Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Inpatient Admissions and Re-Admissions in Medicare Beneficiaries Initiating Umeclidinium/Vilanterol or Tiotropium Therapy
Authors Bogart M, Leung GYH, Cyhaniuk A, DiRocco K
Received 23 August 2023
Accepted for publication 10 January 2024
Published 13 February 2024 Volume 2024:19 Pages 439—450
DOI https://doi.org/10.2147/COPD.S436654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Michael Bogart,1 Gary Yat-Hung Leung,2 Anissa Cyhaniuk,2 Kristi DiRocco3
1Customer Engagement, Value, Evidence and Outcomes (CEVEO) US Medical Affairs, GSK, Research Triangle Park, NC, USA; 2STATinMED Research, Dallas, TX, USA; 3US Medical Affairs, GSK, Collegeville, PA, USA
Correspondence: Kristi DiRocco, US Medical Affairs, GSK, 1250 S. Collegeville Road, Collegeville, PA, 19426, USA, Tel +1 610-412-7175, Email [email protected]
Purpose: Patients with chronic obstructive pulmonary disease (COPD) who are hospitalized are more likely to die from their illness and have increased likelihood of re-admission than those who are not. Subsequent re-admissions further increase the burden on healthcare systems. This study compared inpatient admission rates and time-to-first COPD-related inpatient admission among Medicare beneficiaries with COPD indexed on umeclidinium/vilanterol (UMEC/VI) versus tiotropium (TIO).
Patients and Methods: This retrospective study used the All-Payer Claims Database to investigate hospital admission and re-admission outcomes in Medicare beneficiaries with COPD with an initial pharmacy claim for UMEC/VI or TIO from 1 January 2015 to 28 February 2020. Inpatient admissions, baseline, and follow-up variables were assessed in patients indexed on UMEC/VI and TIO after propensity score matching (PSM), with time-to-first on-treatment COPD-related inpatient admission as the primary endpoint. Re-admissions were assessed among patients with a COPD-related inpatient admission in the 30- and 90-days post-discharge.
Results: Post-PSM, 7152 patients indexed on UMEC/VI and 7069 on TIO were eligible for admissions analysis. The mean (standard deviation [SD]) time-to-first COPD-related inpatient admission was 46.71 (87.99) days for patients indexed on UMEC/VI and 44.96 (85.90) days for those on TIO (p=0.06). The mean (SD) number of inpatient admissions per patient was 1.24 (2.92) for patients indexed on UMEC/VI and 1.26 (3.05) for those on TIO (p=0.49). Proportion of patients undergoing re-admissions was similar between treatments over both 30 and 90 days, excluding a significantly lower proportion of patients indexed on UMEC/VI than those indexed on TIO for COPD-related re-admissions for hospital stays of 4– 7 days and 7– 14 days, and all-cause re-admissions for stays of 4– 7 days.
Conclusion: Patients with COPD using Medicare in the US and receiving UMEC/VI or TIO reported similar time-to-first inpatient admission and similar proportion of re-admissions.
Plain Language Summary: Umeclidinium/vilanterol (UMEC/VI) is associated with improvements in patient outcomes for chronic obstructive pulmonary disease (COPD) versus tiotropium (TIO). Patients with COPD who have hospitalizations have higher healthcare costs and are more likely to be re-hospitalized and die from their illness than those who do not. This study compared hospital admission rates and time to admission among Medicare beneficiaries with COPD treated with UMEC/VI versus TIO.
This study used a medical record database to compare hospital admissions and re-admissions, baseline and follow-up variables were compared in patients prescribed with UMEC/VI and TIO from 1 January 2015 to 28 February 2020. Hospital re-admissions were assessed among patients with a COPD-related inpatient admission in the 30 or 90 days after discharge.
Patients had similar COPD-related hospitalizations and number of hospital admissions per patient regardless of medication. Proportion of patients undergoing re-admissions was similar between treatments, apart from a lower proportion of patients prescribed UMEC/VI than TIO for COPD-related re-admissions for hospital stays of 4– 7 and 7– 14 days, and all-cause re-admissions for 4– 7 days.
