Back to Journals » International Journal of Nanomedicine » Volume 16
Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management
Authors Allemailem KS
Received 8 September 2021
Accepted for publication 29 November 2021
Published 16 December 2021 Volume 2021:16 Pages 8159—8184
DOI https://doi.org/10.2147/IJN.S338272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Khaled S Allemailem
Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
Correspondence: Khaled S Allemailem
Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
Tel +966 53 633 3777
Email [email protected]
Abstract: Conventional therapies for cancer eradication like surgery, radiotherapy, and chemotherapy, even though most widely used, still suffer from some disappointing outcomes. The limitations of these therapies during cancer recurrence and metastasis demonstrate the need for better alternatives. Some bacteria preferentially colonize and proliferate inside tumor mass; thus these bacteria can be used as ideal candidates to deliver antitumor therapeutic agents. The bacteria like Bacillus spp., Clostridium spp., E. coli, Listeria spp., and Salmonella spp. can be reprogrammed to produce, transport, and deliver anticancer agents, eg, cytotoxic agents, prodrug converting enzymes, immunomodulators, tumor stroma targeting agents, siRNA, and drug-loaded nanoformulations based on clinical requirements. In addition, these bacteria can be genetically modified to express various functional proteins and targeting ligands that can enhance the targeting approach and controlled drug-delivery. Low tumor-targeting and weak penetration power deep inside the tumor mass limits the use of anticancer drug-nanoformulations. By using anticancer drug nanoformulations and other therapeutic payloads in combination with antitumor bacteria, it makes a synergistic effect against cancer by overcoming the individual limitations. The tumor-targeting bacteria can be either used as a monotherapy or in addition with other anticancer therapies like photothermal therapy, photodynamic therapy, and magnetic field therapy to accomplish better clinical outcomes. The toxicity issues on normal tissues is the main concern regarding the use of engineered antitumor bacteria, which requires deeper research. In this article, the mechanism by which bacteria sense tumor microenvironment, role of some anticancer agents, and the recent advancement of engineering bacteria with different therapeutic payloads to combat cancers has been reviewed. In addition, future prospective and some clinical trials are also discussed.
Keywords: anticancer payload, cancer, tumor-targeting bacteria, genetic modifications, nanoparticle, targeted drug-delivery
Introduction
Cancer has been one of the main cause of deaths worldwide and poses a serious challenge and threat to human health. The current clinical therapies used for the treatment of different cancers include surgery, radiotherapy, immunotherapy, hormonal therapy, and chemotherapy. The choice can be monotherapy or combination therapy and depends on several factors like cancer origin, stage, location, and grade.1 Even though these anticancer therapies can be effective, they have certain disadvantages, like: (a) they can cause pharmacological adverse effects at normal tissues; (b) they lack the ability of center-point targeting deep within tumor mass; (c) they mostly acquire drug resistance and are unable to eradicate the entire cancer cell population in the tumor.2 Hence, there is an utmost need to develop some innovative therapeutics that should be simple, cost-effective, and could serve as a substitute to conventional treatments to fight cancer. In this regard, recent advancements in the utilization of tumor-targeting bacteria engineered with different therapeutic payloads have been found to be quite unique and effective strategies of cancer therapy.3
Recently, some microbes, cells, bacteria, and viruses have been found to possess unique characteristics of movement towards tumor microenvironment (TME). Thus, these candidates have been utilized as carriers of antitumor payloads including drug-loaded nanoformulations to target the cancer much more efficiently. These properties are not possessed by conventional antitumor nanoparticles (NPs) alone.
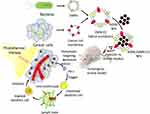
Natural cancer-targeting bacteria have the ability to selectively penetrate, colonize, and degenerate tumors.4 These bacteria can be engineered to perform controlled delivery of specific and diverse therapeutic payloads/drug-loaded nanoformulations into TME at the desired dosage. These therapeutic payloads include cytotoxic proteins, angiogenesis modulators, immunomodulators, prodrug-converting enzymes, small interference RNAs (siRNAs), and drug-loaded nanoformulations, as shown in Figure 1.3,5
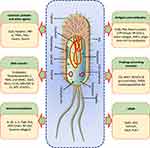 |
Figure 1 Diagrammatic representation of different molecules expressed by engineered-tumor targeting-bacteria, used as therapeutic agents against different cancers. |
The toxicity issues on nearby normal tissue are a main concern for systemic injection of therapeutic agents at the tumor site. These complications have led to improve the center-point target delivery of anticancer drugs and drug nanoformulations to enhance the therapeutic potential and minimize the toxic effects. Rapid advancement in the drug-loaded nanomaterials in the past decade has been a powerful thrust for the innovation of cancer treatment. Some nanomaterials like liposomes, micelles, polymers, metal nanoparticles (NPs), etc., have been widely used as drug-loaded targeted delivery vehicles and play a significant role in cancer treatment. These nanocarriers have been loaded with different antitumor drugs, which include doxorubicin, paclitaxel, cisplatin, tamoxifen, etc.6–8
In comparison to normal tissues, solid tumors are more permeable to therapeutic agents including NPs due to enhanced permeability and retention effect (EPR).9 The EPR-effect is now a well-acknowledged phenomena, validated in different cancer models as well as in cancer patients.10 Cancer tissues with rich blood vessels exhibit a good EPR effect and concomitantly respond to treatments, whereas tumors with reduced blood flow demonstrate poor drug delivery and treatment strategies.11 It has been reported that nitric oxide (NO) is one of the most important factors to enhance the EPR effect through vasodilation, opening of cell junction gaps of endothelial cells, and increasing the blood flow within the hypovascular cancerous mass.
Only a few drug-loaded nanoformulations have shown remarkable success in cancer management, as many challenges still persist in the clinical application of these nanomaterials. The TME is characterized by hypoxia, acidity, immunosuppression, and high interstitial fluid pressure (IFP).12 Therefore, the pinpoint targeted application of nanoformulations at the tumor site is still a challenge which needs to be achieved to effectively eradicate the cancer menace.
Incorporation of specific therapeutic payloads within or on the surface of a particular bacteria as a tool of tumor therapy is now considered as an innovative approach for cancer management. The TME displays a unique environment for an ideal breeding site for some obligate and facultative anaerobic bacteria.13 Bacteria like Bifidobacterium, Clostridium, Escherichia coli (E. coli), and Salmonella typhimurium (S. typhimurium) can preferentially proliferate in immunosuppressive, eutrophic, and hypoxic environments found around tumor tissues. By the use of synthetic biological technology and genetic engineering, these engineered bacteria can achieve center-point targeted delivery of anticancer drugs, specific proteins, antibodies, enzymes, antigens, and cytokines.14
This article reviews the latest developments in engineering some specific tumor-targeting bacteria to enhance further their anticancer potential with immunotherapeutic agents, tumoricidal vectors and enzymes, cytotoxic agents, and drug-loaded NPs. In addition, some bacteria derived therapeutic agents like spores and membrane vesicles to carry different therapeutic payloads to deep sites of diverse tumors are also discussed. Furthermore, the prospects of the future developments and clinical trials for cancer prevention and treatment are also discussed.
Mechanisms by Which Bacteria Can Sense TME
Some bacteria love to accumulate at tumor sites as the TME provides a suitable milieu and such microorganisms can reach this area through flagellar motion.15 The obligate and facultative anaerobic bacteria find a suitable habitat within the TME as it is a nutrient-rich territory.9 S. typhimurium and E. coli, as facultative anaerobes, can sense the nutrient-rich and favorable environment through their chemoreceptors and get accumulated in the periphery as well as the core of tumor region.13 Bacteria preferably colonize in these regions as it displays an immunosuppressive environment, so is not usually cleared by neutrophils and macrophages. In contrast, the immune system quickly clears the bacteria present in the circulatory system and other major organs. In comparison to the normal tissue, the cancerous tissue displays a chaotic vasculature and large capillary spacing that impedes the delivery of therapeutic agents. The powerful motor properties of bacteria help it to pass through the blood vessels to reach the tumor area.
