Back to Journals » Clinical Ophthalmology » Volume 17
Initial Real-World Experience with Faricimab in Treatment-Resistant Neovascular Age-Related Macular Degeneration
Authors Leung EH, Oh DJ, Alderson SE, Bracy J, McLeod M, Perez LI, Bottini A, Chin Yee D, Mukkamala K
Received 23 February 2023
Accepted for publication 28 April 2023
Published 5 May 2023 Volume 2023:17 Pages 1287—1293
DOI https://doi.org/10.2147/OPTH.S409822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ella H Leung, Daniel J Oh, Shannon E Alderson, Joshlynn Bracy, Mia McLeod, Litzi I Perez, Alexander Bottini, David Chin Yee, Krishna Mukkamala
Georgia Retina, Atlanta, GA, USA
Correspondence: Ella H Leung, 833 Campbell Hill St NW, Suite 300, Marietta, GA, 30060, USA, Tel +1 770-218-1888, Email [email protected]
Purpose: To evaluate the initial efficacy and safety of intravitreal faricimab in eyes previously treated for neovascular age-related macular degeneration (nARMD).
Patients and methods: A retrospective review of all patients with nARMD previously treated with anti-vascular endothelial growth factor (anti-VEGF) injections who received at least 3 intravitreal faricimab injections with at least 3 months of follow-up.
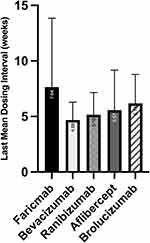
Results: A total of 190 eyes were included. Patients received a mean of 34.2± 23 anti-VEGF injections over 182.41± 128 weeks prior to switching to faricimab. Patients then received a mean of 6.99± 2.3 faricimab injections with an average 34.88± 8.2 weeks of follow-up. The mean best corrected visual acuities improved from 0.33± 0.32 logMAR ≈20/43 to 0.27± 0.32 logMAR ≈20/37 (P=0.0022). The central subfield thickness (CST) improved from 312± 87μm to 287± 71μm (P< 0.0001). At the last clinical visit, 24% had no subretinal fluid or intraretinal fluid on optical coherence tomography. The mean dosing interval between the last two consecutive faricimab injections (7.64± 6.2 weeks) was significantly longer than that for ranibizumab (5.16± 2.0 weeks, P< 0.001) or aflibercept (5.57± 3.6 weeks, P< 0.001). No patients developed idiopathic intraocular inflammation.
Conclusion: Intravitreal faricimab was associated with improved vision and CSTs, even in treatment-resistant nARMD eyes. The mean last dosing interval for faricimab was longer than for ranibizumab or aflibercept. No significant adverse events were directly attributed to faricimab during the study.
Keywords: faricimab, intravitreal injection, neovascular age-related macular degeneration, intraocular inflammation, anti-vascular endothelial growth factor
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) therapies have helped decrease the risk of legal blindness from neovascular age-related macular degeneration (nARMD) by approximately 50%.1 The Food and Drug Administration (FDA) approved faricimab (Vabysmo, RG7716, Roche/ Genentech, Basel, Switzerland) for the treatment of nARMD and DME on January 28, 2022. Faricimab inhibits both VEGF-A and angiopoietin (ang)-2 receptors; Ang-2 is produced in response to hypoxic stress, interrupting the endothelial cell barrier and contributing to neovascularization.1
In the phase 3 clinical trials for faricimab (TENAYA and LUCERNE), patients were given four monthly injections and randomized to either fixed dosing regimens or a personalized treatment interval (PTI) that allowed treatment extension if they met study criteria, even in the presence of subretinal fluid (SRF) or intraretinal fluid (IRF).2,3 While some SRF may be tolerated without adversely affecting the vision,3 14–20% of the 2020 American Society of Retina Specialists survey respondents reported that they would not tolerate any cystoid spaces in patients with nARMD and would adjust their treatment accordingly.4 At 48 weeks, faricimab was found to be non-inferior to aflibercept in improving the visual acuity and CST, with similar risks of ocular adverse events, including intraocular inflammation (IOI).2
The potential risk of IOI has led to a more conservative approach to adopting newer therapies. An independent Safety Review Committee found that brolucizumab (Beovu, Novartis Pharmaceuticals Corporation, East Hanover, NJ) was associated with a 5% risk of IOI, with 0.7% developing moderate vision loss, compared to a 1% risk of IOI and 0.1% risk of moderate vision loss with aflibercept (Eylea, Regeneron Pharmaceuticals, Inc., Tarrytown, NY).5 The FDA did not approve abicipar pegol (Abicipar, Allergan, Troy Hills, NJ) due to the risk of IOI, even after the manufacturing process was modified to reduce the risk from 15% to 9%.6,7 The potential for intraocular inflammation and the differences between research and clinical practice dosing regimens highlight the importance of reviewing post-marketing data to assess efficacy and safety.
