Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Inhibitory Effect of P22077 on Airway Inflammation in Rats with COPD and Its Mechanism
Authors Zeng D , Zhang W, Chen X, Ou G, Huang Y, Yu C
Received 24 November 2023
Accepted for publication 13 March 2024
Published 20 March 2024 Volume 2024:19 Pages 779—788
DOI https://doi.org/10.2147/COPD.S451244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Di Zeng,1,* Wenbo Zhang,2,* Xiaoju Chen,3 Guochun Ou,4 Yuewei Huang,2 Chengxiu Yu2
1Department of General Practice, The Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, 637000, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, 637000, People’s Republic of China; 3Clinical Medical College, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, 610081, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Suining Central Hospital, Suining, Sichuan, 629000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoju Chen, Clinical Medical College, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, 610081, People’s Republic of China, Email [email protected]
Purpose: Here, we studied the pharmacological effect of P22077 on airway inflammation induced by lipopolysaccharide and cigarette smoke and explored the therapeutic mechanism of P22077 in COPD model RAT.
Patients and Methods: The COPD model was established by lipopolysaccharide combined with fumigation; animals were treated with vehicle or P22077. Serum, bronchoalveolar lavage fluid (BALF), and lung tissues were collected for analysis.
Results: Our results showed that P22077 treatment significantly improved the airway inflammation of COPD model RAT and reduced the recruitment of leukocytes in BALF, and hypersecretion of interleukin-18 (IL-18), interleukin-1β (IL-1β) in BALF and serum. H&E staining showed that P22077 treatment could effectively reduce emphysema, immune cell infiltration and airway wall destruction. PAS staining showed that The proliferation of cup cells in the airway wall and the number of bronchial cup cells were significantly reduced in rats treated with P22077. In addition, we found that P22077 treatment suppressed the generation of the NLRP3/ASC/Caspase 1 inflammasome complex to inhibit the inflammatory response caused by IL-1β and IL-18.
Conclusion: Conclusion: P22077 inhibits expression of NLRP3 pathway-related inflammatory factors and proteins and reduces the airway inflammatory response and inflammatory cell aggregation in COPD rats. The underlying mechanism may be related to the down-regulation of NLRP3 inflammatory vesicle signaling pathway expression.
Keywords: COPD, ubiquitinase inhibitors, P22077, airway inflammation, NLRP3
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common diseases of the respiratory system. COPD is a chronic inflammatory disease of the airways, lung parenchyma, and pulmonary vasculature caused by chronic infiltration of inflammatory cells from long-term exposure to harmful gases, dust, and other particles and complex interactions with the host’s factors. The incidence and disease burden of COPD will increase in the coming decades because of ongoing exposure to risk factors caused by environmental and social health issues and an aging population worldwide.1 By 2030, COPD will be the fourth leading cause of death worldwide and the seventh leading cause of the global burden of disease.2 While studies on COPD are increasing, the pathogenesis of COPD is very complex and still not fully understood.
Inflammation has been identified as one of the main causes of COPD onset and recurrent acute exacerbations. Even if the triggers of onset are removed, inflammation caused by an excessive or inadequate immune response will still persist and lead to chronic inflammation3. Repeated irritation of the respiratory tract from various causes enriches inflammatory cells in the airways and lung tissue, stimulating the release of inflammatory mediators to trigger an inflammatory response, which becomes more pronounced and worsens with the development of disease. Inflammatory vesicles are closely related to the inflammatory response.4 Currently, there are five types of inflammasome, among which the NLRP3 inflammasome is the one with the most attention and the most in-depth research. It consists of Nod-like receptor protein 3 as the receptor, ASC as the adaptor, and caspase-1 as the effector.5 The recognition of danger signals by macrophages can lead to the aggregation of NLPR3 molecular complexes and the subsequent maturation of inflammatory vesicles and activation of caspase 1, leading to the release of cytokines such as IL-1β and IL-18 to participate in the inflammatory response. The roles of NLPR3 molecular complexes and the IL-1β and IL-18 cytokines in inflammatory diseases are gaining attention.
The activation of inflammatory vesicles is regulated by post-translational modifications including ubiquitin.6,7 Palazon-Riquelme et al and Cui et al8,9 showed that the deubiquitinating enzymes USP7 and USP47 are involved in the activation of inflammatory vesicles in macrophages. Juliana et al10 have shown that the regulation of inflammatory vesicles by the ubiquitination system has a role in the development and progression of COPD. Song et al11 found that the deubiquitinating enzyme inhibitor P22077, an inhibitor of both USP7 and USP47, can block inflammatory vesicles for anti-inflammatory purposes. Whether deubiquitinating enzymes and inflammatory vesicles play a function in COPD is still unclear.