Since, no oxygen is needed to survive for obligate anaerobic bacteria, they preferably migrate towards the hypoxic areas of the tumor. The flagellar motility enables some bacteria to overcome the diffusion resistance as Bifidobacterium and Clostridium have been located at hypoxic areas around the tumor. Due to the poor lymphoid fluid drainage and blood vessel leaking, the tumor tissues possess higher IFP.16 The increased IFP hinders the conventional therapeutic agents to reach the deeper tumor mass, thus impacts its uptake by the cancer cells. The engineered bacteria with therapeutic payloads can bypass this predicament by their flagellar motion to reach deep inside the necrotic core.4
Bacteria as Cancer Treatment Agents and Their Antitumor Features
Some bacteria like Clostridium spp., Listeria, and Salmonella have innate properties of tumor-targeting, which enables them to target, pierce, proliferate, and reduce solid tumors by different mechanisms.3,4 Clostridium genus bacteria like C. butyricum and C. novyi-NT can survive in hypoxic conditions present around the tumor mass.17 These bacteria can destroy the cancer tissue by exotoxins, which damage the cancer cell membranes and enter these cells and disrupt their essential functions.18 These bacteria can also recruit CD8+ T-cells, macrophages, and granulocytes to the cancerous area and neutrophils mediate the release of TNF-related apoptosis-inducing ligand (TRAIL) (Figure 2).19
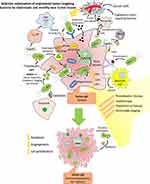 |
Figure 2 Diagrammatic representation of different mechanisms followed by engineered-tumor-targeting-bacteria for cancer therapy. |
Listeria spp. bacteria can target the cancer tissue through tumor-infiltrating myeloid-derived suppressor cells (MDSCs), which wander to the immunosuppressive TME. A unique cell–cell spread mechanism is involved in the transport of Listeria from MDSCs to cancer cells.20 Listeria spp. bacteria and cytotoxic T-cells in combination directly target the cancer cells and lead to shrinkage of the tumor mass.21 These bacteria can activate NADP(+) oxidase within cancer cells and increase the intracellular Ca2+ level, thus triggering the production of reactive oxygen species (ROS). These biochemical changes lead to direct killing of cancer cells.21 In addition, Listeria spp. can transform some infected MDSCs into immune-stimulating phenotypes that can produce interleukin-12 (IL-12), involved in natural killer (NK) and T-cell response (Figure 2).20
Within the TME, some metabolites produced by quiescent cancer cells act as chemo-attractants for S. typhimurium.22 In the presence of tumor environment, these bacteria proliferate and trigger necrosis, apoptosis, and cell rupture, thus kill the surrounding cancer cells.14 The cancer cells are forced to produce gap junction protein (connexin 43) by Salmonella spp. This protein reduces the immunosuppressive expression of indoleamine 2,3-dioxygenase (IDO) and enhances the transfer and cross-presentation of processed tumor antigenic peptides between cancer cells and dendritic cells (DCs).23 In addition, S. typhimurium flagellin reduces the frequency of regulatory T-cells (Tregs) and enhances the antitumor response of NK and CD8+ T-cells (Figure 2).3
Wild-type probiotics have been used to study bladder cancer, cervical cancer, breast cancer, liver cancer, in addition to colorectal cancer.24 These probiotics can be directly delivered at the TME to reduce non-specific pharmacological effects on normal tissues. The tumor-targeting bacteria and probiotics have some limitations in their use as anticancer agents, as it is challenging to balance the bacterial dosage for therapeutic purpose and the measure of toxicity.14,25 In addition, tumor-targeting and probiotics have limitations in eradicating completely the tumor mass and further probiotics lack the intrinsic therapeutic potential of tumor targeting.17 There is still a problem of high risk infection and toxicity by using these bacteria.26 The intratumoral injection of therapeutic bacteria at tumor sites is a good option to reduce the toxicity and infection rate, but it cannot be used during the metastatic tumor phase.27
Mechanism of Bacteria-Mediated Tumor Therapy
Coley used bacilli (Streptococcus pyogenes) for the first time in 1891 for the treatment of osteosarcoma.28 Several mechanisms are involved in bacteria-mediated cancer suppression like the activation of immune system. The concentration of oxygen in the tumor tissue is only 7–28 mm Hg (1–4%), while it is 40–60 mm Hg (5–8%) within the normal tissue.29 Bacteria can also recruit inflammatory cells like NK cells and granulocytes for TME, important for anti-tumor response.30 In addition, bacteria can induce CD4+ T-cells in the TME to produce interferon-γ (IFN-γ) and can also activate CD8+ T-cells to inhibit tumor growth.31
The toxicity of bacteria can be minimized with the aid of genetic modifications in addition to enhanced selective targeting.13 It involves the chromosomal deletion of purI and msbB genes of S. typhimurium (VNP20009) to reduce their septic shock and virulence.32 In addition, the leu-arg-deficient genetically modified S. typhimurium A139 strain possesses exceptional tumor-targeting ability.33
The therapeutic role of bacteria can be classified into three groups as: (a) antitumor immune activation, (b) secretion of bacterial toxins, and (c) swelling and apoptosis of tumor cells by invaded bacteria. Bacteria demonstrate wonderful immune activation capability. For example, dendritic cells and macrophages get colonized in the presence of Salmonella and are induced to produce interleukin-1β (IL-1β). These bacteria also lead to connexin 43 (Cx43) upregulation and the gap junctions formation between tumor and the dendritic cells,34 that leads to significant anticancer immune response. Further, the inflammatory response is also activated through pathogen-associated molecular patterns (PAMPs), which facilitates cytokine release that contributes to cancer immunotherapy.35 For example, toll-like receptor 4 (TLR4) signal transduction is induced by lipopolysaccharides (LPS) that promotes IL-1β production from the macrophages.36 In addition, the NK cells are stimulated by the flagellin that induces the production of IFN-γ (Figure 2).37
The toxins produced from bacteria can activate apoptotic pathways. For example, cytolysin A (ClyA) mediates caspase induced cell death and also forms gaps within the cell membranes.38 ClyA, produced from E. coli K-12, inhibits the cancer growth. In addition, the tumor progression is correlated with nitric oxide (NO) level. The higher level of NO mediates apoptosis of cancer cells, resulting in tumor regression.39
Engineering of Bacteria for Tumor Management
As microscopic robots, bacteria can be reprogrammed following simple genetic rules or sophisticated synthetic bioengineering principles to produce and deliver antitumor agents based on the clinical needs. The engineering of bacteria to combat cancer is performed at different levels as virulence attenuation, enhancement of tumor targeting, targeting the tumor stroma, drug expression strategies, and the expression of cytotoxic agents. In addition, the engineering of tumor-targeting bacteria is also achieved through the biosynthesis of metal NPs and delivery of drug-loaded nanoformulations. Furthermore, the bacterial spores and bacterial membrane vesicles are also utilized as an anticancer strategy. All these strategies of antitumor approaches are briefly discussed here:
Virulence Attenuation
While using specific bacteria against a cancer, it is very important to minimize their virulence against the host immune system, keeping in view that the intrinsic antitumor activity of some bacteria are due to their virulence factors.30,40 Therefore, the antitumor activity of a bacteria should not be lost while attenuating them. Some highly toxic bacterial strains have been attenuated to safer strains through the deletion of major virulence genes. Deletion of purI and msbB genes in S. typhimurium led to the formation of VNP20009 strain, which is extensively used in cancer-bearing mice for different antitumor studies.41 This strain has been accordingly tested in Phase I trials in human cancers, but the outcome has been disappointing.42 The failure is expected to be due to penta-acylated lipid A, a toll-like receptor 4 (TLR4) antagonist.43 New mutant Salmonella strains have been engineered by the deletion of pagL, pagP, and 1pxR genes to produce hexa-acylated lipid A with high affinity for TLR.44
The lipopolysaccharide (LPS)-driven septic shock has also been reduced dramatically by the deletion of msbB gene in Salmonella genus.45 The integration of LPS gene within chromosome in araBAD locus resulted in production of strains with attenuated virulence and enhanced therapeutic effects.46 The downregulation of endotoxin-associated genes led to the formation of another nontoxic Salmonella strain. Salmonella spoT and relA-mutant strains exhibited negligible toxicity as these strains are defective in ppGpp, signaling molecules involved in toxin gene expression. These strains exhibited excellent antitumor activity through the activation of inflammasome (IPAF, NLRP3), which can induce the expression of numerous proinflammatory cytokines.
The cytotoxicity of L. monocytogenes is achieved by the deletion of genes, involved in cell invasion and defects in phagolysosome release, achieved by HIy deletion.47 Mutant strains of L. monocytogenes lacking inIA and inIB are invasion defective and the strains lacking ActA or actA PESTf-like sequences also lack intracellular diffusion ability.48 The additional approach to attenuate virulence with enhanced tumor-specific proliferation is achieved by the introduction of specific nutrient-dependent mutations in bacteria. The examples of some attenuated strains of several tumor-targeting bacteria and their description is listed in Table 1.
 |
Table 1 Description of Some Genetically Modified Bacterial Strains Used for Tumor Therapy |
Enhancement of Tumor Targeting
The approaches to enhance the bacterial tumor targeting can also improve both antitumor efficacy as well as safety aspects. Regarding this approach, the ppGpp-deficient strain SHJ2037 has been genetically engineered to exhibit cancer-specific ligands on its cell surface. An αvβ3 integrin binding with Arg-Gly-Asp peptide has been fused to protein A on the outer membrane to drive its expression.57 The resulted strains possessed enhanced cancer-specific activity and significantly augmented antitumor activity in mDA-MB-435 melanoma xenografts overexpressing αvβ3 integrin and mDA-MB-231 breast cancer cells. The bacteria have also been engineered to target tumor-associated genes like lymphoma-associated antigen CD20 and carcinoembryonic antigen (CEA). These strains possess reduced non-specific accumulation in the spleen and liver and effective antitumor activity.58 The bacteria L. monocytogenes were coated with plasmid-loaded NPs expressing bioluminescence genes to exploit biotin-streptavidin binding. This strain, known as microrobot, could be traced by the bioluminescence imaging as it delivers the functional nucleic acid molecules within the solid tumors.59
A fascinating alternative to enhance the tumor selectivity is achieved by displaying synthetic adhesins (SAs) on the E. coli surface. These adhesins have a modular structure with stable β-domain needed for outer membrane anchoring and surface exposed antibody domains with high specificity and affinity which can be selected from large libraries.60 Some probiotic strains have been designed with enhanced tumor specificity and increased injection capacity of bacteria.61
Targeting the Tumor Stroma
The cancer growth and metastasis is equally supported by angiogenesis, and targeting this tumor neovascularization offers a favorable trend for cancer therapy. Endostatin (20 kDa C-terminal fragment from type XVIII collagen) has been found to possess inhibitory potential on tumor vessel formation with least side-effects or drug resistance.62 The attenuated strain of S. typhimurium was cloned with endostatin and siRNA against transducer and activator of Stat3 and the therapeutic efficacy was investigated on HCC. It showed satisfactory reduction in cancer proliferation and metastasis and reduced the tumor vasculature as well. This strategy led to the downregulation of VEGF expression, regulatory T-cells and TGF-β expression. In addition, there was an enhancement in inflammatory cytokines including TNF-α and IFN-γ and increased CD4+/CD8+ T-cell population.63
VEGF and its receptor (VEGFR) are well known tumor angiogenesis proteins. S. typhimurium (SL3261) expresses the extracellular VEGFR2 domain and the oral administration of this strain led to reduced pulmonary metastasis, neovascularization, and tumor growth. In addition, the administration of this strain led to an increased population of CD4+ and CD8+ T-cells near tumor regions.64
Endoglin (CD105) is a member of the TGF-β receptor family and its gene promoter is overexpressed in tumoral endothelial cells. Hypoxia and TGF-β1 are known to upregulate the endoglin gene promoter. Therefore, targeting the endoglin is considered as a novel strategy of cancer therapy.65 In mouse breast cancer models, Listeria based vaccines have been used against CD105, Lm-LLO-CD105A, and Lm-LLO-CD105B as a treatment strategy. Such vaccines inhibited primary and metastatic tumors by the reduction of angiogenesis and elevated antitumor immune response.66
Drug Expression Strategies
A strict control over the production and targeting of most payloads by tumor-targeting bacteria is of utmost importance as these are toxic to both normal and tumor cells. A precise trigger for the payload expression can minimize its systemic toxicity while maximizing its therapeutic effect. By the insertion of a specific promoter sequence upstream of a drug-encoding gene, a controllable gene expression can be maintained, convening transcriptional control through external signals. The triggering for gene regulation is mainly classified into three categories as (a) internal triggering, (b) self-triggering (quorum sensing-QS), and (c) external triggering.67 The special properties of TME like acidosis, hypoxia, and necrosis are sensed by tumor-targeting bacteria, which are utilized to improve their cancer specificity. It includes hypoxia inducible promoters (HIP-1) and pepT, activated by nitrate and fumarate reduction present in the hypoxic environment of cancerous tissue.68 This hypoxia-inducible expression method was proposed to function during anaerobic conditions only to express essential genes like asd. Furthermore, a glucose sensor has also been engineered in E. coli to sense the glucose level in TME leading to its therapeutic effect.69
Expression of Cytotoxic Agents
The expression of cytotoxic agents can be firmly regulated to check their toxic potential on normal tissues. Bacteria like E. coli, Paratyphi A, and S. typhimurium produce a 34 kDa pore-forming hemolytic protein known as cytolysin A (ClyA), secreted without any post-translational modifications. Several bacterial strains have been engineered to express ClyA from a constitutive promoter.70 In addition, ClyA is programmed to express from inducible promoters activated by doxycycline and arabinose, and excellent tumor inhibition has been reported.
