Wang D, Starr R, Chang WC, et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med. 2020;12(533). doi:10.1126/scitranslmed.aaw2672
91. Dardevet L, Rani D, Aziz TA, et al. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins. 2015;7(4):1079–1101. doi:10.3390/toxins7041079
92. Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27(45):5944–5958. doi:10.1038/onc.2008.272
93. Yang D, Sun B, Dai H, et al. T cells expressing NKG2D chimeric antigen receptors efficiently eliminate glioblastoma and cancer stem cells. J Immunother Cancer. 2019;7(1):171. doi:10.1186/s40425-019-0642-9
94. Zhang P, Zhang Y, Ji N. Challenges in the treatment of glioblastoma by chimeric antigen receptor T-cell immunotherapy and possible solutions. Front Immunol. 2022;13:927132. doi:10.3389/fimmu.2022.927132
95. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. doi:10.1172/JCI83416
96. Yin Y, Rodriguez JL, Li N, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022;30(7):2537–2553. doi:10.1016/j.ymthe.2022.05.011
97. Choi BD, Yu X, Castano AP, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–1058. doi:10.1038/s41587-019-0192-1
98. Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–518. doi:10.1093/neuonc/nox182
99. Choe JH, Watchmaker PB, Simic MS, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. 2021;13(591). doi:10.1126/scitranslmed.abe7378
100. Sabahi M, Jabbari P, Alizadeh Haghighi M, et al. Proposing a tandem AND-gate CAR T cell targeting glioblastoma multiforme. Med Hypotheses. 2020;137:109559. doi:10.1016/j.mehy.2020.109559
101. Johnson A, Townsend M, O’Neill K. Tumor microenvironment immunosuppression: a roadblock to CAR T-cell advancement in solid tumors. Cells. 2022;11(22):3626.
102. Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–1154. doi:10.1517/14712598.2015.1046430
103. Agliardi G, Liuzzi AR, Hotblack A, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444. doi:10.1038/s41467-020-20599-x
104. Lai J, Mardiana S, House IG, et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat Immunol. 2020;21(8):914–926. doi:10.1038/s41590-020-0676-7
105. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3–S9. doi:10.1002/ajh.25418
106. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:10.1038/nrclinonc.2017.148
107. Singh N, Barrett DM, Grupp SA. Roadblocks to success for RNA CARs in solid tumors. Oncoimmunology. 2014;3(12):e962974. doi:10.4161/21624011.2014.962974
108. Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi:10.1097/CJI.0b013e3182829903
109. Ong EZ, Yee JX, Ooi J, et al. Immune gene expression analysis indicates the potential of a self-amplifying Covid-19 mRNA vaccine. NPJ Vaccines. 2022;7(1):154. doi:10.1038/s41541-022-00573-y
110. Francis AG, Elhadd K, Camera V, et al. Acute inflammatory diseases of the central nervous system after SARS-CoV-2 vaccination. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200063. doi:10.1212/NXI.0000000000200063
111. Li Y, Fang H, Zhang T, et al. Lipid-mRNA nanoparticles landscape for cancer therapy. Front Bioeng Biotechnol. 2022;10:1053197. doi:10.3389/fbioe.2022.1053197
112. Saha C, Bojdo J, Dunne NJ, Duary RK, Buckley N, McCarthy HO. Nucleic acid vaccination strategies for ovarian cancer. Front Bioeng Biotechnol. 2022;10:953887. doi:10.3389/fbioe.2022.953887
113. Rurik JG, Tombácz I, Yadegari A, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91–96. doi:10.1126/science.abm0594
114. Gao TA, Chen YY. T cells to fix a broken heart. Science. 2022;375(6576):23–24. doi:10.1126/science.abn0851
115. Meister H, Look T, Roth P, et al. Multifunctional mRNA-based CAR T cells display promising antitumor activity against glioblastoma. Clin Cancer Res. 2022;28(21):4747–4756. doi:10.1158/1078-0432.CCR-21-4384