Despite expectations that patients receiving UMEC/VI would demonstrate increased time-to-first admission and a lower proportion of re-admissions than those receiving TIO, patients reported similar data irrespective of their prescribed medication. Use of head-to-head comparison with claims data and inability to divide patients based on lung function or clinical symptoms may have decreased chances of detecting any significant difference between the treatments, although these results support current recommendations on use of dual therapy.
Keywords: COPD, initial maintenance therapy, umeclidinium/vilanterol dual therapy, tiotropium monotherapy, hospital admissions, hospital re-admissions
Introduction
Chronic obstructive pulmonary disease (COPD) is common both in the United States (US) and globally, with 6.2% of adults in the US having a diagnosis of COPD1 and 212.3 million cases of COPD being reported globally in recent years.2 It is also a leading global cause of death and hospitalization, accounting for 3.3 million deaths in 2019.2 This burden on healthcare is expected to grow in coming years due to increased global exposure to tobacco and noxious particles, aging populations, and lack of awareness and access to diagnosis.3–5
Patients with COPD who are admitted to the hospital have higher healthcare costs and are more likely to die from their illness than those who do not require hospital admission.6 Hospital admission also increases the likelihood of future re-admissions, with almost one-fifth of patients hospitalized following an exacerbation requiring re-admission within 30 days.7 Subsequent re-admissions further increase treatment costs and burden on healthcare systems, being associated with a 20% increase in healthcare costs over the original admission event in the US.7 Exacerbations resulting in hospitalization account for up to 70% of the estimated $40 billion COPD-related medical costs in the US each year.8 Better control of COPD and prevention of exacerbations could therefore help to reduce patient hospital admissions and mortality, and subsequently COPD-related medical costs and healthcare burden.
At the time of this study, for patients receiving maintenance therapy with long-acting muscarinic antagonist (LAMA) or long-acting β2-agonist (LABA) monotherapy, but who still experienced exacerbations, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategic report recommended escalating to LAMA/LABA dual therapy.9 LAMA/LABA dual therapy was also recommended as an initial treatment for patients with at least 2 moderate or 1 severe exacerbation, modified Medical Research Council dyspnea questionnaire (mMRC) score of 2 or less, and who are highly symptomatic (eg, a COPD Assessment Test [CAT] score >10). For all other patients, treatment with a single bronchodilator (LAMA or LABA) or inhaled corticosteroid (ICS)/LABA dual therapy was recommended.9 The current GOLD strategic report (2023) now recommends LAMA/LABA dual therapy as initial maintenance therapy for all patients except those with 0 or 1 moderate exacerbations and a mMRC score of 0–1 or a CAT score of less than 10, where treatment with a single bronchodilator (LAMA or LABA) is recommended.10 The current American Thoracic Society (ATS) guidelines recommend initiating treatment with LAMA/LABA dual therapy for all patients with dyspnea.11
Both tiotropium (TIO), a LAMA monotherapy, and umeclidinium/vilanterol (UMEC/VI), a LAMA/LABA dual therapy, are once-daily, single-dose treatments for COPD administrated as dry powder.12,13 Previous research has found significant improvements in measures of time-to dyspnea, lung function, health outcomes, rescue medication use, and risk of first exacerbation in patients indexed on UMEC/VI versus those indexed on TIO.12,14–16 This study compared inpatient admission rates and time to inpatient admission among Medicare beneficiaries with COPD who initiated treatment with UMEC/VI versus TIO.
Methods
Study Design
This was a retrospective study using an All-Payer Claims Database (APCD), a large database including medical, pharmacy, and dental claims, and eligibility and provider files collected from both private and public payers, which are reported directly by insurers.17 This APCD included Medicare beneficiaries diagnosed with COPD and with an initial pharmacy claim for UMEC/VI or TIO during the identification period, which spanned from 1 January 2015 to 28 February 2020. The index date was the date of the first pharmacy claim for UMEC/VI or TIO during the identification period.