The induction of apoptosis in cancer cells is a novel alternative of tumor management. In this regard, apoptin, a virus-derived protein in chicken, has been selectively used to induce apoptosis in different human cancer cell types through the p53-independent, Bcl-2-insensitive pathway.71 A significant cancer reduction with minimal systemic toxicity has been observed in human laryngeal cancer-bearing mice by the transformation of apoptin-encoding eukaryotic expression plasmid (pCDNA3.1) into the attenuated S. typhimurium strain.
Some other cytotoxic agents for the induction of apoptosis, like Fas ligands, TNF-α, and TRAIL, have limited use due to their hepatotoxicity and short half-life.72 Some bacterial strains have been used to deliver these proteins directly within the cancerous tissues to overcome these limitations.
Yersinia express invasin on its surface which can selectively bind to β1 integrin and triggers bacterial entry into host cells. In mice, the introduction of E. coli strain co-expressing invasin, ovalbumin, as well as LLO has been shown to invade β1-integrin, expressing tumor cells to show strong therapeutic effects.73 Furthermore, azurin is a low-molecular weight redox protein which initiates cancer cell apoptosis through its internalization. This protein helps to release cytochrome c from mitochondria by raising the intracellular level of p53 and Bax. The E. coli based azurin delivery has been reported to suppress 4T1 mouse breast cancer and B16 mouse melanoma, and this approach stimulates inflammatory response and prevents pulmonary metastasis.74
Different Therapeutic Payloads Delivered by Engineered Bacteria
Some specifically engineered bacteria have played a significant role in transporting different types of payloads up to extracellular TME and intracellular locations of tumor cells. Employment of some novel nanocarriers for conventional drugs and therapeutic agents helps to improve their bioavailability and pharmacodynamic and pharmacokinetic parameters. Different types of nanomaterials are used to improve the solubility of anticancer drugs, prolong circulation time, and enhance their accumulation within the TME. Native drug-loaded nanoformulations encounter diffusion limitations in the extracellular matrix and get accumulated in the periphery of the tumor rather than in the hypoxic core of the tumor.
Delivery of Anticancer-Proteins, Enzymes, and Other Agents
Non-pathogenic strains of S. typhimurium have been engineered under the control of prokaryotic radiation-inducible RecA promoter to secrete TRAIL protein. The TRAIL protein induces its toxicity through caspase-3 activation. On irradiation, S. typhimurium secreted TRAIL can lead to caspase-3-mediated apoptosis and death in 4T1 breast cancer cells in culture. In mice, the systemic injection of these engineered bacteria led to TRAIL expression by 2Gy γ-irradiation with delayed breast cancer growth.75
In E. coli, invasin genes have been cloned to express the invasin proteins.76 These proteins are normally exploited by Y. pseudotuberculosis as an entry pass into the host cells during their invasion. The invasins bind with β1-integrin proteins expressed by cancerous and epithelial cells. The invasins enter the host cells through receptor-mediated endocytosis and exploit their anticancerous activity.
In the host cells, E. coli are armed with listeriolysin O (LLO), which forms pores in the lysosomes.76 The expression of invasins in the cytosol results in cancer cell death. In addition, E. coli also helps to boost the immune system at the infection site and systematically with PAMPs expressed, recognized by Pattern Recognition Receptors (PRRs) on immune cells. The interaction of immune cells with PAMPs leads to reactive nitrogen and ROS release. This interaction also leads to the activation of T lymphocytes like CD4+ T-cells and CD8+ T-cells, which are capable of halting further proliferation of tumor cells (Figure 2).
The E. coli derived enzyme asparaginase (L-ASNase) has been utilized for the treatment of acute lymphocytic leukemia.77 This enzyme catalyzes the formation of aspartate from asparagine and to some extent forms glutamate from glutamine and both the reactions are important for cancer treatment.78 A treatment strategy was devised for acute lymphoblastic leukemia by using Salmonella bacteria expressing L-ASNase. The araBAD E. coli inducible promoter was used to design Salmonella cells to deliver L-ASNase to cancer cells.79
Delivery of Gene Therapy and Gene Silencing Agents
A promising approach to cancer therapy has been achieved by silencing specific target genes by using small interference RNAs (siRNAs). The greatest challenge to RNA interference therapy is the requirement of a specific delivery system for siRNAs to the tumor region. Mouse models have been investigated to check the activity of siRNA through bacteria-based delivery systems against indoleamine 2,3-dioxygenase (IDO),80 Stat,63 Sox,81 survivin,82 and the cell cycle-associated polo-like kinase 1 (PLK1).
Recombinant Salmonella has been orally administered in tumor-bearing nude mice, leads to decreased cancer growth, and displayed more sensitivity towards cis-diamine-dichloroplatinum (II) (DDP). Transforming growth factor-α (TGF-α) is a naturally occurring ligand for EGFR, which is overexpressed in tumor cells. A recombinant immunotoxin like PE38 has been constructed by conjugating TGF-α and laboratory-engineered Pseudomonas exotoxin A. Tumors in the mouse model as well as in vitro, PE38 exhibit a toxic effect on cancer cells which express EGFR.83 However, dose-dependent hepatotoxicity has been reported by systemic injection of TGF-α-PE38.84
In one study, DppGpp Salmonella mutant expressing recombinant TGFα-PE38 were investigated, which showed neither attack nor proliferation within mammalian cells,85 but exerted their anticancer effects by the expression of proinflammatory cytokines from neutrophils and macrophages, such as TNFα and IL-1β.79 The study included the construction of a plasmid with DNA encoding TGFα-PE38, inserted into Salmonella cells. Breast and colon tumors with enhanced levels of EGFR expression in mouse models were employed for this study. An inducible system based on PBAD promoter from E. coli was used.86 For the export of TGFα-PE38 recombinant protein from Salmonella, an engineered phage lysis system was employed as a bacterial membrane transport signal, fused to the proteins.87 Both these approaches were found to be effective. It was observed that TGFα-PE38 produced from bacteria reduced cancer progression as compared to non-engineered Salmonella alone.87 Increased expression of EGFR was observed by the treatment with TGFα-PE38 in cancer cells which induced the apoptosis consequently. Therefore, bacteria can be an innovative strategy for enhancing the effectiveness of immunotoxins for cancer treatment.88
A study was performed to investigate the cytotoxic activity of Salmonella strain equipped with salicylate-inducible expression apparatus, that modulates the expression of cytosine deaminase (CD).89 5-FU resistant Salmonella strains were produced for the increased production of bacterial CD. In addition, purD mutation was developed to regulate the intracellular proliferation in the presence of adenine as well as to prevent intracellular Salmonella death. This approach led to the production of Salmonella strains CD to kill cancer cells in the presence of 5-FU.89 As compared to other cancer-targeting bacteria, engineered Salmonella strains have attained a special momentum in the delivery of antitumor payloads within the TME. Table 2 describes some examples of anticancer agents delivered by different Salmonella strains.
 |
Table 2 Some Examples of Anticancer Agents Delivered or Targeted by Different Salmonella Strains |
Delivery of Immunomodulators
Cytokines are well-known to have antitumor potential by inducing apoptosis in tumor cells. These molecules can activate, proliferate, and differentiate immune cells via anti-angiogenesis effects on tumor vasculature. Different cytokines like IL-12, IL-18, and GM-CSF have been checked for clinical trials for tumor therapy.104 Several cytokines have been delivered in the TME by tumor-targeting bacteria, where it augments the antitumor immune response. The primary tumor growth in mice was potentially inhibited by the intravenous administration of attenuated S. typhimurium strain expressing IL-18. This led to increased number of CD4+ T and NK cells and massive leukocyte infiltration (especially granulocyte) at TME. This approach also led to enhanced cytokine production at TME including IFN-γ, IL-1β, TNF-α, and GM-CSF.105
The delivery of tumor associated antigens led by engineered bacteria can sensitize TME and overcome the self-tolerance provoked by the regulatory T-cells, thus elicit effector and memory T-cell response towards the antigen-producing cancer cells.106 Different prostate cancer-associated antigens like prostate-specific antigen (PSA) have been worked out by bacteria-based vaccines tested on several mouse models.107 The gene delivery of endogenous PSA has been performed by using attenuated S. typhimurium (SL7207), which led to alleviated immune response in murine prostate cell antigens and considerably reduced the tumor growth.92
Some promising cancer inhibition effects have also been observed by using a gene therapy approach by using antigens against HER-2/neu,108 Mage-b, NY-ESO,109 and Survivin.110 All these findings led to deep interest in the field of immune checkpoint blockade (ICB) cancer therapy. The success of ICB therapy during clinical trials has been limited to only a few patients, some reasons include host resistance like immunosuppressive TME.111 The bacterial tumor colonization can induce proinflammatory reactions involving enhanced expression of IFN-γ, IL-1β, and TNF-α, as well as NK and T-cell activation, thus a combination of bacterial therapies and ICB can overcome the host resistance.112
Delivery of Prodrug-Converting Enzyme
The conversion of prodrugs into cytotoxic agents by the expression of prodrug-converting enzymes is a smart strategy of tumor eradication. This method reduces the side-effects associated with systemic administration and improves the cancer treatment efficacy. Bacteria have been used to deliver prodrug-converting enzymes.112 These enzymes include cytosine deaminase (CD), which converts nontoxic 5-fluorocytosine (5-FC) into a chemotherapeutic agent, 5-fluorouracil (5-FU) (Figure 2). This drug is highly toxic as it is metabolized to a product which interferes with the DNA and RNA synthesis.113 Another prodrug-converting enzyme/prodrug combination includes the herpes simplex virus type I thymidine kinase/ganciclovir (HSV1-TK/GCV) system, widely studied for tumor therapy. The expression of cancer-specific HSV1-TK can convert nontoxic precursor ganciclovir into a toxic form, ganciclovir-3-phosphate, that kills the cancer cells. The in vivo efficacy of Bifidobacterium infantis strain expressing HSV1-TK and GCV was examined in a rat bladder cancer model. This led to an efficient and targeted approach inhibiting the cancer effectively via apoptosis through the enhanced expression of caspase 3.112
E. coli DH5α is a good example of a prodrug-converting enzyme strain which expresses β-glucuronidase that hydrolyzes glucuronide prodrug 9ACG into 9-aminocamptothecin (9AC), a topoisomerase I inhibitor which efficiently inhibits tumors.114 Furthermore, the attenuated S. typhimurium (VNP20009) has been used as a vector to deliver carboxypeptidase G2 that exhibits enhanced anticancer activity in conjunction with prodrug administration.115
Delivery of Drug-Loaded Liposomes
Liposomes have gained a special importance as active vehicles for the delivery of diverse therapeutic compounds. The surface modifications of conventional liposomes with different ligands have led to the formation of second generation liposomes, with higher drug loading capacity, targeted drug-delivery, and enhanced anticancer activity.116 A novel anticancer therapeutic strategy was designed by using anticancer drug, paclitaxel (PTX) containing liposomes within S. typhimurium. This procedure was initiated by binding biotin molecules on the outer membrane proteins of bacteria and consequently streptavidin molecules were coated on the PTX-loaded liposomes. The motility analysis of bacteria-loaded liposomes exhibited higher average velocity as compared to free bacteria. The cytotoxicity tests were performed on breast cancer cell line (4T1) to figure out the anticancer therapeutic efficacy of the PTX-containing liposome loaded bacteria. In addition, tumor targeting bacteria displayed robust cancer-targeting ability. These findings reveal that engineered bacteria could be an efficient alternative for anticancer therapy.117
Salmonella were loaded with low-temperature sensitive anticancer drug doxorubicin (DOX) loaded within liposomes targeting colon cancer cells to deliver this drug and simultaneously macrophages polarized to M1 phenotype with high intensity focused ultrasound heating (40–42°C). The studies showed that the liposomal loading was highly efficient without affecting the bacterial viability. These drug-loaded liposome-containing bacteria demonstrated efficient intracellular trafficking, excellent nuclear localization of DOX, and induced in vitro pro-inflammatory cytokine expression of colon cancer. By using murine colon tumor models, these engineered bacteria significantly enhanced the therapeutic efficacy and macrophage polarization to M1 phenotypes as compared to control samples. Further, these bacteria focused ultrasound treatments, which have the potential to improve the colon cancer therapy.118
Bacterial Membrane-Based Anticancer Nanoformulations
Bacterial membrane-based nanoformulations include bacteria-derived nanovesicles (BDNVs) and bacterial membrane-coated NPs. BDNVs range in size from 20–400 nm, composed of double lipid layer. BDNVs are mainly classified into four groups based on their source and structure as: outer membrane vesicles (OMVs), outer-inner membrane vesicles (OIMVs), double-layered membrane vesicles (DMBs), and cytoplasmic membrane vesicles (CMVs).119 The BDNVs have been used against cancer, due to their cancer penetration ability, surface modification, and drug loading capacity.
