Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Initial outcomes of one anastomosis gastric bypass at a single institution
Authors Jamal W, Zagzoog MM , Sait SH, Alamoudi AO, Abo’ouf S, Alghamdi AA , Bamashmous RO, Maghrabi AA
Received 13 July 2018
Accepted for publication 18 October 2018
Published 24 December 2018 Volume 2019:12 Pages 35—41
DOI https://doi.org/10.2147/DMSO.S180111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven F. Abcouwer
Wisam Jamal,1 Mohammad M Zagzoog,2 Salma H Sait,2 Ahmed O Alamoudi,2 Shaza Abo’ouf,3 Ayman A Alghamdi,2 Ryan O Bamashmous,2 Ashraf A Maghrabi2
1Department of Surgery, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia; 2Department of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 3Beverly Medical Care Clinics for Obesity Management, Jeddah, Saudi Arabia
Introduction: One anastomosis gastric bypass (OAGB) is an emerging bariatric procedure, which has been reported to be safe and effective. This study aims to evaluate the short-term outcome of OAGB and its midterm effects on weight loss and remission of type 2 diabetes mellitus (T2DM).
Materials and methods: A retrospective review of patients who had undergone OAGB between January 2013 and January 2017 in King Abdulaziz University Hospital, Jeddah, Saudi Arabia, is presented here. Patients’ perioperative characteristics, biochemical profile (fasting blood glucose, HbA1c and iron profile) and details on subsequent weight loss in terms of body mass index (BMI) and excess weight loss percentage (EWL%) along with early and late postoperative complications were evaluated.
Results: Out of the 47 patients who underwent OAGB, 42 were included in this study and completed the 2-year follow-up. Average operative time was 107±21.3 minutes and average length of hospital stay was 2.5±0.53 days. Mean preoperative BMI was 47.6±9.1 kg/m2, and at 1 and 2 years of follow–up, it was 30.5±7.4 and 27.1±5.1, respectively. No mortality, anastomotic leak or bleeding were reported. Most common midterm complication was iron deficiency anemia (n=7/42). Remission of T2DM at 6 months was 80%. Patients with preoperative T2DM for less than 10 years showed better remission (P<0.001).
Conclusion: Our analysis suggests that OAGB is a safe and effective weight loss procedure that carries low perioperative risk and acceptable nutritional complications in the midterm, with a notable remission of T2DM. Preoperative duration of T2DM plays a major role in achieving remission after OAGB.
Keywords: bariatric surgery, weight loss, diabetes mellitus, mini gastric bypass, single anastomosis gastric bypass, omega loop gastric bypass
Introduction
Obesity is a major health problem in Saudi Arabia. It is known that obesity leads to numerous comorbidities such as cardiovascular disease, metabolic syndrome and increased mortality.1–7 Losing weight can be hard to accomplish through traditional nonsurgical methods such as diet, exercise, behavioral changes and medications, and outcome varies between individuals. Therefore, bariatric surgery was introduced as the treatment of choice for morbid obesity3,4 and has been shown to be effective in weight control and remission of comorbidities, particularly type 2 diabetes mellitus (T2DM), leading to decrease in the overall midterm secondary complications and mortality.8,9 Of the variety of bariatric surgical procedures available, laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (RYGB) are the two most commonly performed bariatric operations worldwide with low rates of perioperative complications.10–12
One anastomosis-gastric bypass (OAGB) is a restrictive and malabsorptive weight loss surgery that carries the same characteristics of Roux-en-Y gastric bypass in its status as a weight loss mechanism.13 Yet, it has other advantages as it is considered a simpler technique with a small learning curve and shorter operative time, and similar outcomes in terms of weight loss and remission of comorbidities.14–16
It was first performed in 1997 by Rutledge,17 and his first reported series was in 2001. This procedure involves the formation of a long narrow gastric pouch with a single gastrojejunal anastomosis (omega-loop). Many studies have reported some advantages of OAGB over other bariatric procedures, such as one less anastomosis, shorter operative time and hospital stay, lower risk of anastomotic leakage, easy reversibility18 as well as sustained results regarding weight loss.19
A randomized controlled trial concluded that participants who underwent OAGB are more likely to achieve T2DM remission than those who underwent LSG.20 However, its popularity is limited and the procedure has its critics.21,22 There are doubts as to whether OAGB is an effective procedure when it comes to long-term results such as persistent weight loss.19,23,24 Furthermore, there remain controversies about the side effects of this procedure such as incidence of marginal ulcer, reflux esophagitis and iron deficiency anemia.21
To our knowledge, there are few reports investigating the outcomes of OAGB in the Middle East region.25 This study aims to describe the outcomes of OAGB and its effects on weight loss, and on the remission of T2DM in our center.
