Back to Journals » OncoTargets and Therapy » Volume 12
Initial Therapy of Advanced Anaplastic Thyroid Cancer via Targeting VEGFR-2: A Case Report
Authors Cheng L, Jiao Q, Jin Y, Fu H, Zhang H, Chen L
Received 18 July 2019
Accepted for publication 13 November 2019
Published 2 December 2019 Volume 2019:12 Pages 10495—10500
DOI https://doi.org/10.2147/OTT.S223727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Lin Cheng,1 Qiong Jiao,2 Yuchen Jin,1 Hao Fu,1 Huizhen Zhang,2 Libo Chen1
1Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China; 2Department of Pathology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
Correspondence: Libo Chen
Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yishan Road, Shanghai 200233, People’s Republic of China
Tel +86-21-24058871
Fax +86-21-64941720
Email [email protected]
Abstract: Preclinical studies have demonstrated that Apatinib, major targeting vascular endothelial growth factor receptor-2 (VEGFR-2), could inhibit the proliferation of anaplastic thyroid carcinoma (ATC) cells in vitro and in vivo. The efficacy and safety in ATC patients, however, remains unknown. Here, we report the case of a 93-year-old female with advanced ATC who initially treated with Apatinib. The tumor shrank notably 4 weeks after the initiation of therapy, which sustained for more than 30 weeks. The cervical CT illuminated a stable disease with a best response of 19.7% of the primary lesion and shrinkage of the metastatic lymph node. Adverse events, including hypertension, dental ulcer, hand-foot syndrome, fatigue, and anorexia, were observed and lightened with supportive treatment and dose reductions. The overall survival of the patient was 41 weeks. This is the first report describing the effectiveness of the VEGFR-2 inhibitor for the treatment of advanced ATC, warranting clinical trials to further ascertain its utility in this challenging setting.
Keywords: anaplastic thyroid carcinoma, vascular endothelial growth factor receptor, Apatinib
Introduction
Anaplastic thyroid carcinoma (ATC), one of the most lethal malignant tumors, is characterized by rapid proliferation, extrathyroidal invasion, and distant metastasis. It is the major cause of thyroid carcinoma-related deaths, with a median survival of 5 months and a 1-year survival rate of 20%.1 Surgery and chemoradiation are recommended if the tumor were locoregionally confined,1–3 but more than half of all patients present with advanced disease at the time of diagnosis, and the efficacies of traditional therapies are very poor.4,5 Therefore, new therapeutic strategies urgently need to be explored.
Apatinib, a tyrosine kinase inhibitor, can inhibit multiple tumor-related kinases, such as vascular endothelial growth factor receptor-2 (VEGFR-2), c-Kit, and c-Src6. Our group and others have investigated its safety and efficacy in radioiodine-refractory differentiated thyroid cancer (RR-DTC) patients, which demonstrated an overwhelming metabolic and structural response and tolerable toxicity.7–9 Moreover, preclinical studies demonstrated that Apatinib could inhibit the proliferation of ATC cells in a dose- and time-dependent manner, suggesting a potential in the treatment of patients with ATC.10,11 We, hereby, report an initial attempt to clinically treat ATC with Apatinib.
Case Presentation
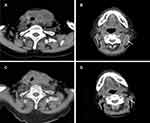
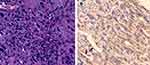
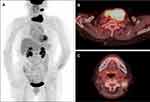
A 93-year-old woman with a rapidly growing left-sided neck mass and hoarseness was referred to our department. Baseline computed tomography images showed a 7.6 × 4.2 cm thyroid mass involving the trachea (Figure 1). Laryngoscope indicated left vocal cord fixation. An ultrasound-guided core-needle puncture followed by pathological examinations including immunohistochemical studies with negative for Epithelial Membrane Antigen, Thyroglobulin, Thyroid Transcription Factor-1, Cytokeratin (CK) 19, CK 20 and Villin, but positive for CKpan, Vimentin, CK 7, Ki 67 (60% +), which revealed the diagnosis of ATC with positive expression of VEGFR-2 (Figure 2; rabbit polyclonal antibody, 1:100 dilution; ZSGB-BIO, China). The staging was performed with a positron emission tomography/CT fusion image showing the hypermetabolic thyroid mass and a left lateral neck lymph node metastasis (Figure 3).
After the Eastern Cooperative Oncology Group performance status of 3 was obtained, the patient was then started on 250 mg Apatinib twice a day as an off-label use with ethical permission and informed consent in January 2018. The mass shrank notably 4 weeks after the initiation of therapy (Figure 4). Along with the cheerful effect, some unpleasant side effects emerged after 2 weeks of treatment, which were evaluated by Common Terminology Criteria for Adverse Events Version 4.0.12 Hypertension first appeared with the highest blood pressure of 170/100 mmHg (grade 3, which was elevated compared to the blood pressure of <150/90mmHg before Apatinib initiation). A dental ulcer (grade 2) and hand-foot syndrome (grade 3) caused notable pain. Other adverse events included fatigue (grade 3) and anorexia (grade 3). Compromising of these undesirable effects, the patient immediately received a calcium ion antagonist (amlodipine, 5 mg daily) and an external use hormone ointment (fluocinonide ointment, twice a day), and a reduced dose of 250 mg daily 4 weeks after the beginning of the treatment was applied. The patient tolerated treatment well thereafter, except for refractory anorexia.