Several genetically modified bacteria including E. coli derived 400 nm nanovesicles have been loaded with chemotherapeutic agents like DOX.120 The feasibility of using BDNVs to transport/deliver siRNA for drug-resistant cancer treatment has also been reported.121 Table 3 lists examples of some cargo items delivered by bacterial membrane vesicles derived from different bacteria for the strategy of cancer management.
 |
Table 3 Efficacy of Different Therapeutic Agents Loaded in Bacterial Membrane and Targeted Against Different Cancers |
In addition to gene and drug carrying potential, BDNVs also hold the capability of activating the immune response against cancer. Diverse immunostimulatory molecules loaded in OMVs have been investigated recently for vaccine and delivery system usage. The anticancer command of genetically modified E. coli derived OMVs exhibited excellent tumor-targeting ability due to their enhanced EPR effect.130 Some immunomodulatory agents induce the production of anticancer agents like CXCL10 and IFN-γ, which can successfully eradicate the established tumors.
The OMVs derived from E. coli BL21 cells have been chemically modified with Calcium phosphate (CaP) shells. These pH-sensitive shells neutralize the acidic TME to polarize the cancer-associated macrophages and avoid the severe systemic inflammation potentially induced by CaP free OMVs. The anti-inflammatory M2 macrophage phenotypes synergized with the intrinsic immunostimulatory effect of OMVs, have eventually led to a 60% survival rate at day 80 compared with day 0 in the group applying naked OMVs.
BDNVs have also been loaded with NPs to provide additional functions like photosensitivity. Bacteria-cancer cell hybrid membrane-coated photosensitizing hollow polydopamine NPs have been synthesized recently for the approach of cancer eradication (Figure 3).131 The anticancer cytokines were potentially produced by bacterial membranes through different immunostimulatory membrane components.
Cancer cell membrane proteins serve as excellent tumor antigens, which synergize with anticancer cytokines and induce a substantial immune response. The combination of photothermal treatment and anticancer immune therapy has been reported to eradicate melanoma. Further, the uploading of NPs within bacterial membranes adds the functionality in photothermal response and also helps to enhance the immune response to fight against cancer (Figure 3).
Delivery of Drug-Conjugated Nanoparticles
Bifidobacterium longum (B. longum) have been engineered to conjugate poly(lactic-co-glycolic acid) (PLGA) NPs (PLGA-NPs) targeting the tumor specifically to achieve precision treatment and imaging. B. longum selectively colonizes in hypoxic regions of the animal body, successfully targeting into solid tumors. Further, perfluorohexane (PFH) has been used to wrap the core of PLGA-NPs to improve its specificity and efficacy for cancer therapy. PFH/PLGA-NPs kills the cancer cells by the deposition of energy by affecting the acoustic environment during High Intensity Focused Ultrasound (HIFU) irradiation. This strategy has been effective in treatment and diagnosis, providing stronger imaging, a longer retention period, and much better tumor therapy.132
A combination of bacteriolytic therapy (COBALT) strategy was applied by using C. novyi devoid of its lethal toxin (C. novyi-NT) spores loaded with conventional chemotherapeutic drugs. It led to extensive antitumor capability against hemorrhagic cancer.133 Bacteria-facilitated NPs delivery into the cancer cells takes the advantage of the invasive property of these microorganisms. The drug-loaded cargos are not carried inside the bacteria, rather these payloads remain attached on the microorganism surface.
S. typhimurium bacteria have been precisely engineered to transport drug-loaded nanoformulations and penetrate prostate cancer cells to deliver their antitumor cargos. Some methods established for the cargo loading and delivery include the attachment of NPs to the Salmonella membrane. The example includes the sucrose-conjugated AuNPs attached to the surface of Salmonella bacteria. The other method includes the attachment of streptavidin-conjugated fluorophores on biotinylated Salmonella membrane, that enhances the transport of and drug delivery.134
Biosynthesis and Delivery of Metal NPs
Bacteria have been significantly employed for the biosynthesis of metal NPs. The bacterial synthesis of NPs involves spontaneous and simple biochemical and biophysical processes leading to the formation of monodisperse and stable formulations. The exact mechanism of its biosynthesis at molecular level is not yet well understood.135 The bacteria exploit different mechanisms like biosorption, solubility changes, extracellular precipitation, bioaccumulation, chelation, and metal complexation for the synthesis of metal NPs involving reducing NAD(P)H-dependent enzymes like cysteine desulfhydrase, glutathione, nitrate reductase, and sulphite reductase.136
Diversified bacteria growing in extreme environmental condition like archaea,137 Deinococcus radiodurans,138 and marine139 ecosystem have been associated with metal NPs biosynthesis. Metal NPs, especially belonging to heavy and toxic group namely Au, Ag, Cd, Ni, Pd, Pt, Se, Ti and some metal oxides like CeO2, Fe3O4, TiO2, Zirconia, and ZnO along with their functional derivatives, have been reported to be synthesized by bacteria.140
The anticancer activity of S. rochei HMM13 synthesized silver NPs (AgNPs) has been checked on different tumor cell lines like breast carcinoma cells (MCF-7), hepatocellular carcinoma cells (HepG-2), prostate carcinoma cells (PC-3), colon carcinoma cells (HCT-116), intestinal carcinoma cells (CACO), lung carcinoma cells (A-549), cervical carcinoma cells (HELA), and larynx carcinoma cells (HEP-2). The percentage of all these different cancer cell lines demonstrated a dose-dependent decrease in their viability percentage by the exposure of these NPs.
The uptake of AgNPs by different tumor cells are catabolized to form amino acids and Ag ions.141 The released Ag+ cations interact with cellular macromolecules like DNA and proteins. These ions lead to protein modifications, DNA damage, and enhanced mitochondrial permeability of cancer cells resulting in enhanced oxidative stress. All these changes in cancer cells push them to apoptosis.142
Biosynthesis and Delivery of Magnetosomes
Magnetically controlled biosensors, contrast agents in MRI diagnosis, and drug delivery system popularly consist of superparamagnetic iron oxide (FeO) nanoparticles (FeONPs).143 Magnetotactic bacteria exclusively contain magnetosomes, unique lipid bound organelles, and provide some special characteristics to these bacteria for cancer management. These magnetosomes possess narrow size distribution, regular morphology, resistance to agglomeration, and low toxicity profile, which makes them excellent for drug and gene delivery applications. The magnetosomes are nanometer-sized crystals, naturally synthesized through cytoplasmic membrane invaginations, followed by influx of iron and certain proteins, leading to magnetite crystal biomineralization.144 These bacteria belong to the α-Proteobacteria group and are mostly Gram-negative, having a micro-aerobic or anaerobic type of metabolism.145 These bacteria are capable to produce naturally iron sulfide (greigite) and iron oxide (magnetite) NPs covered by a lipid bilayer.
The magnetosomes help in aligning the bacteria for external magnetic fields and optimal nutrient and oxygen conditions. The magnetosomes have been isolated from bacteria and have been useful in medical applications like peptide screening in drug development.146 Further, these magnetosomes have been utilized for anticancer gene therapy and drug delivery.147 These specialized bacteria have gained a distinct position as a smart drug delivery system in cancer patients.148
The chain alignment of magnetosomes in Magnetospirillum gryphiswaldense is aligned to enhance the hyperthermia outcome during cancer therapeutics.149 In comparison to FeONPs, magnetosomes have been reported with enhanced efficacy as MRI-contrast agents.150 As a heat sensitive system, bacterial magnetosomes have been used as a smart chemotherapeutic approach.
The magneto-aerotactic behavior of Magnetococcus marinus strain MC-1 has been exploited to transport up to 70 drug-loaded nanoliposomes till extremely low oxygen regions of the cancerous tissue. It has been reported that up to 55% of drug-loaded bacterial cells can penetrate the colorectal xenograft in severe combined immunodeficiency (SCID)-mice.151 Bacterial magnetic nanoparticles (BMN) have been coated with polyethyleneimine (PEI), resulting in a size range of 45–55 nm, used to transfect DNA in mammalian cell lines.152
In comparison with the older methods, the bacterial magnetosomes have been complexed with anticancer antibodies (BM-Ab) to achieve greater antitumor efficacy under the magnetic therapy.153 For the application in drug delivery and imaging protocols, these magnetic and AuNPs have been used as efficient theranostic agents.154 Magnetotactic bacteria derived magnetosomes have been conjugated with Au nanorods and folic acid to form nanohybrids. These nanohybrids serve as effective theranostic agents for the detection and photomechanical killing of cancer cells.155 These NPs have been applied as high contrast probes to seek out even single-cell diagnostics as well as photothermal agents for single-cell therapy (Figure 2). The application, efficacy, and theranostic mechanisms of different types of metal nanoformulations, delivered by diverse tumor targeting strains of bacteria, are summarized further in Table 4.