Materials and methods
Study design and data collection
We retrospectively reviewed all patients who underwent OAGB procedure in our center from January 2013 to January 2017, done by a single surgical team in King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia. Patients were excluded from the study if they had American Society of Anesthesiologists’ (ASA) classification of Physical Health score 4 and more, or a history of major medical problems, such as mental impairment, being pregnant, malignant neoplasm or missing data. Patients were eligible for the OAGB if they had a body mass index (BMI) of 40 kg/m2 or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities such as T2DM, HTN, disk prolapse and sleep apnea.
Patients’ baseline characteristics such as BMI and biochemical data were recorded. Perioperative data (operative duration, length of hospital stay) and complications (early [<30 days] and late [>1 month]) were evaluated. Subsequent biochemical and weight loss changes were recorded in our prospectively collected database from May 2017 to October 2018.
The first postoperative follow-up was done 2 weeks after the surgery. The routine follow-ups were scheduled at 3, 6, 9 and 12 months, and then yearly. Upon discharge, all patients were prescribed multivitamins and iron supplements. At each follow-up visit, weight loss (BMI, excess weight loss percentage [EWL%]), remission of T2DM, glycemic control status (fasting blood glucose [FBG], glycosylated hemoglobin A1C [HbA1c], glycemic therapy and anemia work up (hemoglobin [Hb], mean corpuscular volume [MCV], iron, ferritin, transferrin and total iron-binding capacity [TIBC]). Remission of T2DM was defined as fasting plasma glucose levels less than 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) in addition to HbA1c values less than 6.5% without the use of glycemic therapy (oral hypoglycemics or insulin). Patients with low Hb levels (Hb <12 g/dL in females and Hb <14 g/dL in males), low serum iron (<10 mmol/L), high TIBC (>95 mmol/L) and with total saturation of less than 20% were considered to have iron deficiency anemia. All blood workup measurement took place in one laboratory center. Both informed and written consent was obtained from each patient.
Surgical technique
Surgery took place with the patients under general anesthesia (Cefuroxime 1.5 g IV, Heparin 5,000 IU SQ are given with induction of anesthesia), while they rested in a supine position with separated legs (with the surgeon positioned in-between the patient’s legs). Pneumoperitoneum was induced by the insertion of a veress needle (120 mm in length) into the left subcostal region, until reaching an intra-abdominal pressure of 14 mmHg. The insertion of the first optic trocar (12 mm) mid-way between the xiphoid and the umbilicus, slightly left to the midline, was followed by the insertion of the rest of trocars under vision.
The procedure was initiated by dissecting the angle of His to make sure that the upper part of the abdominal cavity is clearly exposed and technically accessible. To raise the greater omentum and the transverse colon, the first part of jejunum at the duodenojejunal junction (ligament of treitz) was identified, and a length of 200 cm in the small intestine was measured.
Then, the small intestine was hung up at this point by using a tape, and the bowel was raised up into the upper part of the abdomen to make sure that the gastrojejunal anastomosis can be carried out without tension. To identify the lowest part of the incisura angularis of the stomach, the medial to the nerve of Laterjet was found, and dissection was started at the perigastric fat until reaching the lesser sac.