The therapeutic response sustained more than 30 weeks when cervical CT illuminated a stable disease with a best response of 19.7% of baseline in the longest diameter of the primary lesion and shrinkage of metastatic lymph node according to Response Evaluation Criteria in Solid Tumors version 1.1 (Figure 1). No progression evidence was found during the Apatinib treatment. Unfortunately, she died from pneumonia and respiratory failure when the total duration of Apatinib treatment was 41 weeks.
Discussion
In all thyroid cancer types, the prognosis of ATC is most dismal, and the management of ATC remains most challenging. Although previous analyses have reported that the extent of traditional therapeutics, such as surgery, radiotherapy, and chemotherapy, may be associated with the length of survival,13,14 the overall therapeutic effect is awful.
Our patient was not amenable to the above conventional treatment options considering her old age and locally advanced disease at the time of diagnosis.15 New therapeutic strategies were urgently needed. Since increased VEGFR expression had been found in the microvascular endothelial cells of ATC tumor specimens,16 and agents targeting VEGFR could block the effects of vascular endothelial growth factor and play antiangiogenic and antitumor roles in solid tumors,6,17 we hypothesized that agents targeting VEGFR may also play an antitumor role in ATC patients.
Apatinib, a novel tyrosine kinase inhibitor that has highly selective competition in the ATP binding site of VEGFR-2, blocks down pathways and inhibits tumor angiogenesis.18,19 It also mildly inhibits c-Kit and c-Src.20 Preclinical studies have also demonstrated that Apatinib plays an important antitumor role in ATC via suppressing the AKT/GSK3β/ANG signal pathway,10,11 which can be activated when VEGFR-2 activates the phosphatidylinositol 3-kinase.21 Moreover, it has been demonstrated that overwhelming efficacy has been achieved in RR-DTC, and ATC tumors evolve from a background of DTC.7,22 All the above evidence favored Apatinib as a choice for this ATC patient. Additionally, the value of gene examinations needs to be thoroughly evaluated in decision-making using an appropriate sample size of ATC patients since they have succeeded in methodology.23
As a result, gratifying outcomes were achieved regarding a durable response and overall survival of more than 30 and 41 weeks, respectively. Although the degree of tracheal stenosis was slightly more severe than that before treatment due to tumor invasion, the longest diameter of the tumor diminished from 7.6 cm to 6.1 cm. This endeavor may pave a way to new adjuvant therapy for surgery, which has been previously reported in RR-DTC by our team.9 Hypertension, hand-foot syndrome, dental ulcer, fatigue, and decreased appetite were observed, which are known classical side effects of VEGFR-targeted treatments,17 which were lightened with supportive treatment and dose reductions as reported by our group and others.24,25 Pneumonia and respiratory failure beyond of known Apatinib-induced adverse events were considered as the causes of death instead of complications related to the treatment, which may be further explained by decreased cough reflex associated with severely aging, difficulty of expectoration due to tracheal stenosis, and the absence of objective disease progression.17,26
Lenvatinib, another multikinase inhibitor, has been approved for utilization in patients with unresectable ATC in Japan. A phase II clinical trial revealed a median OS of 10.6 months and response rate with partial response and stable disease in 4/17 and 12/17 patients, respectively.27 Another study enrolled 3 patients; 2 were stable, and 1 had a mixed response to Lenvatinib therapy.28 Additionally, for those with BRAF-mutated ATC, the FDA has approved the combination of Dabrafenib and Trametinib for treatment as of May 2018.29 However, these agents are commercially unavailable or unapproved in China by now. In addition, resistance to the above drugs is a common challenge, thereby necessitating second-line or salvage therapies.27,30–33
Conclusion
To the best of our knowledge, this is the first case study of Apatinib treatment for ATC. The prompt and sustained response suggests the possibility that inhibitors targeting VEGFR-2 may be a novel option to control advanced ATC, warranting clinical verifications with larger sample sizes.
Ethics Approval and Consent
The study was under permission of the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was received from the patient for publication including the accompanying data.