The purpose of the current study was to evaluate the initial efficacy and safety of intravitreal faricimab in eyes previously treated for neovascular age-related macular degeneration.
Methods
A retrospective review was performed of all patients who had received an initial intravitreal faricimab at a large retina-only practice from February to August 2022. Inclusion criteria consisted of previously-treated nARMD eyes that had received at least 3 injections of intravitreal faricimab with at least 3 months of follow-up. Exclusion criteria included a history of uveitis, off-label use of faricimab, current enrollment in a clinical trial, incomplete medical records, or insufficient follow-up. The Sterling Institutional Review Board (IRB) waived the IRB approval for the retrospective study. The study and data handling complied with all local and federal laws, the Health Insurance Portability and Accountability Act (HIPAA), and the tenants of the Declaration of Helsinki.
Patient demographics were analyzed, including age, systemic and ocular comorbidities, treatment effects and complications, best corrected visual acuities (BCVA), and CSTs. The macula was deemed “dry” when there was no SRF, no IRF, and no subretinal hemorrhages noted on exam or optical coherence tomography (OCT) imaging. Since patients were often on a treat and extend protocol with varying treatment intervals, the number of weeks between the last two consecutive injections of the same medication was used to estimate the approximate last tolerated treatment interval for each medication. Snellen visual acuities were converted to their logMAR equivalents, with count fingers vision being assigned a value of 1.88 logMAR (≈20/1500), hand motion 2.30 logMAR (≈20/4000),8 light perception 2.70 logMAR, and no light perception or enucleation/ evisceration 3.0 logMAR. The data was analyzed with GraphPad Prism 9 (GraphPad Software, San Diego, CA), and the appropriate parametric or non-parametric tests were used and indicated, with a P value of less than 0.05 being considered statistically significant.
Results
Of the 210 eyes that had received faricimab in the first 6 months after initial FDA approval, twenty eyes were excluded due to insufficient follow-up (n=10), off-label use for vein occlusions (n=6), less than 3 faricimab injections (n=3), and enrollment in a clinical trial (n=1). A total of 190 eyes in 186 patients were therefore included.
The mean age for all patients was 80.1±8.1 years. Approximately 41% were female, 47% were right eyes, and 73% were pseudophakic. The majority of the nARMD patients were Caucasian (93%), followed by 4% African American, and 3% Asian ethnicities.
The mean total follow-up was 215.12±130 weeks. Patients had previously received a mean of 34.2±23 ocular injections over 182.41±128 weeks prior to being switched to faricimab, including an average of 2 different types of anti-VEGFs. Seventy eyes (37%) had ever received bevacizumab (Avastin, Genentech Inc., South San Francisco, CA), with a mean of 2.16±6.1 prior bevacizumab injections per eye, at a mean interval of 4.68±1.6 weeks. Six eyes (3%) were on bevacizumab immediately prior to switching to faricimab, and 29% had no SRF or IRF at the last bevacizumab injection. A total of 127 eyes (67%) had ever received ranibizumab (Lucentis, Genentech Inc., South San Francisco, CA), with an average of 12.6±16 injections, at a mean last dosing interval of 5.16±2.0 weeks. Sixty-three eyes (33%) were on ranibizumab immediately before changing to faricimab, with 34% being dry at the last ranibizumab injection. A total of 129 eyes (68%) had ever received aflibercept, with a mean of 27.0±21 prior injections at an average interval of 5.57±3.6 weeks. One hundred and twelve eyes (59%) were treated with aflibercept immediately before switching to faricimab, with 29% being dry. The last mean dosing intervals were similar between aflibercept and ranibizumab (P=0.60, Mann–Whitney). Twenty-six eyes (14%) had ever received brolucizumab, with a mean of 5.77±4.7 injections, at a mean interval of 6.18±2.6 weeks, with 5% being on brolucizumab immediately prior to faricimab, and 4% were dry at the last brolucizumab injection.