The gastrectomy part of the procedure was started by the horizontal stapling of the stomach, using a 45-mm stapler at incisura level, proceeded by vertical stapling high up until the angle of His, against a calibration tube (36 Fr). The gastric pouch was now created. Gastrostomy was performed at the lower part of the gastric pouch, just anterior to stapler line, and an enterotomy in the small intestine at 2 m from DJ junction was created. Gastrojejunal anastomosis was done by using a 45 mm stapler (anti-mesenteric wall of small bowel with the posterior wall of the stomach). The enterotomy opening was closed using Vicryl 3.0 (absorbable) suture. A leak test with methylene blue was performed at the end of the procedure after the insertion of the nasogastric tube, which was removed at the end of the procedure. No anti-reflux stitch was placed, and no closure of Petersen’s space was carried out. The removal of the trocars under control of vision and the closure of the skin with absorbable Monocryl sutures were the final steps. Most of the patients were encouraged to ambulate 2–4 hours after surgery. No proton pump inhibitor (PPI) was given after surgery unless indicated for symptoms. Patients would receive liquid diet on day 1 postoperative to day 10 and then mashed food starting from day 11 up to day 20, semisolid diet was encouraged from day 21 to day 30 and thereafter full diet was encouraged with special instructions to chew well and eat slow.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. King Abdulaziz University’s ethical and technical committee (Ref: 474-16) approved this study, and all participants provided both informed and written consent prior to participation.
Outcome measures
The primary outcomes were short and midterm complications. Secondary measures included weight loss and remission of T2DM (fasting glucose 126 mg/dL and HbA1c <6.5% without glycemic therapy).
Results
A total of 47 patients underwent OAGB as a primary procedure in KAUH. Five patients were excluded due to missing data and 42 patients (34 female, 8 male) with full data were included in the study. The mean age was 37.3±10 years (range 24–68), mean weight was 125.7±27.2 kg and mean BMI was 47.6±9.1 kg/m2 (range 34.2–79.7). All patients successfully completed the 2-year follow-up for weight loss. Patients’ baseline characteristics are presented in Table 1.
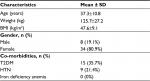  | Table 1 Patients Characteristics at Baseline Notes: Data are presented as the mean±SD. Abbreviations: BMI, body mass index; T2DM, type 2 diabetes mellitus. |
Perioperative characteristics
As can be seen from Table 2, 39 out of the 42 patients (92.8%) underwent OAGB as a single procedure with a mean operative time of 107±21.3 minutes. One case underwent OAGB with laparoscopic cholecystectomy with an operative time of 143 minutes. Two patients had OAGB with gastric band removal with a mean operative time of 180±28.3 minutes. All operations were carried out using laparoscopic technique with zero conversion rates. Mean hospital stay was 2.5±0.53 days.
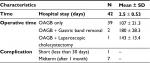  | Table 2 Perioperative Characteristics Notes: Data are presented as the mean±SD. Abbreviation: OAGB, one anastomosis-gastric bypass procedure. |
Short and midterm complications
Within 30 days post OAGB, no mortality, anastomotic leak or bleeding was reported. Only one patient had a complication requiring reoperation on postoperative day 2 due to small bowel strangulation within a trocar site. All patients had their follow-up visits for midterm (>1 month) observation. Of those, 16.6% (7/42) patients were diagnosed with iron deficiency anemia and none of the patients required revision of the procedure.
Weight loss outcomes
Postoperative EWL% and BMI reduction were observed at all follow-up assessment points (M3, M6, M9, M12 and M24). This is demonstrated in Figures 1 and 2. The BMI decreased from 47.6±9.1 to 40.2±9.4, 35.2±8, 33.3±8.1, 30.5±7.4 and 27.1±5.1 at 3, 6, 9, 12 and 24 months, respectively (Figure 1). EWL% was 34±15%, 58±21.7%, 70±23%, 77.2±22.7% and 89.8±19.6% at 3, 6, 9, 12, and 24 months, respectively.