Two analyses were performed, the first focusing on initial patient hospital admissions (admissions analysis), and the second on any subsequent re-admissions following discharge from the initial admission (re-admissions analysis) (Figure 1). For the admissions analysis, inpatient admissions during the on-treatment period (which spanned from the index date to the first stopping point) were assessed (Figure 1A). Stopping points were a pharmacy fill for a non-index medication, discontinuation of index medication (defined as a gap of ≥45 days between the end of a dispensing and the next fill, or between the end of the last dispensing and the end of data), end of continuous enrollment, patient death, or end of patient data availability. For the re-admissions analysis, re-admissions were assessed among patients with at least one COPD-related inpatient admission in the 30- or 90-days post index date (discharge date of first admission). Identification periods were therefore 1 January 2015–29 January 2020 for the 30-day analysis, and 1 January 2015–30 November 2019 for the 90-day analysis (Figure 1B).
 |
Figure 1 Study design for (A) admissions and (B) re-admissions analysis. Abbreviations: COPD, chronic obstructive pulmonary disease; TIO, tiotropium bromide; UMEC, umeclidinium; VI, vilanterol. |
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Informed consent and ethics committee or Institutional Review Board approval were not required for the study as no direct patient contact or primary collection of patient data occurred.
Study Population
To be included in this study patients were required to be ≥65 years of age at the start of the pre-index period, have ≥1 pharmacy claim for fixed-dose UMEC/VI or TIO during the identification period, and have ≥1 medical claim with an International Classification of Diseases (ICD) diagnosis code for COPD (ICD-9-Clinical Modification [CM]: 491.x, 492.x, 496.x, or ICD-10-CM: J41–J44) in any position during the pre-index period (a minimum of 12 months prior to and including the index date). Continuous capture of the patient’s health plan throughout the pre-index period was also required, as well as Medicare insurance both at index and throughout pre- and post-index periods. For additional inclusion in the re-admission analyses, patients were also required to have ≥1 on-treatment inpatient admission (ie, hospitalization) and be alive at discharge. Continuous enrollment with medical and pharmacy coverage of ≥30 days after the index discharge date for the analysis of 30-day re-admissions, or ≥90 days after the index discharge date for the analysis of 90-day re-admissions was also required for inclusion in re-admission analyses.
Patients were excluded from the study if they had ≥1 pharmacy claim for ICS-, LABA-, or LAMA-containing controller therapy during the pre-index period, pharmacy claims for both UMEC/VI and TIO, for non-index controller medications, or fixed-dose triple therapy on the index date, or ≥1 medical claim with a diagnosis for asthma in the pre-index period, including index date. After application of inclusion and exclusion criteria, patients were divided into two cohorts: those indexed on UMEC/VI and those indexed on TIO.
Outcomes
The primary endpoint of the study was time-to-first on-treatment COPD-related inpatient admission. The secondary endpoints were mean number of admissions per patient, proportion of patients undergoing re-admission (COPD-related and all-cause, within 30 days and within 90 days, overall and stratified by stay length), and mean re-admissions per patient (over 30 and 90 days, COPD-related and all-cause).
COPD-related admissions and re-admissions were defined as any hospitalization claim with a primary diagnosis of COPD. Patient characteristics were obtained during the pre-index period (baseline characteristics) and during follow-up. Moderate exacerbations were defined as an outpatient or emergency room visit with a COPD exacerbation diagnosis code in the primary position. At least one dispensing/administration of a systemic corticosteroid or guideline-recommended antibiotic within five days following, or prior to the visit was also required. Severe exacerbations were defined as an inpatient hospitalization with a COPD exacerbation diagnosis code in the primary position.
Statistical Analysis
A power calculation based on the normal approximation to the binomial distribution, with 80% power, type I error alpha of 0.05 and a two-sided test determined the required sample size to obtain a power of 80% to detect a difference in the proportion of patients with a COPD-related admission is 10,198 patients per group (20,396 patients total). Propensity score matching (PSM) was conducted to balance patient characteristics between cohorts at baseline and follow-up. Patients were matched on their demographic and clinical characteristics. For this analysis, a 1:3 matching of patients indexed on UMEC/VI to patients indexed on TIO was used given sample sizes. A Poisson model was used to evaluate rate of inpatient hospitalization, and Kaplan–Meier curves were utilized to assess time to hospitalization and re-admission rates. Characteristics at baseline and follow-up were compared using t-tests for age, exacerbations during baseline, Quan Charlson Comorbidity Index (CCI) score, medication use, and healthcare resource utilization (HCRU), and Chi-squared tests for patient sex, year of index claim, and geographic region.