The decision to switch from another anti-VEGF agent to faricimab was at the discretion of the treating physician, most commonly due to persistent fluid (64%) and/or an inability to extend the treatment interval beyond 4–6 weeks. There was no pre-set dosing protocol, but patients received approximately monthly (5.16±2.8 weeks) doses of faricimab for the first 3 injections, with the dosing intervals being increased by approximately 2 weeks when there was no fluid present or there was a minimal stable amount of fluid on OCT based on volume, location, and CST, as determined by the treating physician.
A total of 1328 faricimab injections were administered, with an average follow-up of 34.88±8.2 weeks between the initial faricimab and the last clinical visit. There was a mean of 6.99±2.3 intravitreal faricimab injections per eye. Approximately 38% achieved resolution of their SRF/IRF at least once during the follow-up period, occurring after an average of 3.01±2.0 faricimab injections, and 45% remained dry on subsequent treat and extend regimens. At the last follow-up visit, 24% of all patients had no SRF/IRF in their maculas.
The mean last dosing interval for faricimab was 7.64±6.2 weeks, which was longer than for ranibizumab (P<0.0001, Wilcoxon matched-pairs signed rank test and Mann–Whitney) or aflibercept (P<0.0001, Wilcoxon matched-pair and Mann–Whitney, Figure 1). The dosing interval was 12 weeks or longer in 14 patients (7%) and longer than 16 weeks in 5 patients (3%) at the last visit.
Faricimab was discontinued in 11 patients (6%) after an average of 5.09±2.4 injections due to worsened SRF or IRF (n=7), no improvement (n=3), or the patient decision to discontinue all treatments (n=1). All patients maintained their baseline visions and returned to their baseline OCT findings after switching back to their previous anti-VEGFs.
The mean best corrected visual acuities improved from the initial to final visits (0.33±0.32 logMAR ≈20/43 initially to 0.27±0.32 logMAR ≈20/37, P=0.0022, Wilcoxon matched-pairs). The CST also improved from a mean initial thickness of 312±87μm to 287±71μm (P<0.0001, Wilcoxon matched-pairs).
No patients developed idiopathic intraocular inflammation or retinal vasculitis during the study. Two patients (1%) developed culture-negative presumed endophthalmitis 2–4 days after their fourth doses of intravitreal faricimab. Four patients (2%) developed retinal pigment epithelial (RPE) tears after 5.25±2.06 injections. Three patients (1.6%) developed subretinal hemorrhages after an average of 7.33±2.08 injections; two of them had previously developed subretinal hemorrhages with ranibizumab and/or aflibercept.
Discussion
Anti-VEGF therapies are effective at resolving SRF and IRF while decreasing vision loss. Most of the visual gains occur within the first few months of initiating therapy, with the goal of maintenance in the secondary phase of treatment.9 Some patients may be poorly responsive or non-responsive to anti-VEGFs, with less than 25% reductions in central retinal thicknesses, persistent or new fluid or hemorrhages, and minimal visual improvements or even vision loss.9,10 Drug therapies may be switched in these treatment-resistant eyes.9
In the phase 3 clinical studies for faricimab, all of the ARMD patients were treatment naïve and were therefore more likely to experience an improvement in their BCVAs and CSTs. One real-world case series of 11 treatment naive and previously treated nARMD eyes found that faricimab improved fluid and vision.11 All 190 eyes in the current study had been previously treated for an average of 3–4 years with persistent fluid and/or high treatment demands at 4–6 weeks. Nevertheless, there were statistically significant improvements in the BCVAs and CSTs during the study. The mean 0.06 logMAR visual gain and −25μm change in CST, however, were more similar to the changes reported in the 5 year follow-up studies for CATT (−3.3 ETDRS letters and −20μm)3 than those in the faricimab research trials (5.8 ETDRS letters gained and −137μm adjusted CST improvement).2 The differences may reflect the effects of long-standing neovascular ARMD, with the associated risks of photoreceptor damage, retinal atrophy, fibrovascular scars, and patients reaching treatment plateaus or experiencing tachyphylaxis.3,10
The switch to faricimab resulted in approximately 38% achieving fluid resolution at least once during the course of the study. At the last follow-up visit, 24% of these treatment-resistant eyes remained dry on OCT, which was slightly higher than the 17% found in the CATT extension study.3 During the limited 35 weeks of follow-up in the current study, 7% were able to tolerate a ≥12 week dosing interval (Figure 2), and 6% had their faricimab treatments discontinued due to no improvement or worsening fluid; fortunately, the vision remained stable. In contrast, 70–80% of treatment naïve patients in the faricimab clinical trials were able to tolerate a 12 week dosing interval by week 48, and 45% tolerated a 16 week interval.2 The difference likely reflects the poorly responsive nature of recalcitrant nARMD, as well as the more stringent and slower treatment protocol employed in the current study; patients were not extended if there was worsening fluid on OCT (even if the CST change was less than 50μm), and once the fluid resolved, the dosing intervals were usually extended by 2 weeks instead of 4–8 weeks. As the follow-up period is extended, the proportions of patients who are able to tolerate longer dosing intervals will likely change.