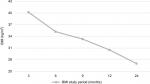  | Figure 1 Patients’ BMI (kg/m2) at 3, 6, 9, 12, and 24 months postoperative (n=42). Abbreviation: BMI, body mass index. |
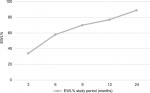  | Figure 2 Patients’ EWL% at 3, 6, 9, 12, and 24 months postoperative (n=42). Abbreviation: EWL%, excess weight loss percentage. |
Remission of type 2 diabetes mellitus
Remission of T2DM at 6 months follow-up after surgery is shown in Table 3. Out of 42 patients, 15 were with preoperative T2DM and 80% (12/15) achieved remission with statistical significance (P<0.001). The mean of HbA1c significantly decreased from preoperative (8.3±2.3) to postoperative (5.8±0.7) (P<0.001) (Table 3). Interestingly, patients with preoperative T2DM for <10 years showed better remission than patients with preoperative T2DM for more than 10 years (P<0.001).
  | Table 3 Postoperative follow-up for diabetic patients Notes: Data are presented as the mean±SD. Abbreviations: BMI, body mass index; HbA1C, glycosylated hemoglobin a1c. |
Discussion
In this manuscript, we evaluated the outcomes of OAGB in our study group. We observed a significant weight loss in the first and second years of follow-up when compared to preoperative values. Furthermore, perioperative results regarding morbidity and mortality demonstrated OAGB as a technically safe and efficient procedure.
The mean excess weight loss in our series at 1 year was 77.2%, and at 2 years was 89.8%. These results corroborate previous reports regarding OAGB performance. A systematic review of OAGB studies revealed that the mean EWL% at 12 months ranged between 55% and 88%.13,16,26,27 Among an European experience, two studies have compared the outcome of OAGB with SG in term of weight loss, which reported excellent weight loss following OAGB in 1 year of follow-up.28,29 While, a randomized control trial published in 2005 reported EWL% due to OAGB after 1 year postoperative to be significantly higher (64.9%) compared to RYGB (58.7%).30 These differences in weight loss following OAGB among the previous reports and the current study may be related to the study group’s culture, their eating habits and genetic variabilities among individuals.
As far as co-morbidity remission is concerned, the present study has shown acceptable metabolic improvement and weight loss following OAGB among T2DM patients with remission rate of 80% after a mean follow-up of 6 months. These results are similar to the results of the study carried out by Rutledge among 2,410 patients in the United States, with 6 years follow-up, which revealed that remission rate of T2DM and hypertension following OAGB was 83% and 80%, respectively.17 Lastly, a study conducted by Milone et al in 2013 with a comparable number of patients as in this study gave 87.5% remission of diabetes with OAGB at 1 year in comparison to 66.7% with LSG. The latter study suggested better DM remission with OAGB.9 Similar findings were concluded by Musella et al in their study, which suggested that the type of surgery plays an important role in the remission of T2DM.29 These results appear to be promising in the remission of T2DM. Therefore, we recommend that OAGB should be included as a treatment of choice for obese individuals with T2DM.
In a study with a cohort of 110 patients, Hall et al revealed that a preoperative duration of T2DM greater than 10 years was shown to significantly reduce the chances of T2DM remission following RYGB.31 Our study confirms this observation with OAGB; eleven patients with a preoperative T2DM for <10 years showed better remission than patients with T2DM for more than 10 years duration. However, explanation for dependence of remission rate on the length of T2DM prior to surgery remains elusive and requires further investigations.