Acknowledgment
The authors thank the patient and her family members for their participation in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22(6):486–497. doi:10.1016/j.clon.2010.03.013
2. Prasongsook N, Kumar A, Chintakuntlawar AV, et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metabol. 2017;102(12):4506–4514. doi:10.1210/jc.2017-01180
3. Foote RL, Molina JR, Kasperbauer JL, et al. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid. 2011;21(1):25–30. doi:10.1089/thy.2010.0220
4. Ain KB, Egorin MJ, Desimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel phase 2 trial using ninety-six-hour infusion collaborative anaplastic thyroid cancer health intervention trials. Thyroid. 2000;10:587–594. doi:10.1089/thy.2000.10.587
5. Kawada K, Kitagawa K, Kamei S, et al. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol. 2010;40(6):596–599. doi:10.1093/jjco/hyq025
6. Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:1. doi:10.1186/1471-2407-10-529
7. Lin Y, Wang C, Gao W, Cui R, Liang J. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget. 2017;8(26):42252–42261. doi:10.18632/oncotarget.15036
8. Chen W, Xin Z, Xue Y, et al. PET response assessment in apatinib-treated radioactive iodine-refractory thyroid cancer. Endocr Relat Cancer. 2018;25(6):653–663. doi:10.1530/ERC-18-0007
9. Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. 2018;125:111–120. doi:10.1016/j.critrevonc.2018.03.012
10. Jin Z, Cheng X, Feng H, et al. Apatinib inhibits angiogenesis via suppressing Akt/GSK3beta/ANG signaling pathway in anaplastic thyroid cancer. Cell Physiol Biochem. 2017;44(4):1471–1484. doi:10.1159/000485583
11. Feng H, Cheng X, Kuang J, et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018;9(10):1030. doi:10.1038/s41419-018-1054-3
12. Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013;98(1):31–42. doi:10.1210/jc.2012-2909
13. Swaak-Kragten AT, de Wilt JH, Schmitz PI, Bontenbal M, Levendag PC. Multimodality treatment for anaplastic thyroid carcinoma–treatment outcome in 75 patients. Radiother Oncol. 2009;92(1):100–104. doi:10.1016/j.radonc.2009.02.016
14. Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006;107(8):1786–1792. doi:10.1002/(ISSN)1097-0142
15. Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. doi:10.1089/thy.2012.0302
16. Viglietto G, Maglione D, Rambaldi M, et al. Upregulation of vascular endothelial growth factor (VEGF) and downregulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene. 1995;11(8):1569–1579.
17. Hu X, Cao J, Hu W, et al. Multicenter phase II study of Apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14(1):820. doi:10.1186/1471-2407-14-820
18. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi:10.1200/JCO.2005.06.081
19. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi:10.1111/j.1349-7006.2011.01939.x
20. Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–229. doi:10.1358/dot.2015.51.4.2320599
21. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi:10.1038/nrm1911
22. Van der Laan B, Freeman JL, Tsang RW, Sylvia L, The association of well-differentiated thyroid carcinoma with insular or anaplastic thyroid carcinoma: evidence for dedifferentiation in tumor progression. Endocr Pathol. 1993;4:215–221. doi:10.1007/BF02915464
23. van der Tuin K, Ventayol Garcia M, Corver WE, et al. Targetable gene fusions identified in radioactive iodine refractory advanced thyroid carcinoma. Eur J Endocrinol. 2019;180(4):235–241. doi:10.1530/EJE-18-0653
24. Zhang X, Wang C, Lin Y. Pilot dose comparison of Apatinib in Chinese patients with progressive radioiodine-refractory differentiated thyroid cancer. J Clin Endocrinol Metabol. 2018;103(10):3640–3646. doi:10.1210/jc.2018-00381
25. Liu M, Shen Y, Ruan M, Li M, Chen L. Notable decrease of malignant pleural effusion after treatment with sorafenib in radioiodine-refractory follicular thyroid carcinoma. Thyroid. 2014;24(7):1179–1183. doi:10.1089/thy.2013.0703
26. Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190(1):29–33. doi:10.1007/s00408-011-9334-z
27. Tahara M, Kiyota N, Yamazaki T, et al. Lenvatinib for anaplastic thyroid cancer. Front Oncol. 2017;7:25. doi:10.3389/fonc.2017.00025
28. Iniguez-Ariza NM, Ryder MM, Hilger CR, Bible KC. Salvage lenvatinib therapy in metastatic anaplastic thyroid cancer. Thyroid. 2017;27(7):923–927. doi:10.1089/thy.2016.0627
29. Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7–13. doi:10.1200/JCO.2017.73.6785
30. Iyer PC, Dadu R, Gule-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6(1):68. doi:10.1186/s40425-018-0378-y
31. Iyer PC, Dadu R, Ferrarotto R, et al. Real-world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid. 2018;28(1):79–87. doi:10.1089/thy.2017.0285
32. Kollipara R, Schneider B, Radovich M, Babu S, Kiel PJ. Exceptional response with immunotherapy in a patient with anaplastic thyroid cancer. Oncologist. 2017;22(10):1149–1151. doi:10.1634/theoncologist.2017-0096
33. Dadu R, Devine C, Hernandez M, et al. Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab. 2014;99(6):2086–2094. doi:10.1210/jc.2013-3588
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.