Results
Study Population and Baseline Characteristics
A total of 61,547 patients were included at baseline: 17,742 (29%) patients indexed on UMEC/VI and 43,805 (71%) patients indexed on TIO (Supplementary Figure 1). During PSM, the patients initially included at baseline were matched into equal groups of 17,729 patients for both cohorts. Of these, 7152 patients indexed on UMEC/VI and 7069 patients indexed on TIO were eligible for admissions analysis following a COPD-related inpatient admission.
Post-PSM, at baseline, patients had similar mean ages regardless of treatment at index (UMEC/VI mean [standard deviation (SD)]: 73.8 [5.5], TIO mean [SD]: 73.8 [5.6]), and a similar proportion of patients indexed on UMEC/VI were female (51.2%) to those indexed on TIO (51.5%) (Table 1). CCI scores were identical between treatments (UMEC/VI mean [SD]: 3.2 [2.6], TIO mean [SD]: 3.2 [2.6]). Patients indexed on UMEC/VI had significantly fewer exacerbations (mean [SD]: 0.9 [1.8] vs 1.0 [1.9], p=0.0145), less methylxanthine use, more short-acting muscarinic antagonist/short-acting β2-agonist use, and more systemic corticosteroid use than patients indexed on TIO at baseline period (Table 1).
 |
Table 1 Baseline Patient Characteristics |
Post-PSM, at baseline, a significantly lower proportion of patients indexed on UMEC/VI had COPD-related inpatient admissions (29.9% vs 32.6%, p<0.0001), outpatient visits (68.9% vs 67.1%, p=0.0005, long-term care charges (25.0% vs 31.5%, p<0.0001) and lab claims (33.2% vs 33.8%, p=0.2513) compared with patients indexed on TIO. Similar results were seen for all-cause HCRU (Table 1).
Time-to-First On-Treatment COPD-Related Inpatient Admission and Mean Number of Admissions per Patient
Post-PSM, the mean (SD) time-to-first COPD-related inpatient admission was 46.71 (87.99) days for patients indexed on UMEC/VI and 44.96 (85.90) days for patients indexed on TIO (p=0.06). Post-PSM, mean (SD) number of inpatient admissions per patient was 1.24 (2.92) for patients indexed on UMEC/VI and 1.26 (3.05) for patients indexed on TIO (p=0.49).
COPD-Related and All-Cause On-Treatment Inpatient Re-Admissions
Of the 17,846 patients (29% of patients included at baseline) with a COPD-related inpatient admission, the 30-day re-admission cohort consisted of 12,954 (73%) patients who had data available for at least 30 days following the discharge date (UMEC/VI: n=3086 [24%], TIO: n=9868 [76%]), and the 90-day re-admission cohort consisted of 12,032 (67%) patients who had data available for at least 90 days following the discharge date (UMEC/VI: n=2854 [24%]; TIO: n=9178 [76%]).
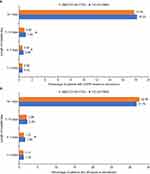
A similar proportion of patients had a COPD-related inpatient re-admission for both treatments, within both 30 (UMEC/VI: 4.25%, TIO: 4.02%) and 90 days (UMEC/VI: 8.40%, TIO: 8.92%). Mean (SD) number of re-admissions per patient were similar between cohorts, within both 30 (UMEC/VI: 0.06 [0.41], TIO: 0.07 [0.49]) and 90 days (UMEC/VI: 0.15 [0.73], TIO: 0.18 [0.86]) (Figure 2). Similar results were shown for all-cause on-treatment inpatient re-admissions (Figure 3).