Rate of complication of this study is the same or lower than other bariatric procedures.16,32,33 The only early postoperative complication in our study was an incarcerated bowel, which was reported in only one patient, no leaks or revision was observed. However, this could be attributed to the small number of patients enrolled in the study. DVT or pulmonary embolism was not reported in this study. This may be related to the short operating time, early mobilization of patients and short hospital stay following the procedure. A study conducted by Kular et al in 2014 reported a similar low incidence of complications.34
Many studies have been done to address the postoperative complications following OAGB. In a large cohort study among (1,000) patients of OAGB, 3.4% were found to have early postoperative complications and midterm complications was 4.7%.15 The most common midterm complication in the latter study was incisional hernia (4.16%). In the Rutledge study of 1,274 patients, there was one hospital death, and the complication rate was 4% in the last 200 cases.17 In comparison to other bariatric procedures, Milone et al reported more incidence of postoperative bleeding in the LSG group as compared to the OAGB group (3.3% vs 1%),9 while, also, Lee et al reported OAGB to be safer than RYGB.30
There has always been a fear of iron deficiency anemia following OAGB. Kular et al reported five cases (6.9%) of iron deficiency anemia 5 years postoperative, and this concords with our study in which iron deficiency anemia was observed in seven (7/42) patients.34 Other available studies also show similar incidence of iron deficiency anemia with OAGB.16,17,35 The percentage of iron deficiency anemia in our study is relatively high which could be related to the small number of patients in the study. We could hypothesize that some patients did not take their prescribed multivitamin and iron supplements, although patients denied noncompliance. Others have explained the iron deficiency anemia following OAGB as being due to the duodenal bypass with the malabsorption of iron.34
This cohort is focusing on the outcomes of OAGB in terms of weight loss and the remission of T2DM. However, it has several limitations. First, this is a retrospective study with limited follow-up of 2 years and a small number of patients, although prospectively collected. Second, there is the lack of gut hormonal profile, such as glucagon, gastric inhibitory peptide and glucagon-like peptide 1, as without this data we cannot elicit the underlying mechanism for the remission of T2DM. Other limitations of the presented study are that symptoms of biliary reflex, vitamins and minerals deficiency were not reported. The study has 2 years of follow-up, although we stated the remission of T2DM for 6 months postoperatively, mainly due to the missing data regarding the HbA1c for some patients.
Information regarding midterm weight loss, durability and safety of biochemical profile in this group will require a larger number of patients and longer follow-up period. Further prospective studies are needed to elucidate this issue.
Conclusion
Based on the pervious supporting literatures and current study, one anastomosis gastric bypass operation is technically safe and effective on weight loss and remission of T2DM with minimal morbidity and mortality. Although no one has yet required revision in our study, the design of the OAGB offers the possibility for laparoscopic revision if indicated. Short-term results appear promising; however, in the long term, follow-up for iron deficiency anemia is recommended.
Acknowledgment
We would like to thank Charles V. Rajadurai, and Anoud R. Omer who helped us with editing and revising the manuscript, Afnan H. Altowaireb, Esraa Alzahrani and Ibtihal A. Alghamdi for their contribution in collecting the data. This research was self-funded and received no grant from any funding agency.
Author contributions
As per the guidelines of the International Committee of Medical Journal Editors (ICMJE), all authors made substantive intellectual contributions to the study, collaboratively designing, analyzing, and interpreting the data, and writing and revising the manuscript. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Toghaw P, Matone A, Lenbury Y, De Gaetano A. Bariatric surgery and T2DM improvement mechanisms: a mathematical model. Theor Biol Med Model. 2012;9:16. | ||
Poirier P, Giles TD, Bray GA. Obesity and cardiovascular disease: pathophysiology evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. | ||