In this study, we explored the relationship between COPD, NLRP3 inflammatory vesicles and deubiquitinating enzymes using an experimental rat model of COPD. Our results may provide new ideas in the clinical treatment of COPD.
Material and Methods
Animals and Experimental Protocol
Ethical approval was obtained from the Ethics Committee of the North Sichuan Medical College, and all animal experiments were carried out in accordance with the requirements of the Institutional Animal Care and Use Committee. Twenty-one healthy adult male SD rats (240–260 g) were obtained from North Sichuan Medical College (Nanchong, Sichuan, China). Rats were given standard laboratory chow and water.
Main Reagents and Instruments
The reagents in this study were obtained from the following companies: Niu cigarettes (Sichuan China Tobacco Industry Co. LTD), P22077 (Cayman Corporation), lipopolysaccharide (Sigma Corporation), Rui-style-Kimsa staining solution (Nanjing Jiancheng Technology Co. Ltd.), enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Enzyme Link Biotechnology Co. Ltd.), rabbit anti-rat IgG secondary antibody (Wuhan PhD Biological Engineering Co. Ltd.), anti-ASC-antibody (Wuhan PhD Biological Engineering Co. Ltd.), anti-NLRP3-antibody (Gene Tex), HE staining (Wuhan PhD Biological Engineering Co. Ltd.), PSA staining kit (KGI Biology), self-made glass staining kit (Shanghai Lishen Scientific Instrument Factory), and high-speed frozen centrifuge (Shanghai Lishen Scientific Instrument Factory).
Animal Groups and Treatment
The animals were randomly assigned to three groups: the control group, COPD group, and P22077 group (n=7 per group). The COPD rat model was established as described by Song et al12. Both the COPD group and the P22077 group were subjected to the same smoke stimulation and lipopolysaccharide airway instillation at days D1-D30. Lipopolysaccharide (200 μg/200 μL) was given intratracheally on day 1 (d1) and d14. For the remaining time periods (d2–d13 and d15–d30), animals were placed in a homemade glass fumigation box (50 x 40×40 cm, 80 L) for 30 min (10 cigarettes each time), twice a day, with at least a 6 h interval). During d31–35, 1 mL of saline was injected intraperitoneally in the COPD group once a day, while 2 mg/kg of P22077 was injected intraperitoneally in the P22077 group once a day according to the drug instructions. In the control group, 200 μL of saline was given intratracheally on d1 and d14 and 1 mL of saline was injected intraperitoneally on d31–d35; no treatment was given for the remaining time. All animals were maintained on standard rat food. Within 24 hours after the last administration, the rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital (50 mg/kg) and sacrificed, and BALF, blood and lung tissues were collected for subsequent experiments.
BALF and Differential Cell Counts
After tracheal intubation, bilateral lung lavage was performed three times by slowly injecting 5 mL of 37 °C BPS solution from the trachea using a disposable syringe. Supernatants of BALF were stored at −80°C after centrifugation (4°C, 1200 rpm/min, 10 min). The cell pellets were resuspended in 200μL of PBS and four cell smears were prepared. Cell counts and sorting counts (at least 200 cells) were conducted using Wright-Giemsa staining. Absolute counts and ratios of macrophages (AMC), neutrophils (NEU), and lymphocytes (LYM) were calculated.
Tissue Staining
For HE staining, the thoracic cavity of rats was opened, and lung tissues were isolated and removed. Tissues were rinsed in sterile saline, fixed in 4% paraformaldehyde for 24 h, and dehydrated in gradient ethanol. Paraffin-embedded sections were made and sections were dewaxed and washed, followed by HE staining following kit instructions. The pathological features of the bronchi and lung tissues were observed under light microscopy. For PAS, sections were dewaxed and washed in water and then examined by light microscopy to observe the proliferation of primary bronchial cupped cells following PAS kit instructions.
Enzyme-Linked Immunosorbent Assay
IL-18 and IL-1β in rat serum and BALF were measured by ELISA using a double sandwich method according to the instruction manual. Samples were read at 450 nm and evaluated using a standard curve.