 |
Table 4 Summary of Different Metal Based Drug-Nanoformulations Loaded in Various Tumor-Targeting Bacteria for Cancer Therapy |
Bacterial Spores
The majority of anaerobic bacteria produce highly resistant spores which can survive even in an oxygen-rich environment. Once the favorable conditions like that of TME are met, these spores germinate and the bacteria thrive accordingly, targeting the nearby cancer cells. C. novyi-NT bacteria are genetically modified to be devoid of lethal toxins which target cancer cells without involving side-effects.173 An intratumoral injection of C. histolyticum spores in mice resulted in marked lysis of cancerous tissue. A similar phenomenon has been observed by intravenous injection of C. sporogenes spores in mice.156 The spores of C. novyi-NT are rapidly cleared by the reticuloendothelial system from circulation as observed by toxicological and pharmacological evaluation. Injection of these spores in healthy rabbits or mice even with large doses showed no clinical toxicity. However, the toxicity was related to spores dosage and tumor size in diseased mice.174 In addition, bacterial spores have also been used as carriers of anticancer drug delivery agents, therapeutic proteins, gene therapy vectors, and cytotoxic peptides.175
A brief description of some important anticancer agents delivered by tumor-targeting bacteria near or within cancer cells and their concise mechanism of action has been described in various articles and is illustrated in Figure 4.
 |
Figure 4 Some examples of anticancer agents delivered by different tumor-targeting bacteria and their brief mechanism of action. |
Tumor-Directed Remote Control Guidance of Bacteria
It has been reported that only a few bacteria reach TME on their own, so active research is going on in engineering other bacteria to carry or produce and deliver anticancer compounds within the tumor regions. The clinicians need to effectively navigate bacterial therapies near cancer sites, as most tumors are inaccessible by direct injection of antitumor agents. Further, the engineered bacteria should controllably and reliably release their anticancer drugs they carry or encode.176 The incorporation of synthetic compounds within the live bacteria can allow remote control guidance of certain actions or functionality. The light has a limited ability to penetrate the cancerous tissues which hampers its approach, even though optically triggered navigation and control have enormous potential. The use of ultrasound has filled some gaps, as it has a broad range of applications in medical diagnostics and monitoring.177
Recently, to augment the ultrasound images of tissues, gas-filled microbubbles have been used due to their distinct and strong acoustic response. In addition, some special forms of super-powered and focused ultrasound have been used to boost the transport of drug-loaded nanobubbles by the use of acoustic pressure waves as an external energy source to push it to deeper regions of TME. This tactic has achieved some promising results in glioblastoma, as the blood–brain barrier (BBB) is a challenge to overcome for drug transport.178
In the recent past, ultrasound has been used to track the bacteria for therapeutic purposes in vivo. Bacteria have been genetically engineered to express the acoustic reporter gene (ARG), which encodes the compounds of gas vesicles that scatter ultrasound waves, thus generating an echo to enable the bacterial location deep inside living mice.179 The application of magnetic fields is another source of external energy which can be remotely and safely used in the human body.
The advantage that anaerobic bacteria tend to shift to low oxygen environment, coupled with anticancer drugs and the natural homing mechanism of an externally directing magnetic field, has demonstrated enhanced penetration and accumulation for therapy in mouse tumors. The magnetotactic bacteria act like little propellers on a rotating magnetic field with tissue models on a chip, creating a flow that pushes nanomedicine out of the blood vessels and deeper in tissues.
Attaching magnetic materials to non-magnetic bacteria is an alternative to control such bacteria by the external magnetic field.180 Tiny magnetic NPs have been attached to E. coli in addition with DOX and upon treatment with cancer cells, it has been reported that such bacteria are remotely controlled by the magnetic field to improve their tumor targeting.181 The science of external energy source and controllable genetically engineered bacteria are a fascinating new direction in the field of cancer management. The convergence of mechanical engineering, synthetic biology, and robotics has opened up a new approach of using tiny robots to destroy different cancer types.182
Clinical Trials
For the management of cancer in human subjects, different bacterial strains have been selected since the use of live bacteria by Dr. Coley in 1891.183 Among different bacterial species, Listeria vaccine strains have shown promising results, and some strains are tested in Phase II and Phase III clinical trials.184 The attenuated strain of S. typhimurium (VNP20009) was the first strain to enter a phase I human clinical trial in 1999, tested on 24 patients with metastatic melanoma and metastatic renal carcinoma. Although different proinflammatory cytokines like IL-1β, IL-6, IL-12, and TNF-α were reported to be raised in some patients, no objective tumor regression was reported.185 S. typhimurium (VNP20009) was used in another clinical trial involving metastatic melanoma patients, but no remarkable tumor response was reported.186 To enhance the therapeutic potential, S. typhimurium (VNP20009) was engineered to express E. coli CD, that converts 5-FC to toxic 5-FU. An intratumoral injection of these bacteria was used in three patients suffering from esophageal adenocarcinoma and head and neck squamous carcinoma. Even after the six treatment cycles, no significant adverse response was observed in these patients.
Recently, some other phase I clinical trials have been reported by using S. typhimurium (VNP20009) and S. typhimurium (χ4550) expressing IL-2, as summarized in Table 5. The conclusion of these trials disclosed that the differences between human patients and preclinical animal models might be due to dissimilarities in tumor structure and growth rates that might alter bacterial TME behavior. The clinical trials by Salmonella spp. have demonstrated that TLR4-mediated signaling is important for tumor colonization and antitumor activity, as a VNP20009 strain missing lipid A function was unsuccessful to colonize tumor sufficiently to suppress tumor growth. Although limited, these clinical trials have revealed some significant hurdles and some challenges that must be overcome for successful human application in the future. Some examples of clinical trials using several bacteria are listed in Table 5.
 |
Table 5 Previous and Ongoing Clinical Trials Involving Tumor-Targeting Bacteria and Cancer Bearing Human Subjects |
Future Perspective
The complete treatment of cancer is considered a challenging task as hypovascular areas provide inadequate access to drug-loaded nanoformulations. Even though some tumor-targeting bacteria have been genetically engineered to combat various cancers, several future studies are needed to address and expediate the further advancement of nanobiohybrid systems in tumor therapy.
These prospective studies need to know the shape of the nanoformulations as it is a significant parameter for nanobiohybrid systems, which impacts on bacterial transport efficiency. The loading quantity and volume of nanomaterials also affect the bacterial movement. In addition, the performance of nanobiohybrid interaction between nanomaterials and bacteria is of utmost importance to adopt varied loading strategies based on different nanomaterials to augment the performance. The attachment of NPs on the bacterial surface can affect bacterial chemoreceptors in response to TME. Therefore, abiotic/biological interfaces need to be carefully designed to conserve the chemotaxis and bacterial mobility.
The role of exogenous and endogenous stimuli is very important for the release of nanomaterials from the bacteria at tumor regions. It is very significant to known the spatiotemporal control of drug action at the heterogeneous environment of tumors. Furthermore, the limitations of metal toxicity in living systems need an act of balancing between the positive therapeutic effects of metal oxide NPs and their toxic side-effects.190 Any delayed elimination or absence of dissolution/biodegradation can be followed by generation of intracellular ROS, DNA damage that triggers apoptotic cell death.191
The possession of bacterial immunogenicity and toxicity is very important to ensure the safety aspects. Even though a variety of bacteria are non-pathogenic, the possible toxicity may threaten immunocompromised patients with advanced stage cancer. Engineering bacteria to knock out virulence genes is of utmost importance. In addition, the complexity of the biological environment makes it necessary to develop feasible methods to control the noncatalytic therapy process to inhibit adverse catalytic reactions and prevent any damage to normal tissue. The lack of information on diverse mechanisms and side-effects of bacterial cancer therapy with development of smart microorganisms to treat specific cancers remains a significant challenge.
Conclusion
The therapeutic potential of different bacteria for the cancer management has been taken into significant consideration in the recent decade. Numerous bacteria possess great potential as anticancer strategies, however, this novel therapeutic approach has both advantages as well as disadvantages. The tumor-targeting bacteria possess several unique features like tumor selectivity and genetic modification capabilities. The center-point targeting of anticancer therapeutic payloads through specific bacteria is still a challenging task which can be resolved by a proper understanding about drug-nanoformulation design and its loading within bacteria, bacterial genetic setup, modifications, etc. Recent advancement in microbiology, drug-nanoformulations, and genetic engineering on the same desk have guided some anticancer bacteria to deliver different anticancer payloads at tumor sites with high precision. The bacterial anticancer therapy is still at its basic stage and more future research needs to be conducted to bypass the limitations and side-effects of this therapy by using genetic engineering and precise modifications of some antitumor agents. Despite the promising in vivo and in vitro results of anticancer bacteriotherapy, a few studies have led to clinical trials. In spite of some remarkable achievements, several critical issues like inflammation and toxicity must be resolved before the possible translation of this anticancer strategy into clinical use.
Acknowledgments
The researcher would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Disclosure
The author reports no conflicts of interest for this work.
References
1. Liang P, Ballou B, Lv X, et al. Monotherapy and combination therapy using anti‐angiogenic nanoagents to fight cancer. Adv Mater. 2021;33(15):2005155. doi:10.1002/adma.202005155
2. Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharma Bull. 2017;7(3):339. doi:10.15171/apb.2017.041
3. Liu S, Xu X, Zeng X, Li L, Chen Q, Li J. Tumor-targeting bacterial therapy: a potential treatment for oral cancer. Oncol Lett. 2014;8(6):2359–2366. doi:10.3892/ol.2014.2525
4. Duong MT, Qin Y, You S-H, et al. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51:1–15. doi:10.1038/s12276-019-0297-0
5. Sarotra P, Medhi B. Use of bacteria in cancer therapy. Recent Results Cancer Res. 2016;209:111–121.
6. Khan AA, Allemailem KS, Almatroudi A, Almatroodi SA, Alsahli MA, Rahmani AH. Novel strategies of third level (organelle-specific) drug targeting: an innovative approach of modern therapeutics. J Drug Deliv Sci Technol. 2020;61:102315.
7. Allemailem KS, Almatroudi A, Alrumaihi F, et al. Novel approaches of dysregulating lysosome functions in cancer cells by specific drugs and its nanoformulations: a smart approach of modern therapeutics. Int J Nanomedicine. 2021;16:5065. doi:10.2147/IJN.S321343
8. Allemailem KS, Almatroudi A, Alsahli MA, et al. Novel strategies for disrupting cancer-cell functions with mitochondria-targeted antitumor drug–loaded nanoformulations. Int J Nanomed. 2021;16:3907. doi:10.2147/IJN.S303832
9. Golombek SK, May J-N, Theek B, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi:10.1016/j.addr.2018.07.007
10. Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi:10.1016/j.addr.2015.01.002
11. Wu J. The Enhanced Permeability and Retention (EPR) effect: the significance of the concept and methods to enhance its application. J Personal Med. 2021;11(8):771.