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. | ||
Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. | ||
Musella M, Milone M, Bellini M, et al. The potential role of intragastric balloon in the treatment of obese-related infertility: personal experience. Obes Surg. 2011;21(4):426–430. | ||
Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F. Effect of bariatric surgery on obesity-related infertility. Surg Obes Relat Dis. 2012;8(4):445–449. | ||
Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19(2):217–229. | ||
Noria SF, Grantcharov T. Biological effects of bariatric surgery on obesity-related comorbidities. Can J Surg. 2013;56(1):47–57. | ||
Milone M, Di Minno MN, Leongito M, et al. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol. 2013;19(39):6590–6597. | ||
Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822–1832. | ||
Stroh C, Weiner R, Horbach T, et al. KompetenznetzAdipositas, ArbeitsgruppeAdipositaschirurgie. New data on quality assurance in bariatric surgery in Germany. ZentralblChir. 2013;138:180–188. | ||
Gagner M, Deitel M, Erickson AL, Crosby RD. Survey on laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on Sleeve Gastrectomy. Obes Surg. 2013;23(12):2013–2017. | ||
Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg. 2005;15(5):648–654. | ||
Mahawar KK, Jennings N, Brown J, Gupta A, Balupuri S, Small PK. “Mini” gastric bypass: systematic review of a controversial procedure. Obes Surg. 2013;23(11):1890–1898. | ||
Noun R, Skaff J, Riachi E, Daher R, Antoun NA, Nasr M. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg. 2012;22(5):697–703. | ||
Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15(9):1304–1308. | ||
Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg. 2001;11(3):276–280. | ||
Mahawar KK, Carr WR, Balupuri S, Small PK. Controversy surrounding ‘mini’ gastric bypass. Obes Surg. 2014;24(2):324–333. | ||
Yang X, Yang G, Wang W, Chen G, Yang H. A meta-analysis: to compare the clinical results between gastric bypass and sleeve gastrectomy for the obese patients. Obes Surg. 2013;23(7):1001–1010. | ||
Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143–148. | ||
Fisher BL, Buchwald H, Clark W, et al. Mini-gastric bypass controversy. Obes Surg. 2001;11(6):773–777. | ||
Olchowski S, Timms MR, O’Brien P, Bauman R, Quattlebaum JK, O’Brien P. More on mini-gastric bypass. Obes Surg. 2001;11(4):532. | ||
Braghetto I, Csendes A, Lanzarini E, Papapietro K, Cárcamo C, Molina JC. Is laparoscopic sleeve gastrectomy an acceptable primary bariatric procedure in obese patients? Early and 5-year postoperative results. Surg Laparosc Endosc Percutan Tech. 2012;22(6):479–486. | ||
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–324. | ||
Al-Shurafa H, Elzaafarany AH, Albenmousa A, Balata MG. Primary experience of bariatric surgery in a newly established private obesity center. Saudi Med J. 2016;37(10):1089–1095. | ||
Lee WJ, Pj Y, Wang W, et al. Laparoscopic Roux-en-Y versus mini- gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005;242(1):20–28. | ||
Carbajo M, García-Caballero M, Toledano M, Osorio D, García-Lanza C, Carmona JA. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005;15(3):398–404. | ||
Disse E, Pasquer A, Espalieu P, Poncet G, Gouillat C, Robert M. Greater weight loss with the omega loop bypass compared to the Roux-en-Y gastric bypass: a comparative study. Obes Surg. 2014;24(6):841–846. | ||
Musella M, Apers J, Rheinwalt K, et al. Efficacy of bariatric surgery in type 2 diabetes mellitus remission: the role of mini gastric bypass/one anastomosis gastric bypass and sleeve gastrectomy at 1 year of follow-up. A European survey. Obes Surg. 2016;26(5):933–940. | ||
Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity. Ann Surg. 2005;242(1):20–28. | ||
Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20(9):1245–1250. | ||
Chakhtoura G, Zinzindohoué F, Ghanem Y, Ruseykin I, Dutranoy JC, Chevallier JM. Primary results of laparoscopic mini-gastric bypass in a French obesity-surgery specialized university hospital. Obes Surg. 2008;18(9):1130–1133. | ||
Lanyon RI, Maxwell BM, Kraft AJ. Prediction of long-term outcome after gastric bypass surgery. Obes Surg. 2009;19(4):439–445. | ||
Kular KS, Manchanda N, Rutledge R. Analysis of the five-year outcomes of sleeve gastrectomy and mini gastric bypass: a report from the Indian sub-continent. Obes Surg. 2014;24(10):1724–1728. | ||
Lee WJ, Ser KH, Lee YC, Tsou JJ, Chen SC, Chen JC. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22(12):1827–1834. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