Western Blot Assays
Lung tissue (100 mg) was lysed in 1 mL of lysis solution (1 mL of enhanced RIPA lysis solution + 10 μL of broad-spectrum protease inhibitor mixture) and homogenized on ice. Protein concentration was calculated by the BCA protein quantification method. Equal amounts of protein samples were separated by SDS-PAGE and transferred to a a 0.45 µm PVDF membrane. The membrane was blocked with 5% skim milk for 1 h and then incubated with primary antibody (1:1000) overnight at 4°C. The membrane was washed and incubated with secondary antibody for rabbit anti-rat IgG secondary antibody (1:5000). Then the PVDF membrane was washed 5 times in TBST solution for 10 min. Finally, the protein content of the PVDF membrane was measured by gel analysis software.
Statistical Analysis
The SPSS statistical package was used for statistical analyses. Data are expressed as mean ± standard deviation.( ). For comparison between multiple samples, differences between groups were analyzed by one-way ANOVA or H-test if non-normal distribution or variance was not equal; SNK method was used for multiple comparisons. P < 0.05 indicated statistical significance.
). For comparison between multiple samples, differences between groups were analyzed by one-way ANOVA or H-test if non-normal distribution or variance was not equal; SNK method was used for multiple comparisons. P < 0.05 indicated statistical significance.
Results
Establishment of the COPD Model
While establishing the COPD model, the rats gradually developed poor mental health, with slowed movement, poor feeding, hydrophobia, and yellowing and withering of the hair during the nebulization process from d2 to d30. The animals became drowsy during the fogging, accompanied by flowing delay and cyanosis of the lips and mouth. Paroxysmal sputum and wheezing sounds were detected during d31–d35 in the COPD group. We observed less sputum chirping and wheezing frequency on d31–d35 in P22077-treated rats, with a gradual improvement in diet. The control rats maintained good condition, with regular breathing, without coughing or wheezing, and were responsive when moving around. No changes in body weight were observed among the three groups. No changes in body weight were observed among the three groups (Figure 1).
 |
Figure 1 Effect of P22077 on body weight in rats with COPD, The body weight change at three groups was not significantly different on statistics. |
Effect of P22077 on the Recruitment of Inflammatory Cells in BALF of Rats with COPD
The numbers of inflammatory cells in the BALF of rats in both the COPD and P22077 groups were significantly higher than those of controls (both P < 0.01) (Figure 2). The number of inflammatory cells in the BALF of rats in the P22077 group was significantly lower compared with controls (P < 0.01). The neutrophil and lymphocyte counts and the proportion of BALF were significantly higher in the COPD group while the alveolar macrophage count and proportion were slightly lower compared with numbers in controls (P < 0.01). However, the number and proportion of neutrophils and lymphocytes in the BALF of P22077 rats decreased and the number and proportion of alveolar macrophages increased compared with results in the COPD group (P < 0.01). In addition, the total number of cells, the proportion of alveolar macrophages, and the proportion of lymphocytes were all increased in the P22077 group compared with results in the COPD group (P < 0.01). There was no significant difference in the proportion of neutrophils.
IL-18 and IL-1β Inflammatory Factors in BALF and Serum of Rats
The levels of NLRP3-related inflammatory factors IL-18 and IL-1β were significantly higher in BALF and serum of rats in the COPD group compared with controls (both P < 0.01) (Figure 3). The BALF and serum levels of IL-18 and IL-1β were reduced in COPD rats treated with P22077; the differences in IL-18 and IL-1β levels between the CON and P22077 groups were not significant (P > 0.05). These results show that the COPD model exhibited increased secretion of IL-18 and IL-1β inflammatory factors, leading to an enhanced systemic inflammatory response in COPD rats. P22077 inhibited NLRP3 inflammatory pathway activation, resulting in lower levels of IL-18 and IL-1β BALF and serum levels in the P22077 group compared with the COPD group.
 |
Figure 3 (A) ELISA analysis of IL-1β and IL-18 in BALF. (B) ELISA analysis of IL-1β and IL-18 in serum. Data expressed as mean ± SEM (n=7 per group). ##P < 0.01 vs control group; **P < 0.01 vs COPD. |
Pathological Changes in the Lungs of Rats
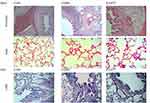
PAS and H&E staining were used to assess the effect of P22077 on the lung tissue of rats with lipopolysaccharide combined with fumigation–induced COPD (Figure 4). HE staining showed that the alveolar structure of the rats in the COPD group was disorganized compared with the controls. some alveoli were of different sizes, alveolar septa were seen to be broken and fused, airway epithelial cells were degenerated and necrotic, and the airway wall was infiltrated with a large number of inflammatory cells such as lymphocytes and neutrophils. The damaged alveolar and airway in P22077 group were not repaired, but the number of inflammatory cells in the airway wall was significantly reduced, and the airway inflammation was significantly reduced in rats. The proliferation of airway epithelial cup cells was observed by PAS staining of paraffin sections of rat lung tissues. It was difficult to find cup cells in the airway epithelium of control rats, while obvious and abundant strong positive PAS staining was seen in the bronchial tube wall of rats in the COPD group. The proliferation of cup cells in the airway wall and the number of bronchial cup cells were significantly reduced in rats treated with P22077.