There were no adverse events directly attributed to faricimab itself. The rates of RPE tears, submacular hemorrhages, and endophthalmitis were similar to those previously reported. Approximately 4–13% of patients with neovascular membranes may develop RPE tears, with or without anti-VEGF treatment.12,13 The risk of submacular hemorrhage has been estimated to be approximately 4.6% per year per 1000 patients, regardless of whether the eyes have been treated with bevacizumab, ranibizumab, or aflibercept.14 In the CATT trials, the infection rate was 0.9% of all eyes and 0.06% of all injections.15
The risk of endophthalmitis underscores the importance of trying to decrease the number of injections whenever possible. Fortunately, the dosing interval was able to be extended further with faricimab, even in patients who did not tolerate longer injection intervals with ranibizumab or aflibercept. Furthermore, the longer dosing intervals for faricimab could improve its cost-effectiveness compared to the other anti-VEGFs due to the decreased time and travel burdens for patients and their caretakers with less injections.16
The limitations of the study include the retrospective nature and relatively short follow-up. The injection intervals were determined by the treating physician, though most patients were on the popular treat and extend protocol.17 The outcomes may differ on a fixed dosing regimen or PTI, but a meta-analysis found similar efficacy between treat and extend protocols and fixed dosing regimens with other anti-VEGFs.18 The mean last dosing intervals were used for comparisons, but the overall mean interval of 5.33 weeks for all prior anti-VEGF injections was still less than that for faricimab. The study interval was only 35 weeks in order to assess the early safety and efficacy of the newly approved medication, but it was still long enough to detect early trends in the dosing intervals, and 98% of patients had more than 3 faricimab injections and 92% had 6 months or more of follow-up. No patients developed IOI in over 1300 faricimab injections, but the study size was not large enough to accurately assess the risk of intraocular inflammation. Furthermore, the CSTs were not available for all patients, and some CSTs may have been affected by underlying fibrovascular scars or media artifacts; nevertheless, the CST changes were similar to those of previously published large studies.3 Patients were not refracted, and there may have been some test-retest variability;19,20 however, pinhole visions were obtained, and BCVAs were similar to those achieved in other studies.3 There were also too few patients who had received all four anti-VEGF medications to allow meaningful comparisons of the dosing intervals for brolucizumab and bevacizumab, but there were similar treatment responses between ranibizumab and aflibercept.
Conclusion
Intravitreal faricimab slightly improved vision and CSTs even in treatment-resistant neovascular ARMD eyes and was associated with longer dosing intervals than for ranibizumab or aflibercept. Intravitreal faricimab was not associated with an increased risk of intraocular inflammation during the study. Larger longitudinal studies will help determine the long-term efficacy and safety of faricimab, especially in patients with recalcitrant disease.