12. Szebeni J, Simberg D, Gonzalez-Fernandez A, Barenholz Y, Dobrovolskaia MA. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat Nanotechnol. 2018;13:1100–1108. doi:10.1038/s41565-018-0273-1
13. Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:784–793. doi:10.1038/nrc2934
14. Zheng JH, Nguyen VH, Jiang S-N, et al. Two step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537. doi:10.1126/scitranslmed.aak9537
15. Peters L, Weidenfeld I, Klemm U, et al. Phototrophic purple bacteria as optoacoustic in vivo reporters of macrophage activity. Nat Commun. 2019;10:1191. doi:10.1038/s41467-019-09081-5
16. Heldin C-H, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi:10.1038/nrc1456
17. Staedtke V, Roberts NJ, Bai R-Y, et al. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3:144. doi:10.1016/j.gendis.2016.01.003
18. Bettegowda C, Huang X, Lin J, et al. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol. 2006;24:1573–1580. doi:10.1038/nbt1256
19. Shinnoh M, Horinaka M, Yasuda T, et al. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol. 2013;42:903–991. doi:10.3892/ijo.2013.1790
20. Chandra D, Jahangir A, Quispe-Tintaya W, et al. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 2013;108:2281–2290. doi:10.1038/bjc.2013.206
21. Kim SH, Castro F, Paterson Y, et al. High efficacy of a listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860–5866. doi:10.1158/0008-5472.CAN-08-4855
22. Kasinskas RW, Forbes NS. S. typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007;67:3201–3209. doi:10.1158/0008-5472.CAN-06-2618
23. Lin HC, Yang C-J, Kuan Y-D, et al. The inhibition of indoleamine 2, 3-dioxygenase 1 by connexin 43. Int J Med Sci. 2017;14:1181–1188. doi:10.7150/ijms.20661
24. Lenoir M, Del Carmen S, Cortes-Perez NG, et al. Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J Gastroenterol. 2016;51:862–873. doi:10.1007/s00535-015-1158-9
25. Patyar S, Joshi R, Byrav DP, et al. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17:21. doi:10.1186/1423-0127-17-21
26. Zheng JH, Min -J-J. Targeted cancer therapy using engineered S. typhimurium. Chonnam Med J. 2016;52:173–184. doi:10.4068/cmj.2016.52.3.173
27. Hatefi A, Canine BF. Perspectives in vector development for systemic cancer gene therapy. Gene Ther Mol Biol. 2009;13:15–19.
28. Moese JR, Moese G. Oncolysis by clostridia. I. activity of clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 1964;24:212–216.
29. West C, Slevin F. Tumour hypoxia. Clin Oncol. 2019;31:595.
30. Ozdemir T, Fedorec AJH, Danino T, Barnes CP. Synthetic biology and engineered live biotherapeutics: toward increasing system complexity. Cell Syst. 2018;7:5–16. doi:10.1016/j.cels.2018.06.008
31. Binder DC, Wainwright DA. The boosting potential of bacteria in cancer immunotherapy. Trends Mol Med. 2017;23:580–582. doi:10.1016/j.molmed.2017.05.008
32. Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi:10.1086/315497
33. Zhao M, Yang M, Ma H, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi:10.1158/0008-5472.CAN-06-0716
34. Saccheri F, Pozzi C, Avogadri F, et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med. 2010;2:44–57. doi:10.1126/scitranslmed.3000739
35. Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi:10.1038/s41416-018-0328-y
36. Phan TX, Nguyen VH, Duong MTQ, Hong Y, Choy HE, Min -J-J. Activation of inflammasome by attenuated Salmonella typhimurium in bacteria-mediated cancer therapy: bacteriotherapy and inflammasome. Microbiol Immunol. 2015;59:664–675. doi:10.1111/1348-0421.12333
37. Kupz A, Curtiss R, Bedoui S, Strugnell RA, Zamboni DS. In vivo IFN- secretion by NK cells in response to Salmonella typhimurium requires NLRC4 inflammasomes. PLoS One. 2014;9:e97418. doi:10.1371/journal.pone.0097418
38. Lai X-H, Arencibia I, Johansson A, et al. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect Immun. 2000;68:4363–4367. doi:10.1128/IAI.68.7.4363-4367.2000
39. Vannini F, Kashfi K, Nath N. The dual role of INOS in cancer. Redox Biol. 2015;6:334–343. doi:10.1016/j.redox.2015.08.009
40. Felgner S, Kocijancic D, Frahm M, et al. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2018;7:e1382791. doi:10.1080/2162402X.2017.1382791
41. Lee CH, Lin S-T, Liu -J-J, et al. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014;21:309–316. doi:10.1038/gt.2013.86
42. Nemunaitis J, Cunningham C, Senzer N, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–744. doi:10.1038/sj.cgt.7700634
43. Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J Immunol. 2005;175:4669–4676. doi:10.4049/jimmunol.175.7.4669
44. Liang K, Liu Q, Li P, et al. Endostatin gene therapy delivered by attenuated Salmonella typhimurium in murine tumor models. Cancer Gene Ther. 2018;25:167–183. doi:10.1038/s41417-018-0021-6
45. Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther. 2001;12:1594–1596.
46. Frahm M, Felgner S, Kocijancic D, et al. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. MBio. 2015;6:e00254–15. doi:10.1128/mBio.00254-15
47. Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi:10.1083/jcb.200201081
48. Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science. 2000;290:992–995. doi:10.1126/science.290.5493.992
49. Kazuyuki Y, Fujimori M, Amano J, Kano Y, Taniguchi SI. Bifidobacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000;2:269–274.
50. Dang Long H, Bettegowda C, Huso DL, et al. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci. 2001;98:15155–15160. doi:10.1073/pnas.251543698
51. Lemmon MJ, van Zijl P, Fox ME, et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997;8:791–796. doi:10.1038/sj.gt.3300468
52. Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Ther. 2002;9(4):291–296. doi:10.1038/sj.gt.3301659
53. Grillot-Courvalin C, Goussard S, Courvalin P. Bacteria as gene delivery vectors for mammalian cells. Horizontal Gene Transfer. 2002;261–265. doi:10.1016/B978-012680126-2/50029-3
54. Na HS, Kim HJ, Lee HC, Hong Y, Rhee JH, Choy HE. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine. 2006;12:2027–2034.
55. Wang Y, Chen J, Tang B, Zhang X, Hua ZC. Systemic administration of attenuated Salmonella typhimurium in combination with interleukin-21 for cancer therapy. Mol Clin Oncol. 2013;3:461–465.
56. Yano S, Zhang Y, Zhao M, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle. 2014;24:3958–3963.
57. Park SH, Zheng JH, Nguyen VH, et al. RGD peptide cell-surface display enhances the targeting and therapeutic efficacy of attenuated Salmonella-mediated cancer therapy. Theranostics. 2016;6:1672–1682. doi:10.7150/thno.16135
58. Dai YM, Toley BJ, Swofford CA, Forbes NS. Construction of an inducible cell-communication system that amplifies Salmonella gene expression in tumor tissue. Biotechnol Bioeng. 2013;110:1769–1781. doi:10.1002/bit.24816
59. Zhang Y, Ni Q, Xu C, et al. Smart bacterial magnetic nanoparticles for tumor-targeting magnetic resonance imaging of HER2-positive Breast cancers. ACS Appl Mater Interfaces. 2019;11:3654–3665. doi:10.1021/acsami.8b15838
60. Pinero-Lambea C, Bodelón G, Fernández-Periáñez R, et al. Programming controlled adhesion of E. coli to target surfaces, cells, and tumors with synthetic adhesins. ACS Synth Biol. 2015;4:463–473. doi:10.1021/sb500252a
61. Zhang Y, Ji W, He L, et al. E. coli Nissle 1917-derived minicells for targeted delivery of chemotherapeutic drug to hypoxic regions for cancer therapy. Theranostics. 2018;8:1690–1705. doi:10.7150/thno.21575
62. Zhang HY, Man J-H, Liang B, et al. Tumor-targeted delivery of biologically active TRAIL protein. Cancer Gene Ther. 2010;17:334–343. doi:10.1038/cgt.2009.76
63. Jia H, Li Y, Zhao T, et al. Antitumor effects of Stat3-siRNA and endostatin combined therapies, delivered by attenuated Salmonella, on orthotopically implanted hepatocarcinoma. Cancer Immunol Immunother. 2012;61:1977–1987. doi:10.1007/s00262-012-1256-y
64. Zuo SG, Chen Y, Wu ZP, et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol Pharm Bull. 2010;33:174–182. doi:10.1248/bpb.33.174
65. Nassiri F, Cusimano MD, Scheithauer BW, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290.
66. Wood LM, Pan ZK, Guirnalda P, Tsai P, Seavey M, Paterson Y. Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother. 2011;60:931–942.
67. Liang K, Liu Q, Li P, Luo H, Wang H, Kong Q. Genetically engineered salmonella typhimurium: recent advances in cancer therapy. Cancer Lett. 2019;448:168–181. doi:10.1016/j.canlet.2019.01.037
68. Javan B, Shahbazi M. Hypoxia-inducible tumour-specific promoters as a dual-targeting transcriptional regulation system for cancer gene therapy. Ecancermedicalscienc. 2017;11. doi:10.3332/ecancer.2017.751
69. Baumgartner JW, Kim CH, Brissette RE, Inouye M, Park CH, Hazelbauer GL. Transmembrane signalling by a hybrid protein: communication from the domain of chemoreceptor Trg that recognizes sugar-binding proteins to the kinase/phosphatase domain of osmosensor EnvZ. J Bacteriol. 1994;176:1157–1163. doi:10.1128/jb.176.4.1157-1163.1994
70. Jiang SN, Phan TX, Nam T-K, et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18:635–642. doi:10.1038/mt.2009.295
71. Danen-van Oorschot AA, Fischer DF, Grimbergen JM, et al. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci USA. 1997;94:5843–5847. doi:10.1073/pnas.94.11.5843
72. Wu X, Wang S, Li M, et al. Nanocarriers for TRAIL delivery: driving TRAIL back on track for cancer therapy. Nanoscale. 2017;9:13879–13904. doi:10.1039/C7NR04959E
73. Critchley-Thorne RJ, Stagg AJ, Vassaux G. Recombinant Escherichia coli expressing invasin targets the Peyer’s patches: the basis for a bacterial formulation for oral vaccination. Mol Ther. 2006;14:183–191. doi:10.1016/j.ymthe.2006.01.011
74. Uchugonova A, Zhao M, Zhang Y, et al. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012;32:4331–4337.
75. Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer. 2009;101:1683–1691. doi:10.1038/sj.bjc.6605403
76. Grillot-Courvalin C, Goussard S, Huetz F, Ojcius DM, Courvalin P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol. 1998;9:862–866.
77. Wriston JC, Yellin TO. L-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol. 1973;39:185–248. doi:10.1002/9780470122846.ch3
78. Willems L, Jacque N, Jacquel A, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122:3521–3532. doi:10.1182/blood-2013-03-493163
79. Kim K, Jeong JH, Lim D, et al. L-Asparaginase delivered by Salmonella typhimurium suppresses solid tumors. Mol Ther Oncolytics. 2015;2:15007. doi:10.1038/mto.2015.7
80. Manuel ER, Chen J, D’Apuzzo M, et al. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immunol Res. 2015;3:1096–1107. doi:10.1158/2326-6066.CIR-14-0214
81. Liu B, Jiang Y, Dong T, et al. Blockage of autophagy pathway enhances Salmonella tumor-targeting. Oncotarget. 2016;7:22873–22882. doi:10.18632/oncotarget.8251
82. Kong Q, Six DA, Liu Q, et al. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect Immun. 2012;80:3215–3224. doi:10.1128/IAI.00123-12
83. Siegall CB, FitzGerald DJ, Pastan I. Selective killing of tumor cells using EGF or TGF alpha-Pseudomonas exotoxin chimeric molecules. Semin Cancer Biol. 1990;1:345–50. 51.
