Back to Journals » Clinical Ophthalmology » Volume 16
Intravitreal Faricimab for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration
Received 28 October 2022
Accepted for publication 30 November 2022
Published 9 December 2022 Volume 2022:16 Pages 4041—4046
DOI https://doi.org/10.2147/OPTH.S395279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Ryan B Rush,1– 3 Sloan W Rush1,2
1Panhandle Eye Group, Amarillo, TX, USA; 2Department of Surgery, Texas Tech University Health Science Center, Amarillo, TX, USA; 3Southwest Retina Specialists, Amarillo, TX, USA
Correspondence: Ryan B Rush, Southwest Retina Specialists, 7411 Wallace Blvd, Amarillo, TX, 79106, USA, Tel +1 806 351-1870, Email [email protected]
Purpose: To evaluate the short-term effects of intravitreal faricimab (IVF) in treatment-resistant neovascular age-related macular degeneration (nAMD) subjects previously treated with intravitreal aflibercept (IVA).
Methods: A retrospective review was conducted on nAMD patients undergoing IVA therapy at a single private practice institution. Subjects were divided into Study and Control groups. Both Study and Control subjects had undergone ≥ 6 IVA treatments during the previous 12 months, ≥ 4 IVA treatments during the previous 6 months, had a central macular thickness (CMT) on optical coherence tomography (OCT) of ≥ 300 microns, and had observable intraretinal and/or subretinal fluid on OCT prior to group assignment. Study subjects were switched from IVA to IVF and received 3 treatments within 4 months. Control subjects remained on IVA during the same time period and received 3 treatments within 4 months.
Results: There were a total of 55 subjects analyzed. There were 39.3% (11/28) in the Study Group and 7.4% (2/27) in the Control Group attaining a CMT of less than 300 microns without retinal fluid on OCT at the end of the 4-month study period (p = 0.004). There were 35.7% (10/28) in the Study Group and 7.4% (2/27) in the Control Group gaining 2 or more lines of visual acuity at the end of the 4-month study period (p = 0.008).
Conclusion: IVF can improve the visual and anatomic outcomes in a significant minority of treatment-resistant nAMD subjects previously managed with IVA. A greater follow-up period is needed to determine if such improvements can be maintained.
Keywords: faricimab, neovascular age-related macular degeneration, recalcitrance, treatment-resistant
Introduction
Intravitreal therapies neutralizing vascular endothelial growth factor (VEGF) have considerably reduced the extent of vision loss in patients with neovascular age-related macular degeneration (nAMD).1 Nevertheless, there exist a number of limitations to anti-VEGF therapy, including the requirement of repetitive injections and inadequate response in a subset of subjects. Even with monthly anti-VEGF injections over 12 months, one-fifth of subjects experience declining best-corrected visual acuity (BCVA).2 As a consequence, novel treatments and delivery systems are being developed to target other complementary mediators implicated in angiogenesis.
Faricimab (Vabysmo; Roche/Genentech; Basel, Switzerland) is a bispecific antibody targeting VEGF-A and Angiopoietin-2 (Ang-2). Based on the results of the two multi-center Phase 3 trials, LUCERNE and TENAYA,3 Faricimab gained approval by the Food and Drug Administration for the treatment of nAMD in January 2022. In these pivotal trials,3 78% of subjects receiving intravitreal faricimab (IVF) at an interval of ≥12 weeks demonstrated non-inferior BCVA compared to subjects receiving intravitreal aflibercept (Eylea/Regeneron; NY, USA) (IVA) at an 8 week fixed interval after 48 weeks of follow up in treatment-naïve nAMD subjects. Although the results of LUCERNE and TENAYA suggest that IVF may be more efficacious to IVA in regards to allowing longer treatment intervals when employing an optical coherence tomography (OCT)-guided management protocol, a significant weakness in these clinical trials rests in the study design that did not allow subjects randomized to the IVA treatment group to extend out longer than 8 weeks between treatments, regardless of the clinical findings. Thus, subjects receiving IVF at an interval of ≥12 weeks cannot be compared to subjects receiving IVA at an interval of ≥12 weeks, allowing only speculation as to how these might have compared. If IVF is indeed more efficacious than IVA, it stands to reason that intraretinal and/or subretinal fluid may be reduced to a greater extent by IVF in subjects who are not treatment-naïve. In this study, the authors evaluate the short-term benefits of switching nAMD subjects from IVA to IVF when treatment-resistance is encountered using an OCT-guided management protocol in a real-world setting.