116. Foster JB, Griffin C, Rokita JL, et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J Immunother Cancer. 2022;10(9):e004450. doi:10.1136/jitc-2021-004450
117. Cherkassky L, Hou Z, Amador-Molina A, Adusumilli PS. Regional CAR T cell therapy: an ignition key for systemic immunity in solid tumors. Cancer Cell. 2022;40(6):569–574. doi:10.1016/j.ccell.2022.04.006
118. Vitanza NA, Ronsley R, Choe M, et al. Locoregional CAR T cells for children with CNS tumors: clinical procedure and catheter safety. Neoplasia. 2023;36:100870. doi:10.1016/j.neo.2022.100870
119. Kovacs ZI, Kim S, Jikaria N, et al. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A. 2017;114(1):E75–E84. doi:10.1073/pnas.1614777114
120. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. doi:10.1038/s41586-020-2209-9
121. Sanchez-Perez LA, Choi BD, Archer GE, et al. Myeloablative temozolomide enhances CD8⁺ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One. 2013;8(3):e59082. doi:10.1371/journal.pone.0059082
122. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi:10.1038/nri3191
123. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi:10.1126/scitranslmed.aac5415
124. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi:10.1182/blood-2017-02-769208
125. Davies DM, Maher J. Crosstown traffic: lymphodepleting chemotherapy drives CAR T cells. Cancer Cell. 2021;39(2):138–140. doi:10.1016/j.ccell.2020.12.019
126. Straathof K, Flutter B, Wallace R, et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med. 2020;12(571). doi:10.1126/scitranslmed.abd6169
127. Hong LK, Chen Y, Smith CC, et al. CD30-redirected chimeric antigen receptor T cells target CD30(+) and CD30(-) embryonal carcinoma via antigen-dependent and fas/fasl interactions. Cancer Immunol Res. 2018;6(10):1274–1287. doi:10.1158/2326-6066.CIR-18-0065
128. DeSelm C, Palomba ML, Yahalom J, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542–2552. doi:10.1016/j.ymthe.2018.09.008
129. Thomas P, Galopin N, Bonérandi E, Clémenceau B, Fougeray S, Birklé S. CAR T cell therapy’s potential for pediatric brain tumors. Cancers. 2021;13(21):5445. doi:10.3390/cancers13215445
130. Murty S, Haile ST, Beinat C, et al. Intravital imaging reveals synergistic effect of CAR T-cells and radiation therapy in a preclinical immunocompetent glioblastoma model. Oncoimmunology. 2020;9(1):1757360. doi:10.1080/2162402X.2020.1757360
131. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi:10.1158/0008-5472.CAN-17-1788
132. Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972–984. doi:10.1158/1078-0432.CCR-13-0709
133. Huan T, Li H, Tang B. Radiotherapy plus CAR-T cell therapy to date: a note for cautions optimism. Front Immunol. 2022;13:1033512. doi:10.3389/fimmu.2022.1033512
134. Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor microenvironment as A “Game Changer” in cancer radiotherapy. Int J Mol Sci. 2019;20(13):3212. doi:10.3390/ijms20133212
135. Siegel R, Burock S, Wernecke KD, et al. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer. 2009;9:50. doi:10.1186/1471-2407-9-50
136. Lanitis E, Rota G, Kosti P, et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J Exp Med. 2021;218(2). doi:10.1084/jem.20192203
137. Al-Haideri M, Tondok SB, Safa SH, et al. CAR-T cell combination therapy: the next revolution in cancer treatment. Cancer Cell Int. 2022;22(1):365. doi:10.1186/s12935-022-02778-6
138. Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. doi:10.1038/leu.2010.75
139. Alizadeh D, Wong RA, Yang X, et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res. 2019;7(5):759–772. doi:10.1158/2326-6066.CIR-18-0466
140. Tang L, Zheng Y, Melo MB, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol. 2018;36(8):707–716. doi:10.1038/nbt.4181