84. Wright SE, Rewers-Felkins KA, Quinlin I, et al. TGFalpha-PE38 enhances cytotoxic T-lymphocyte killing of breast cancer cells. Oncol Lett. 2014;7:2113–2117. doi:10.3892/ol.2014.1969
85. Song M, Kim H-J, Ryu S, Yoon H, Yun J, Choy HE. ppGpp-mediated stationary phase induction of the genes encoded by horizontally acquired pathogenicity islands and cob/pdu locus in Salmonella enterica serovar Typhimurium. J Microbiol. 2010;48:89–95. doi:10.1007/s12275-009-0179-6
86. Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE, Min JJ. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi:10.1158/0008-5472.CAN-09-3453
87. Jeong JH, Kim K, Lim D, et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS One. 2014;9:e80050. doi:10.1371/journal.pone.0080050
88. Lim D, Kim KS, Kim H, et al. Anti-tumor activity of an immunotoxin (TGFalpha-PE38) delivered by attenuated Salmonella typhimurium. Oncotarget. 2017;8:37550–37560. doi:10.18632/oncotarget.17197
89. Mesa-Pereira B, Medina C, Camacho EM, Flores A, Santero E. Improved cytotoxic effects of Salmonella-producing cytosine deaminase in tumour cells. Microb Biotechnol. 2015;8:169–176. doi:10.1111/1751-7915.12153
90. Zhang L, Gao L, Zhao L, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res. 2007;67:5859–5864. doi:10.1158/0008-5472.CAN-07-0098
91. Chen G, Tang B, Yang BY, et al. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl Microbiol Biotechnol. 2013;97:4393–4401. doi:10.1007/s00253-012-4321-8
92. Ahmad S, Casey G, Cronin M, et al. Induction of effective antitumor response after mucosal bacterial vector mediated DNA vaccination with endogenous prostate cancer specific antigen. J Urol. 2011;186:687–693. doi:10.1016/j.juro.2011.03.139
93. Shao C, Yang B, Zhao L, Wang S, Zhang J, Wang K. Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J Surg Oncol. 2013;11:123. doi:10.1186/1477-7819-11-123
94. Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med. 2004;6:1382–1393. doi:10.1002/jgm.626
95. Tian Y, Guo B, Jia H, et al. Targeted therapy via oral administration of attenuated Salmonella expression plasmid-vectored Stat3- shRNA cures orthotopically transplanted mouse HCC. Cancer Gene Ther. 2012;19:393–401. doi:10.1038/cgt.2012.12
96. Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2ʹ-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008;15:474–484. doi:10.1038/cgt.2008.19
97. Fu W, Lan H, Liang S, Gao T, Ren D. Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2ʹ-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Sci. 2008;99:1172–1179. doi:10.1111/j.1349-7006.2008.00808.x
98. Zhao C, He J, Cheng H, Zhu Z, Xu H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J Exp Clin Cancer Res. 2016;35:107. doi:10.1186/s13046-016-0381-4
99. Lewen S, Zhou H, Hu HD, et al. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi:10.1007/s00262-007-0389-x
100. Chou CK, Hung JY, Liu JC, Chen CT, Hung MC. An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Ther. 2006;13:746–752. doi:10.1038/sj.cgt.7700927
101. Xiong G, Husseiny MI, Song L, et al. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int J Cancer. 2010;126:2622–2634. doi:10.1002/ijc.24957
102. Shi L, Yu B, Cai C-H, et al. Combined prokaryotic–eukaryotic delivery and expression of therapeutic factors through a primed autocatalytic positive-feedback loop. J Controll Release. 2016;222:130–140. doi:10.1016/j.jconrel.2015.12.005
103. Ye J, Li L, Zhang Y, Zhang X, Ren D, Chen W. Recombinant Salmonella-based 4-1BBL vaccine enhances T cell immunity and inhibits the development of colorectal cancer in rats: in vivo effects of vaccine containing 4-1BBL. J BioMed Sci. 2013;20:8. doi:10.1186/1423-0127-20-8
104. Le UN, Kim H-S, Kwon J-S, et al. Engineering and visualization of bacteria for targeting infarcted myocardium. Mol Ther. 2011;19:951–959. doi:10.1038/mt.2011.25
105. Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother. 2009;58:769–775. doi:10.1007/s00262-008-0555-9
106. Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clin Vaccin Immunol. 2011;18:23–34. doi:10.1128/CVI.00286-10
107. Shahabi V, Reyes-Reyes M, Wallecha A, et al. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1313. doi:10.1007/s00262-008-0463-z
108. Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/ neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56:927–938. doi:10.1007/s00262-006-0237-4
109. Meng JZ, Dong Y-J, Huang H, et al. Oral vaccination with attenuated Salmonella enterica strains encoding T-cell epitopes from tumor antigen NY-ESO-1 induces specific cytotoxic T-lymphocyte responses. Clin Vaccin Immunol. 2010;17:889–894. doi:10.1128/CVI.00044-10
110. Martínez‐García D, Manero‐Rupérez N, Quesada R, Korrodi‐Gregório L, Soto‐Cerrato V. Therapeutic strategies involving survivin inhibition in cancer. Med Res Rev. 2019;39(3):887–909. doi:10.1002/med.21547
111. Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44:1255–1269. doi:10.1016/j.immuni.2016.06.001
112. Tang W, He Y, Zhou S, Ma Y, Liu G. A novel Bifidobacterium infantis mediated TK/GCV suicide gene therapy system exhibits antitumor activity in a rat model of bladder cancer. J Exp Clin Cancer Res. 2009;28:155. doi:10.1186/1756-9966-28-155
113. Sasaki T, Fujimori M, Hamaji Y, et al. Genetically engineered Bifidobacterium longum for tumor targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 2006;97:649–657. doi:10.1111/j.1349-7006.2006.00221.x
114. Cheng CM, Lu Y-L, Chuang K-H, et al. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Ther. 2008;15:393–401. doi:10.1038/cgt.2008.10
115. Friedlos F, Lehouritis P, Ogilvie L, et al. Attenuated salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin Cancer Res. 2008;14:4259–4266. doi:10.1158/1078-0432.CCR-07-4800
116. Khan AA, Allemailem KS, Almatroodi SA, Almatroudi A, Rahmani AH. Recent strategies towards the surface modification of liposomes: an innovative approach for different clinical applications. 3 Biotech. 2020;10(4):1–5. doi:10.1007/s13205-020-2144-3
117. Han JW, Choi YJ, Cho S, et al.Active tumor-therapeutic liposomal bacteriobot combining a drug (paclitaxel)-encapsulated liposome with targeting bacteria (Salmonella typhimurium) sensors and actuators B. Sensors Actuat B. 2016;224:217–224.
118. Ektate K, Munteanu MC, Ashar H, Malayer J, Ranjan A. Chemo-immunotherapy of colon cancer with focused ultrasound and Salmonella-laden temperature sensitive liposomes (thermobots). Sci Rep. 2018;8. doi:10.1038/s41598-018-30106-4
119. Wang S, Gao J, Li M, Wang L, Wang Z, Facile A. Approach for development of a vaccine made of bacterial double-layered membrane vesicles (DMVs). Biomaterials. 2018;187:28–38. doi:10.1016/j.biomaterials.2018.09.042
120. MacDiarmid JA, Mugridge NB, Weiss JC, et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell. 2007;11:431–445. doi:10.1016/j.ccr.2007.03.012
121. MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, et al. Sequential treatment of drug-resistant tumors with targeted minicells containing SiRNA or a cytotoxic drug. Nat Biotechnol. 2009;27:643–651. doi:10.1038/nbt.1547
122. Gujrati V, Kim S, Kim S-H, et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8:1525–1537. doi:10.1021/nn405724x
123. Qing S, Lyu C, Zhu L, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32:2002085. doi:10.1002/adma.202002085
124. Peng L-H, Wang MZ, Chu Y, et al. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci Adv. 2020;6:eaba2735. doi:10.1126/sciadv.aba2735
125. Huang W, Shu C, Hua L, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–312. doi:10.1016/j.actbio.2020.03.030
126. Kim OY, Dinh NTH, Park HT, Choi SJ, Hong K, Gho YS. Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics. Biomaterials. 2017;113:68–79. doi:10.1016/j.biomaterials.2016.10.037
127. Gao J, Wang S, Dong X, Wang Z. RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting. Theranostics. 2021;11:3301–3316. doi:10.7150/thno.51988
128. Chen Q, Bai H, Wu W, et al. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2020;20:11–21. doi:10.1021/acs.nanolett.9b02182
129. Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS. Antineoplastic activity of salmonella typhimurium outer membrane nanovesicles. Exp Cell Res. 2021;399:112423. doi:10.1016/j.yexcr.2020.112423
130. Kim OY, Park HT, Dinh NTH, et al. Bacterial outer membrane vesicles suppress tumor by interferon- -mediated antitumor response. Nat Commun. 2017;8:626. doi:10.1038/s41467-017-00729-8
131. Wang D, Liu C, You S, et al. Bacterial vesicle-cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl Mater Interfaces. 2020;12:41138–41147. doi:10.1021/acsami.0c13169
132. Luo Y, Xu D, Gao X, et al. Nanoparticles conjugated with bacteria targeting tumors for precision imaging and therapy. Biochem Biophys Res Commun. 2019;514:1147–1153. doi:10.1016/j.bbrc.2019.05.074
133. Barb S, Van Mellaert L, Ann J. The use of clostridial spores for cancer treatment. J Appl Microbiol. 2006;101:571–578. doi:10.1111/j.1365-2672.2006.02886.x
134. Kazmierczak R, Choe E, Sinclair J, Eisenstark A. Direct attachment of nanoparticle cargo to Salmonella typhimurium membranes designed for combination bacteriotherapy against tumors. In: Salmonella. New York, NY: Humana Press; 2015:151–163.
135. Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi:10.1016/j.cis.2010.02.001
136. Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int J Mol Sci. 2018;19:4100. doi:10.3390/ijms19124100
137. Srivastava P, Bragança J, Ramanan SR, Kowshik M. Synthesis of silver nanoparticles using haloarchaeal isolate Halococcus salifodinae BK3. Extremophil Life Under Extreme Conditions. 2013;17:821–831. doi:10.1007/s00792-013-0563-3
138. Li J, Webster TJ, Tian B. Functionalized nanomaterial assembling and biosynthesis using the extremophile Deinococcus radiodurans for multifunctional applications. Small. 2019;15:e1900600. doi:10.1002/smll.201900600
139. Patil MP, Kim GD. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloid Surf B Biointerfaces. 2018;172:487–495. doi:10.1016/j.colsurfb.2018.09.007
140. Saratale RG, Karuppusamy I, Saratale GD, et al. A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloid Surf B Biointerfaces. 2018;170:20–35. doi:10.1016/j.colsurfb.2018.05.045
141. Kalaiselvan V, Rajasekaran A. Biosynthesis of silver nanoparticles from Aspergillus Niger and evaluation of its wound healing activity in experimental rat model. IJPRIF. 2009;1:1523–1529.