Methods
This retrospective, case-controlled series of subjects treated from February to May 2022 at a private practice in Amarillo, TX was approved by the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-08), and all research components followed the Declaration of Helsinki and were in conformity with human research standards and regulations. Informed consent from patients in the study was waived because data was collected retrospectively and all identifying patient information was omitted. Subjects were divided into a Study Group and a Control Group based on whether or not the subject was switched from IVA to IVF during the designated study interval.
The criteria of inclusion for both Study and Control Groups were as follows: 1) the subject had been actively receiving IVA for nAMD up to the beginning of the study period in February 2022, 2) the subject was being managed by a treat-and-extend protocol primarily based on the presence/absence of intraretinal and/or subretinal fluid, 3) the subject had received ≥6 IVA treatments during the previous 12 months (370 days), 4) the subject had undergone ≥4 IVA treatments during the previous 6 months (180 days), and 5) the subject had a central macular thickness (CMT) on OCT of ≥300 microns with observable intraretinal and/or subretinal fluid at the beginning of the study period.
Subjects included in the Study Group were switched to IVF between February and March 2022 and received 3 IVF treatments within 4 months (120 days) after the switch with corresponding follow up during the study interval. Subjects included in the Control Group remained on IVA between February and May 2022 and received 3 IVA injections within 4 months (120 days) with corresponding follow up during the study interval.
The criteria of exclusion for both Study and Control Groups were as follows: 1) the subject’s baseline Snellen BCVA was worse than 20/200, 2) the subject underwent an ocular treatment other than anti-VEGF therapy 6 months prior to or during the study interval (ie, cataract surgery, pars plana vitrectomy, intravitreal steroid injection, etc.), and 3) the subject had a condition considered by the examiner to be responsible for a loss ≥2 Snellen lines of visual acuity unrelated to the diagnosis of nAMD (ie, cataract, epiretinal membrane, glaucoma, stroke-related vision loss, etc.).
The treat-and-extend protocol employed during this study was based primarily on the appearance of retinal fluid and has been reported previously by the authors.4 In brief, subjects received monthly (28–34 days) anti-VEGF injections until all observable intraretinal and/or subretinal fluid resolved on OCT. Once the retinal fluid reabsorbed on OCT, subjects were extended out 1–2 week intervals until recurrence of the retinal fluid on OCT was observed and the treatment interval was then fine-tuned to maintain a fluid-free macula. Subjects continued receiving monthly injections indefinitely if the retinal edema could not be resolved. Similar to the authors' previous work, subjects were considered recalcitrant to treatment if a fluid-free macula on OCT could not be achieved despite ≥6 anti-VEGF injections over a 12-month period.5 When both eyes of Study and Control Group subjects satisfied the above inclusion/exclusion criteria, a random number generating program (simple randomization) determined which eye would be analyzed. For the purpose of this study, the baseline examination was the evaluation immediately prior to the study’s start interval (February 2022). OCT was performed using the Heidelberg Spectralis system (Heidelberg Engineering, Heidelberg, Germany). Baseline and final OCT images were evaluated by 2 masked fellowship-trained vitreoretinal specialists for the presence/absence of intraretinal and/or subretinal fluid. If disagreement between the two specialists occurred, a third masked specialist made the final determination.