Acknowledgments
The authors are extremely grateful to the contributions of Dr. Hyung Cho, Dr. Michael Jacobson, Dr. Sean Koh, Dr. Rahul Komati, Dr. Scott Lampert, Dr. Gregory Lee, Dr. John Miller, Dr. Yogin Patel, Dr. Mark Rivellese, Dr. Atul Sharma, Dr. Jay Stallman, Dr. Robert Stoltz, Dr. Stephanie Vanderveldt, and Dr. Harpreet (Paul) Walia.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
Dr. Chin Yee is a speaker for Genentech, and Dr. Mukkamala is a speaker for Regeneron; neither company had any involvement in the study. The authors report no other conflicts of interest in this work.
References
1. Adamis AP, Brittain CJ, Dandekar A, Hopkins JJ. Building on the success of anti-vascular endothelial growth factor therapy: a vision for the next decade. Eye. 2020;34(11):1966–1972. doi:10.1038/s41433-020-0895-z
2. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet Lond Engl. 2022;399(10326):729–740. doi:10.1016/S0140-6736(22)00010-1
3. Maguire MG, Martin DF, Ying GS, et al.; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. 5-Year outcomes with anti-VEGF treatment of neovascular age-related Macular Degeneration (AMD): the comparison of AMD treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi:10.1016/j.ophtha.2016.03.045
4. American Society of Retina Specialists. 2020 membership survey preferences and trends; 2020. Available from: https://www.asrs.org/content/documents/_2020-pat-survey-results-for-website.pdf.
5. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011
6. Kunimoto D, Yoon YH, Wykoff CC, et al. Efficacy and safety of abicipar in neovascular age-related macular degeneration: 52-week results of phase 3 randomized controlled study. Ophthalmology. 2020;127(10):1331–1344. doi:10.1016/j.ophtha.2020.03.035
7. Khurana RN, Kunimoto D, Yoon YH, et al. Two-year results of the phase 3 randomized controlled study of abicipar in neovascular age-related macular degeneration. Ophthalmology. 2021;128(7):1027–1038. doi:10.1016/j.ophtha.2020.11.017
8. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
9. Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye. 2015;29(6):721–731. doi:10.1038/eye.2015.48
10. Broadhead GK, Hong T, Chang AA. Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92(8):713–723. doi:10.1111/aos.12463
11. Stanga PE, Valentín-Bravo FJ, Stanga SEF, Reinstein UI, Pastor-Idoate S, Downes SM. Faricimab in neovascular AMD: first report of real-world outcomes in an independent retina clinic. Eye. 2023;1–8. doi:10.1038/s41433-023-02505-z
12. Pauleikhoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2002;240(7):533–538. doi:10.1007/s00417-002-0505-8
13. Gelisken F, Ziemssen F, Voelker M, Bartz-Schmidt KU, Inhoffen W. Retinal pigment epithelial tears after single administration of intravitreal bevacizumab for neovascular age-related macular degeneration. Eye. 2009;23(3):694–702. doi:10.1038/sj.eye.6703098
14. Gabrielle PH, Maitrias S, Nguyen V, et al. Incidence, risk factors and outcomes of submacular haemorrhage with loss of vision in neovascular age-related macular degeneration in daily clinical practice: data from the FRB registry. Acta Ophthalmol. 2022;100(8):e1569–e1578. doi:10.1111/aos.15137
15. Meredith TA, McCannel CA, Barr C, et al. Postinjection endophthalmitis in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2015;122(4):817–821. doi:10.1016/j.ophtha.2014.10.027
16. Meer EA, Oh DH, Brodie FL. Time and distance cost of longer acting anti-VEGF therapies for macular degeneration: contributions to drug cost comparisons. Clin Ophthalmol Auckl NZ. 2022;16:4273–4279. doi:10.2147/OPTH.S384995
17. Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679–680. doi:10.1016/j.ajo.2007.02.024
18. Fallico M, Lotery AJ, Longo A, et al. Treat and extend versus fixed regimen in neovascular age related macular degeneration: a systematic review and meta-analysis. Eur J Ophthalmol. 2021;31(5):2496–2504. doi:10.1177/1120672120964699
19. Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice. Trans Am Ophthalmol Soc. 2009;107:311–324.
20. Lim LA, Frost NA, Powell RJ, Hewson P. Comparison of the ETDRS logMAR, ‘compact reduced logMar’ and Snellen charts in routine clinical practice. Eye. 2010;24(4):673–677. doi:10.1038/eye.2009.147
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.