The proportion of patients indexed on UMEC/VI with COPD-related inpatient re-admissions was significantly lower than the proportion of patients indexed on TIO for hospital stays of 4–7 days (UMEC/VI: 0.50%, TIO: 0.68%, p=0.02) and 7–14 days (UMEC/VI: 0.82%, TIO: 1.04%, p=0.04). The proportion of patients indexed on UMEC/VI with all-cause inpatient re-admissions was also significantly lower than the proportion of patients indexed on TIO for hospital stays of 4–7 days (UMEC/VI: 1.31%, TIO: 1.65%, p=0.01) (Figure 4). COPD-related on-treatment inpatient re-admissions with lengths of 1–3 days and 14+ days and all-cause on-treatment inpatient re-admissions with lengths of 1–3 days, 8–14 days, and 14+ days were not statistically significant between indexed treatments in the 30-day or 90-day re-admission timeframes (Figure 4).
Follow-Up
Regardless of treatment at index, the post-PSM mean (SD) numbers of both moderate (0.5 [1.4]) and severe (0.5 [1.2]) exacerbations at follow-up were the same. Patients indexed on UMEC/VI had significantly less phosphodiesterase-4 inhibitor, systemic corticosteroid, and montelukast use than patients indexed on TIO at follow-up (Supplementary Table 1).
Post-PSM, a significantly lower proportion of patients indexed on UMEC/VI had COPD-related inpatient admissions and long-term care charges compared with patients indexed on TIO (inpatient admissions: 24.8% vs 26.2%, p=0.0024, long-term care charges: 26.5% vs 31.1%, p<0.0001); however, the opposite was true for outpatient visits (58.9% vs 55.9%, p<0.0001). A significantly lower proportion of patients indexed on UMEC/VI had all-cause long-term care charges compared with patients indexed on TIO (39.9% vs 44.7%, p<0.0001), while a significantly higher proportion had all-cause outpatient visits (82.4% vs 79.3%, p<0.0001), lab claims (49.6% vs 47.9%, p=0.0020), and other HCRUs (61.7% vs 60.6%, p=0.0364).
Discussion
This claims-based study in a US population of Medicare recipients found that overall, time-to-first on-treatment COPD-related inpatient admission after treatment initiation was similar between patients receiving UMEC/VI and those receiving TIO. Patients indexed on UMEC/VI also had similar re-admission rates and duration of re-admission episodes than those indexed on TIO.
The similarity between UMEC/VI and TIO when used as an initial maintenance therapy in this study supports the current recommendations of the GOLD strategic report and ATS guidelines regarding the use of LAMA/LABA dual therapy as initial maintenance therapy in many patients with COPD.10,11 Results from this study are in line with a recent meta-analysis which found no significant difference in hazard ratio of time-to-first moderate/severe exacerbation between UMEC/VI and TIO,18 and with a recent retrospective matched cohort study in the US that also found similar time-to-first overall, moderate, and severe exacerbations between UMEC/VI and TIO, as well as similar rates of on-treatment exacerbations.19 In contrast with our findings, a recent matched cohort study found significantly reduced risk of severe exacerbation and numerically reduced risk of overall exacerbation in patients indexed on UMEC/VI compared with those indexed on TIO.20 However, the patient population in that recent study had high costs and comorbidities and did not have Medicaid, which may explain the disparities between the studies.
Several clinical trials have shown significant improvements in dyspnea, lung function, health outcomes, rescue medication use, and risk of first exacerbation in patients indexed on UMEC/VI versus those on TIO.12,14,15 These previous findings would lead us to expect a similar difference in time-to-first inpatient admission, re-admission rates, and duration of re-admission episodes between treatments, which were not present in our findings.