Outcomes and Statistical Analysis
The primary outcome was the percentage of patients who achieved a CMT less than 300 microns without observable intraretinal and/or subretinal fluid on OCT at the end of the 4-month study interval. The secondary outcome was the percentage of patients who gained 2 or more lines of BCVA at the end of the 4-month study interval. Snellen visual acuity was changed into logMAR for statistical analysis. One-way analysis of the variance and likelihood ratios were employed to evaluate for statistical significance at the alpha <0.05 level.
Results
There were a total of 55 subjects included in the analysis, of which 28 were in the Study Group and 27 in the Control Group. The study population’s baseline demographics and characteristics are displayed in Table 1. There were no significant differences between cohorts at baseline. The concordance rate between masked OCT reviewers was 92.7% (51/55).
There were 39.3% (11/28) in the Study Group and 7.4% (2/27) in the Control Group attaining a CMT of less than 300 microns without intraretinal and/or subretinal fluid on OCT at the end of the 4-month study period (p = 0.004). The CMT on OCT changed from 393.3 (375.9–410.7) microns at baseline to 328.7 (311.3–346.1) microns at the end of the 4-month study interval in the Study Group (p < 0.001). The CMT on OCT changed from 399.9 (382.1–417.7) microns at baseline to 379.4 (361.6–397.3) microns at the end of the 4-month study interval in the Control Group (p = 0.11).
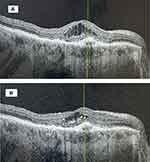
There were 35.7% (10/28) in the Study Group and 7.4% (2/27) in the Control Group gaining 2 or more lines of visual acuity at the end of the 4-month study period (p = 0.008). The visual acuity changed from 0.75 (0.68–0.83) logMAR (Snellen 20/114) at baseline to 0.62 (0.55–0.69) logMAR (Snellen 20/83) at the end of the 4-month study interval in the Study Group (p = 0.01). The visual acuity changed from 0.70 (0.63–0.77) logMAR (Snellen 20/100) at baseline to 0.65 (0.58–0.72) logMAR (Snellen 20/89) at the end of the 4-month study interval in the Control Group (p = 0.37). A case example of a study patient successfully switched from IVA to IVF is displayed in Figure 1.
Discussion
To the knowledge of the authors, this study is the first to report comparative outcomes in nAMD subjects switched from aflibercept to faricimab. Both visual and anatomic outcomes were improved in a clinically significant minority when subjects were switched to faricimab from aflibercept in cases of recalcitrant nAMD. This suggests that faricimab potentially offers fewer intravitreal injections without compromising long-term outcomes in this difficult-to-treat subgroup of treatment-resistant nAMD subjects when a treat-and-extend protocol is employed, as is the preference for a majority of vitreoretinal specialists.6,7 The authors consider this benefit clinically meaningful since 27.6–70.3% of subjects undergoing anti-VEGF therapy for nAMD have been reported to persist with intraretinal and/or subretinal fluid on OCT despite monthly injections,2,8,9 and studies suggest that long-term visual outcomes are optimal when all residual retinal fluid is resolved.10 Fewer intravitreal injections with stable visual and anatomic outcomes in this elderly population may benefit the patient (fewer doctor visits), the specialist (more opportunity to see other vitreoretinal patients requiring attention), and society (lower overall cost to the payer).
Faricimab has theoretical advantages over aflibercept, including a stronger affinity for VEGF-A as well as blocking Ang-2, which has been implicated in the pathophysiology of neovascularization.11–13 However, as alluded to above in the Introduction, the pivotal trials LUCERNE and TENAYA did not permit patients randomized to treatment with aflibercept to extend beyond 8 weeks between injections regardless of the subject’s examination findings.3 The authors would have preferred a head-to-head comparison between IVF and IVA with the exact same treatment protocol in order to better assess comparative efficacy between drugs. The authors made it a point to case-control our study to give the reader a robust comparison. Since there were no significant differences between study groups at baseline and both groups underwent a similar treat-and-extend protocol, the authors believe our comparison is valid. However, our study evaluated only treatment-resistant nAMD cases and not treatment-naïve cases as in LUCERNE and TENAYA. Therefore, our results and conclusions may not be applicable to treatment-naïve nAMD cases.