142. Jeyaraj M, Renganathan A, Sathishkumar G, et al. Biogenic metal nanoformulations induce Bax/Bcl2 and caspase mediated mitochondrial dysfunction in human breast cancer cells (MCF-7). RSC Adv. 2015;5:2159–2166. doi:10.1039/C4RA11686K
143. Ashraf N, Ahmad F, Da-wei L, Zhou RB, Feng-Li H, Yin DC. Iron/iron oxide nanoparticles: advances in microbial fabrication, mechanism study, biomedical, and environmental applications. Crit Rev Microbiol. 2019;45:278–300. doi:10.1080/1040841X.2019.1593101
144. Firlar E, Ouy M, Bogdanowicz A, et al. Investigation of the magnetosome biomineralization in magnetotactic bacteria using graphene liquid cell - transmission electron microscopy. Nanoscale. 2019;11:698–705. doi:10.1039/C8NR08647H
145. Vargas G, Cypriano J, Correa T, Leão P, Bazylinski DA, Abreu F. Applications of magnetotactic bacteria, magnetosomes and magnetosome crystals in biotechnology and nanotechnology: mini-review. Molecules. 2018;23:2438. doi:10.3390/molecules23102438
146. Tanaka T, Kokuryu Y, Matsunaga T. Novel method for selection of antimicrobial peptides from a phage display library by use of bacterial magnetic particles. Appl Environ Microbiol. 2008;74:7600–7606. doi:10.1128/AEM.00162-08
147. Geng Y, Wang J, Wang X, et al. Growth-inhibitory effects of anthracycline-loaded bacterial magnetosomes against hepatic cancer in vitro and in vivo. Nanomedicine. 2019;14:1663–1680. doi:10.2217/nnm-2018-0296
148. Kuzajewska D, Wszołek A, Zwierełło W, Kirczuk L, Maruszewska A. Magnetotactic bacteria and magnetosomes as smart drug delivery systems: a new weapon on the battlefield with cancer? Biology. 2020;9:102. doi:10.3390/biology9050102
149. Gandia D, Gandarias L, Rodrigo I, et al. Unlocking the potential of magnetotactic bacteria as magnetic hyperthermia agents. Small. 2019;15:e1902626. doi:10.1002/smll.201902626
150. Erdal E, Demirbilek M, Yeh Y, et al. A comparative study of receptor-targeted magnetosome and HSA-coated iron oxide nanoparticles as MRI contrast-enhancing agent in animal cancer model. Appl Biochem Biotechnol. 2018;185:91–113. doi:10.1007/s12010-017-2642-x
151. Felfoul O, Mohammadi M, Taherkhani S, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11:941–947. doi:10.1038/nnano.2016.137
152. Xiang L, Bin W, Huali J, et al. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J Gene Med. 2007;9:679–690. doi:10.1002/jgm.1068
153. Tang YS, Wang D, Zhou C, Zhang S. Preparation and anti-tumor efficiency evaluation of bacterial magnetosome-anti-4–1BB antibody complex: bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol Appl Biochem. 2019;66:290–297. doi:10.1002/bab.1724
154. Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159–1166. doi:10.1016/j.copbio.2013.02.020
155. Nima ZA, Watanabe F, Jamshidi-Parsian A, et al. Bioinspired magnetic nanoparticles as multimodal photoacoustic, photothermal and photomechanical contrast agents. Sci Rep. 2019;9:887. doi:10.1038/s41598-018-37353-5
156. Liu L, He H, Luo Z, et al. In situ photocatalyzed oxygen generation with photosynthetic bacteria to enable robust immunogenic photodynamic therapy in triple-negative breast cancer. Adv Funct Mater. 2020;30:1910176. doi:10.1002/adfm.201910176
157. Vidya SM, Mutalik S, Bhat KU, Huilgol P, Avadhani K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016;153:171–179. doi:10.1016/j.lfs.2016.04.022
158. Deng X, Yang W, Shao Z, Zhao Y. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials. 2021;272:120809. doi:10.1016/j.biomaterials.2021.120809
159. Akin D, Sturgis J, Ragheb K, et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotech. 2007;2:441–449. doi:10.1038/nnano.2007.149
160. Chen F, Zang Z, Chen Z, et al. Nanophotosensitizer-engineered salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019;214:119226. doi:10.1016/j.biomaterials.2019.119226
161. Portilla-Arias JA, Camargo B, García-Alvarez M, de Ilarduya AM, Muñoz-Guerra S. Nanoparticles made of microbial poly(gamma-glutamate) s for encapsulation and delivery of drugs and proteins. J Biomater Sci Polym. 2009;20:1065–1079. doi:10.1163/156856209X444420
162. Suh S, Jo A, Traore MA, et al. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci. 2019;6:1801309. doi:10.1002/advs.201801309
163. Fan J, Peng M, Wang H, et al. Engineered bacterial bioreactor for tumor therapy via fenton-like reaction with localized H2O2 generation. Adv Mater. 2019;31:1808278. doi:10.1002/adma.201808278
164. Rajinikanth PS, Mishra B. Stomach-site specific drug delivery system of clarithromycin for eradication of Helicobacter pylori. Chem Pharm Bull. 2009;57:1068–1075. doi:10.1248/cpb.57.1068
165. Sivakumar B, Aswathy RG, Sreejith R, et al. Bacterial exopolysaccharide based magnetic nanoparticles: a versatile nanotool for cancer cell imaging, targeted drug delivery and synergistic effect of drug and hyperthermia mediated cancer therapy. J Biomed Nanotechnol. 2014;10:885–899. doi:10.1166/jbn.2014.1820
166. Dai Q, Long R, Wang S, et al. Bacterial magnetosomes as an efficient gene delivery platform for cancer theranostics. Microb Cell Fact. 2017;16:216. doi:10.1186/s12934-017-0830-6
167. Cheng L, Ke Y, Yu S, Jing J. Co-delivery of doxorubicin and recombinant plasmid pHSP70-Plk1-shRNA by bacterial magnetosomes for osteosarcoma therapy. Int J Nanomed. 2016;11:5277–5286. doi:10.2147/IJN.S115364
168. Long R, Liu Y, Dai Q, Wang S, Deng Q, Zhou X. A natural bacterium-produced membrane-bound nanocarrier for drug combination therapy. Materials. 2016;9:889. doi:10.3390/ma9110889
169. Kotelnikova PA, Shipunova VO, Aghayeva UF, et al. Synthesis of magnetic nanoparticles stabilized by magnetite-binding protein for targeted delivery to cancer cells. Doklady Biochem Biophys. 2018;481:198–200. doi:10.1134/S1607672918040051
170. Zheng D-W, Chen Y, Li Z-H, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun. 2018;9:1680. doi:10.1038/s41467-018-03233-9
171. Raveendran S, Poulose AC, Yoshida Y, Maekawa T, Kumar DS. Bacterial exopolysaccharide based nanoparticles for sustained drug delivery, cancer chemotherapy and bioimaging. Carbohydr Polym. 2013;91:22–32. doi:10.1016/j.carbpol.2012.07.079
172. Chen Q, Wang J, Wang X, et al. Inhibition of tumor progression through the coupling of bacterial respiration with tumor metabolism. Angew Chem Int Ed. 2020;59:21562–21570. doi:10.1002/anie.202002649
173. Weerakkody LR, Witharana C. The role of bacterial toxins and spores in cancer therapy. Life Sci. 2019;235:116839. doi:10.1016/j.lfs.2019.116839
174. Diaz LA, Cheong I, Foss CA, et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol Sci. 2005;88(2):562–575. doi:10.1093/toxsci/kfi316
175. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4(9):548–556. doi:10.1016/S1470-2045(03)01194-X
176. Yang H, Jiang F, Ji X, et al. Genetically engineered bacterial protein nanoparticles for targeted cancer therapy. Int J Nanomedicine. 2021;16:105. doi:10.2147/IJN.S292432
177. Schnell C. Gas vesicles enable ultrasound imaging. Nat Methods. 2018;15(3):159. doi:10.1038/nmeth.4621
178. Mehta N, Lyon JG, Patil K, Mokarram N, Kim C, Bellamkonda RV. Bacterial carriers for glioblastoma therapy. Mol Ther Oncolytics. 2017;4:1–7. doi:10.1016/j.omto.2016.12.003
179. Bourdeau RW, Lee-Gosselin A, Lakshmanan A, et al. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts. Nature. 2018;553(7686):86–90. doi:10.1038/nature25021
180. Martín M, Carmona F, Cuesta R, Rondón D, Gálvez N, Domínguez‐Vera JM. Artificial magnetic bacteria: living magnets at room temperature. Adv Funct Mater. 2014;24(23):3489–3493. doi:10.1002/adfm.201303754
181. Nemani KV, Ennis RC, Griswold KE, Gimi B. Magnetic nanoparticle hyperthermia induced cytosine deaminase expression in microencapsulated E. coli for enzyme–prodrug therapy. J Biotechnol. 2015;203:32–40. doi:10.1016/j.jbiotec.2015.03.008
182. Wu MR, Jusiak B, Lu TK. Engineering advanced cancer therapies with synthetic biology. Nat Rev Cancer. 2019;19(4):187–195. doi:10.1038/s41568-019-0121-0
183. Coley WB. Contribution to the knowledge of sarcoma. Ann Surg. 1891;14:199–220. doi:10.1097/00000658-189112000-00015
184. Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18:727–743. doi:10.1038/s41568-018-0070-z
185. Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi:10.1200/JCO.2002.20.1.142
186. Heimann DM, Rosenberg SA. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J Immunother (1991). 2003;26:179–180. doi:10.1097/00002371-200303000-00011
187. Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and listeria monocytogenes–expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi:10.1200/JCO.2014.57.4244
188. Basu P, Mehta A, Jain M, et al. A randomized Phase 2 study of ADXS11-001 listeria monocytogenes-Listeriolysin O immunotherapy with or without cisplatin in treatment of advanced cervical cancer. Int J Gynecol Cancer. 2018;28:764–772. doi:10.1097/IGC.0000000000001235
189. Schmitz-Winnenthal FH, Hohmann N, Schmidt T, et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology. 2018;7:e1303584. doi:10.1080/2162402X.2017.1303584
190. Nikolova MP, Chavali MS. Metal oxide nanoparticles as biomedical materials. Biomimetics. 2020;5:27. doi:10.3390/biomimetics5020027
191. Shevtsov M, Nikolaev B, Marchenko Y, et al. Targeting experimental orthotopic glioblastoma with chitosan based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int J Nanomed. 2018;13:1471–1482. doi:10.2147/IJN.S152461
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.