A recent retrospective matched cohort study found significantly reduced risk and rates of COPD-related inpatient admissions, as well as a numerically lower proportion of short inpatient stays (1–3 days) at both 30 and 90 days and a 22-day longer mean time-to-first admission, for UMEC/VI versus TIO.6 While this study was also in a US population, it was not focused specifically on Medicare recipients, which may have contributed to the disparity in findings. The cohorts in this study also had a lower baseline mean rate of exacerbations (0.49–0.5 per year) than those in our study (1.7–2.5 over the 12-month baseline period), which may have also impacted findings.6
Rates of all-cause hospital re-admission after 30 days also differ in comparison to previous research, with one previous study finding overall rates of 19.2% for patients in the US.7 However, a previous analysis of patients in California found similar re-admission rates to our study; 14.77% for patients under 65 years of age, and 11.80% for patients at least 65 years of age, although this study only covered one state and required a specific prescription for COPD management.16 A study specifically investigating US patients also found rates of 11.7% for both indexed on UMEC/VI and those indexed on TIO, a similarity in re-admission rates which are supported by our findings.6
The significantly lower proportion of COPD-related re-admissions for patients with hospital stays of 4–7 and 7–14 days treated with UMEC/VI compared with TIO may indicate that patients indexed on UMEC/VI may be experiencing less severe exacerbations or improved symptom control versus those indexed on TIO. This finding would align with previous research, which found patients had a numerically lower proportion of re-admissions following short inpatient stays for UMEC/VI versus TIO.6 This study had several strengths, including the use of APCD data, which allows for easy comparisons of healthcare costs and utilization between treatments, and provides detailed data on patients’ sociodemographic characteristics.21 The database repository is nationally representative of the US and includes patients from all geographic regions, including over 300 million unique patients. The APCD is also updated daily, providing near-real-time data. Use of PSM and group matching ensured that differences in many baseline variables were accounted for. There were also some limitations of the study design which should be considered. Methodological limitations may have influenced the ability of the study to detect any significant differences in time-to-readmission and re-admission between UMEC/VI and TIO post-PSM. Use of head-to-head comparison with claims data may have resulted in lack of sufficient power to detect treatment differences. This study also used PSM to ensure as close to an appropriate comparison of UMEC/VI and TIO cohorts as possible. The inability to further stratify by lung function or clinical symptoms may also have limited the detectability of any significant differences. As this study focused on Medicare recipients, care must be taken with generalizing findings more broadly across patients with COPD in the US. A previous patient analysis found a significant difference in re-admission rates in patients under 65 years of age depending on payer status, with private insurance having 0.67 times likelihood of re-admission relative to Medicare.16 This higher risk of re-admission could also have contributed to masking the treatment effect in this study. In addition, some clinical endpoints, such as a complete list of comorbidities and smoking history, are not available in the APCD.
Conclusion
UMEC/VI and TIO have similar performance in respect to re-admissions duration, frequency, and time-to-first inpatient admission following treatment initiation in patients with COPD using the Medicare system in the US. Our results support the recommendations of the current GOLD strategic report and ATS guidelines regarding the use of LAMA/LABA dual therapy as initial maintenance therapy in all patients except those with 0 or 1 moderate exacerbations and a mMRC score of 0–1 or a CAT score of less than 10, and all patients with dyspnea, respectively.
Abbreviations
APCD, All-Payer Claims Database; ATS, American Thoracic Society; CAT, COPD Assessment test; CCI, Quan Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HCRU, healthcare resource utilization; ICD-CM, International Classification of Diseases Clinical Modification; ICS, inhaled corticosteroid; IP, inpatient; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnea questionnaire; NAC, N-acetylcysteine; N/A, not applicable; PSM, Propensity Score Matching; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation; TIO, tiotropium bromide; UMEC, umeclidinium; US, United States; VI, vilanterol.
Data Sharing Statement
The data analyzed in this publication are derived from the APCD. Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time.
Ethics Approval and Informed Consent
Approval of this study was provided by the GSK Protocol Review Committee, which reviewed the protocol. No personal subject contact or primary collection of individual human data occurred, and anonymized patient-level data were used in this analysis; patient consent was therefore not required.
Acknowledgments
Editorial support (in the form of writing assistance including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Christopher Heath, PhD, of Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK. Michael Bogart was employed at Customer Engagement, Value, Evidence and Outcomes (CEVEO) US Medical Affairs, GSK, Research Triangle Park, NC, USA, at the time of the study; Gary Yat-Hung Leung and Anissa Cyhaniuk were employed at STATinMED Research, Dallas, TX, USA, at the time of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (GSK study 214310). GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report and GSK funded the article processing charges and open access fee.