Strengths of our study include its case-control design with well-matched Study and Control Groups at baseline, its moderately large number of cases involved considering the novelty of IVF, and its real-world setting employing a typical treat-and-extend regimen used by most specialists, thereby allowing for a practical application to other specialists treating this patient population. Weaknesses of our study include its retrospective design, its utilization of logMAR visual acuity as opposed to ETDRS letter scoring, and its relatively short follow up period. Longer follow up is needed to determine how our study’s subjects would fare once treatment extension is introduced into their treat-and-extend protocol. Though it is certainly advantageous to achieve a fluid-free macula in nAMD subjects undergoing monthly anti-VEGF therapy, it is even better when subjects can be successfully extended out longer between treatments and still preserve their visual and anatomical outcomes.
In conclusion, switching nAMD subjects who are recalcitrant to aflibercept treatment to faricimab may result in better visual and anatomical outcomes in a statistically significant portion of subjects, thereby leading to potentially longer treatment intervals when a real-world treat-and-extend management protocol is followed. Further research is warranted to confirm these findings, especially with a longer follow up period.
Abbreviations
OCT, optical coherence tomography; BCVA, corrected visual acuity; nAMD, neovascular age-related macular degeneration; VEGF, vascular endothelial growth factor; CMT, central macular thickness.
Declarations
The study was approved by the Panhandle Eye Group Institutional Review Board (IORG0009239; IRB00011013-08) in accordance with the Ethical Standards laid down in the Declaration of Helsinki. Informed consent from study participants was waived because this was a retrospective study with no identifying patient information presented.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
2. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006
3. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi:10.1016/S0140-6736(22)00010-1
4. Rush RB, Simunovic MP, Vandiver L, Aragon AV, Ysasaga JE. Treat-and-extend bevacizumab for neovascular age-related macular degeneration: the importance of baseline characteristics. Retina. 2014;34(5):846–852. doi:10.1097/IAE.0000000000000033
5. Rush RB, Rush SW. Predictability of recalcitrance in neovascular age-related macular degeneration with indocyanine green angiography. Asia Pac J Ophthalmol. 2015;4(4):187–190. doi:10.1097/APO.0000000000000111
6. Koh A, Lanzetta P, Lee WK, et al. Recommended guidelines for use of intravitreal aflibercept with a treat-and-extend regimen for the management of neovascular age-related macular degeneration in the Asia-Pacific region: report from a consensus panel. Asia Pacific J Ophthalmol. 2017;6(3):296–302.
7. Ross AH, Downey L, Devonport H, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye. 2020;34(10):1825–1834. doi:10.1038/s41433-019-0747-x
8. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and Bevacizumab for treatment of Neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi:10.1016/j.ophtha.2012.03.053
9. Wykoff CC, Brown DM, Croft DE, et al. Two year SAVE outcomes: 2.0 mg ranibizumab for recalcitrant neovascular AMD. Ophthalmology. 2013;120(9):1945–6 e1941. doi:10.1016/j.ophtha.2013.06.030
10. Brown DM, Tuomi L, Shapiro H. Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina. 2013;33:23–34. doi:10.1097/IAE.0b013e318263cedf
11. Hussain RM, Neiweem AE, Kansara V, et al. Tie-2/Angiopoietin pathway modulation as a therapeutic strategy for retinal disease. Expert Opin Investig Drugs. 2019;28(10):861–869. doi:10.1080/13543784.2019.1667333
12. Ferro Desideri L, Traverso CE, Nicolò M. The emerging role of the Angiopoietin-Tie pathway as therapeutic target for treating retinal diseases. Expert Opin Ther Targets. 2022;26(2):145–154. doi:10.1080/14728222.2022.2036121
13. Sahni J, Dugel PU, Patel SS, et al. Safety and efficacy of different doses and regimens of faricimab vs ranibizumab in neovascular age-related macular degeneration: the AVENUE Phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138(9):955–963. doi:10.1001/jamaophthalmol.2020.2685
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.