141. Brown CE, Rodriguez A, Palmer J, et al. Off-The-shelf, steroid-resistant, IL13Rα2-specific CAR T cells for treatment of glioblastoma. Neuro Oncol. 2022;24(8):1318–1330. doi:10.1093/neuonc/noac024
142. Yoon DH, Osborn MJ, Tolar J, Kim CJ. Incorporation of immune checkpoint blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci. 2018;19(2). doi:10.3390/ijms19020340
143. Tang OY, Tian L, Yoder T, et al. PD1 expression in EGFRvIII-directed CAR T cell infusion product for glioblastoma is associated with clinical response. Front Immunol. 2022;13:872756. doi:10.3389/fimmu.2022.872756
144. DiDomenico J, Lamano JB, Oyon D, et al. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology. 2018;7(7):e1448329. doi:10.1080/2162402X.2018.1448329
145. Gargett T, Yu W, Dotti G, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24(6):1135–1149. doi:10.1038/mt.2016.63
146. Davidson TB, Lee A, Hsu M, et al. Expression of PD-1 by T cells in malignant glioma patients reflects exhaustion and activation. Clin Cancer Res. 2019;25(6):1913–1922. doi:10.1158/1078-0432.CCR-18-1176
147. Kuramitsu S, Ohno M, Ohka F, et al. Lenalidomide enhances the function of chimeric antigen receptor T cells against the epidermal growth factor receptor variant III by enhancing immune synapses. Cancer Gene Ther. 2015;22(10):487–495. doi:10.1038/cgt.2015.47
148. Wang Z, Zhou G, Risu N, et al. Lenalidomide enhances CAR-T cell activity against solid tumor cells. Cell Transplant. 2020;29:963689720920825. doi:10.1177/0963689720920825
149. Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi:10.1038/s41586-019-1593-5
150. Ma L, Dichwalkar T, Chang J, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365(6449):162–168. doi:10.1126/science.aav8692
151. Sun T, Li Y, Yang Y, Liu B, Cao Y, Yang W. Enhanced radiation-induced immunogenic cell death activates chimeric antigen receptor T cells by targeting CD39 against glioblastoma. Cell Death Dis. 2022;13(10):875. doi:10.1038/s41419-022-05319-1
152. Altinoz MA, Ozpinar A, Hacker E, Ozpinar A. Combining locoregional CAR-T cells, autologous + allogeneic tumor lysate vaccination and levamisole in treatment of glioblastoma. Immunopharmacol Immunotoxicol. 2022;44(6):797–808. doi:10.1080/08923973.2022.2086136
153. Huang M, Deng J, Gao L, Zhou J. Innovative strategies to advance CAR T cell therapy for solid tumors. Am J Cancer Res. 2020;10(7):1979–1992.
154. Watanabe K, Luo Y, Da T, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3(7). doi:10.1172/jci.insight.99573
155. Tanoue K, Rosewell Shaw A, Watanabe N, et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. 2017;77(8):2040–2051. doi:10.1158/0008-5472.CAN-16-1577
156. Wing A, Fajardo CA, Posey AD Jr, et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res. 2018;6(5):605–616. doi:10.1158/2326-6066.CIR-17-0314
157. Huang J, Zheng M, Zhang Z, et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother. 2021;70(9):2453–2465. doi:10.1007/s00262-021-02856-0
158. Evgin L, Kottke T, Tonne J, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci Transl Med. 2022;14(640):eabn2231. doi:10.1126/scitranslmed.abn2231
159. Liu Z, Zhou J, Yang X, et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol Cancer. 2023;22(1):3. doi:10.1186/s12943-022-01711-9
160. Zhang G, Zhao Y, Liu Z, et al. GD2 CAR-T cells in combination with Nivolumab exhibit enhanced antitumor efficacy. Transl Oncol. 2023;32:101663. doi:10.1016/j.tranon.2023.101663
161. Nakagawa T, Kijima N, Hasegawa K, et al. Identification of glioblastoma-specific antigens expressed in patient-derived tumor cells as candidate targets for chimeric antigen receptor T cell therapy. Neurooncol Adv. 2023;5(1):vdac177. doi:10.1093/noajnl/vdac177
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.