Disclosure
MB and KD are employees of GSK and hold stock and shares at GSK or were employees and did hold stock and shares at the time of the study. GL and AC were employees of STATinMED Research at the time of the study, which received funding from GSK to conduct the study. STATinMED Research is a business that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. STATinMED Research employees work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered.
References
1. Wheaton AG, Liu Y, Croft JB, et al. Chronic obstructive pulmonary disease and smoking status—United States, 2017. MMWR. 2019;68(24):533. doi:10.15585/mmwr.mm6824a1
2. Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;2022;378. doi:10.1136/bmj-2021-069679
3. Akwe J, Fair N, Fongeh T. Overview of epidemiology, pathophysiology, diagnosis and staging with 2020 Updates. Med Res Arch. 2020;8(2). doi:10.18103/mra.v8i2.2046
4. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
5. Miravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–868. doi:10.1136/thx.2009.115725
6. Slade D, Ray R, Moretz C, et al. Hospital admission and readmission among US patients receiving umeclidinium/vilanterol or tiotropium as initial maintenance therapy for chronic obstructive pulmonary disease. Pulm Ther. 2021;7(1):203–219. doi:10.1007/s41030-021-00151-y
7. Jacobs DM, Noyes K, Zhao J, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thoracic Soc. 2018;15(7):837–845. doi:10.1513/AnnalsATS.201712-913OC
8. Tkacz J, Evans KA, Touchette DR, et al. PRIMUS–prompt initiation of maintenance therapy in the US: a real-world analysis of clinical and economic outcomes among patients initiating triple therapy following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:329. doi:10.2147/COPD.S347735
9. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD; 2022. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
11. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
12. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi:10.1016/S2213-2600(14)70065-7
13. Donohue JF, Maleki-Yazdi M, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi:10.1016/j.rmed.2013.06.001
14. Kerwin EM, Kalberg CJ, Galkin DV, et al. Umeclidinium/vilanterol as step-up therapy from tiotropium in patients with moderate COPD: a randomized, parallel-group, 12-week study. Int J Chron Obstruct Pulmon Dis. 2017;12:745. doi:10.2147/COPD.S119032
15. Maleki-Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med. 2014;108(12):1752–1760. doi:10.1016/j.rmed.2014.10.002
16. Simmering JE, Polgreen LA, Comellas AP, Cavanaugh JE, Polgreen PM. Identifying patients with COPD at high risk of readmission. Chron Obstr Pulm Dis. 2016;3(4):729. doi:10.15326/jcopdf.3.4.2016.0136
17. Agency for Healthcare Research and Quality. All-payer claims databases; 2018. Available from: https://www.ahrq.gov/data/apcd/index.html.
18. Ismaila AS, Haeussler K, Czira A, et al. Comparative efficacy of umeclidinium/vilanterol versus other bronchodilators for the treatment of chronic obstructive pulmonary disease: a network meta-analysis. Adv Ther. 2022;39(11):4961–5010. doi:10.1007/s12325-022-02234-x
19. Slade D, Ray R, Moretz C, et al. Time-to-first exacerbation, adherence, and medical costs among US patients receiving umeclidinium/vilanterol or tiotropium as initial maintenance therapy for chronic obstructive pulmonary disease: a retrospective cohort study. BMC Pulm Med. 2021;21(1):1–10. doi:10.1186/s12890-021-01612-5
20. Kalhan R, Slade D, Ray R, et al. Umeclidinium/vilanterol compared with fluticasone propionate/salmeterol, budesonide/formoterol, and tiotropium as initial maintenance therapy in patients with COPD who have high costs and comorbidities. Int J Chron Obstruct Pulmon Dis. 2021;2021:1149–1161 doi:10.2147/COPD.S298032.
21. Blewett LA, Mac Arthur NS, Campbell J. The future of state all-payer claims databases. J Health Polit Policy Law. 2023;48(1):93–115. doi:10.1215/03616878-10171104
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.