Effect of P22077 on ASC, Caspase-1 and NLRP3 Protein Expression in Rat Lung Tissue
To further investigate the effect of the deubiquitinating enzyme inhibitor P22077 on the NLRP3 inflammatory pathway in lung tissues of rats with COPD, ASC, caspase-1, and NLRP3 protein expression in lung tissues of each group were examined by Western blot (Figure 5). The expression of ASC, caspase-1, and NLRP3 in the lung tissues of rats in the COPD group was significantly higher compared with levels in controls (P < 0.01). In the P22077 group, the expression of ASC and caspase-1 protein decreased significantly compared with levels in the COPD group (P < 0.01), and NLRP3 protein also decreased compared with levels in the COPD group (P < 0.05). ASC and NLRP3 protein contents in lung tissues of rats treated with P22077 were increased compared with controls, but the difference was not statistically significant (P > 0.05). In the P22077 group, caspase-1 protein expression was significantly increased compared with the CON group (P < 0.01). This suggests that in the COPD rats, the NLRP3 inflammatory vesicle pathway was activated, and P22077 reduced the expression of inflammatory vesicle pathway–related proteins and even to levels of that in controls.
Discussion
COPD is a chronic inflammatory airway disease characterized by irreversible airflow limitation. The inflammation of the bronchial mucosa and surrounding tissues results from damage to the lung parenchyma and small airways. The typical clinical manifestations are chronic cough, sputum, shortness of breath or dyspnea, wheezing, and chest tightness. The prevalence and mortality rates are increasing worldwide of COPD, causing a huge social and economic burden. At present, drug research has been stagnant for a long time, and the research of new therapeutic direction is an important focus.
The establishment of COPD rat models by lipopolysaccharide combined with fumigation has been previously demonstrated.11 In this study, with the increase of fumigation days, COPD model rats gradually showed symptoms such as wheezing, sneezing and audible sputum in the throat. Their activity tolerance decreased significantly. In our study, HE staining of the COPD samples showed that the bronchial epithelial cells of rats were partially necrotic and detached, cilia were inverted, the tracheal wall was thickened, a large number of inflammatory cells gathered and infiltrated, the alveolar wall was thinned, the lung septum was destroyed and broken, and some alveoli were fused and enlarged to form alveoli. PAS staining revealed a significantly higher number of airway cupped cells in the COPD group compared with controls. All the above pathological changes were consistent with the pathological changes of COPD rats. The total number of inflammatory cells, macrophages, lymphocytes and neutrophil counts in the BALF were significantly higher in the COPD group compared with controls. Notably, COPD model rats treated with P22077 showed a significant reduction in wheezing symptoms, a decrease in inflammatory cell infiltration around the tracheal wall, a reduction in PAS-positive staining of the tracheal wall, and a decrease in the number of multiple inflammatory cells in BALF.
Inflammation is a complex process in which the inflammatory response is designed to eliminate danger and stimulate the maintenance of homeostasis in the body’s tissues and organs Research has shown that the development and progression of COPD disease is closely related to the chronic inflammatory response of the body. This chronic inflammation allows inflammatory cells such as neutrophils, macrophages, and lymphocytes to accumulate in the respiratory system and secrete a variety of inflammatory mediators over a long period of time. Damage-associated or pathogen-associated molecules can trigger pattern recognition receptors (PRRs) to activate these immune cells and cause a downstream cascade of signaling pathway responses, resulting in increased expression of pro-inflammatory cytokines, leading to inflammation. Inflammatory vesicles respond to a variety of endogenous or exogenous danger signals and control the maturation and release of pro-inflammatory cytokines, which are important for protecting the host from infection. When induced by activating factors such as infection, they become over-activated and are a feature of many inflammatory diseases. The NLRP3 inflammatory vesicle signaling pathway is activated in COPD patients and experimental models during smoke exposure and infection13 Pro-inflammatory IL-1-like cytokines (ie IL-1β and IL-18) were significantly elevated in sputum and BALF of COPD patients, and the induction of IL-18, IL-1β, and accumulation of ASC spots during inflammasome activation sustained the inflammatory response.14 In this study, the levels of IL-18 and IL-1β, inflammatory factors downstream of the NLRP3 inflammatory pathway, were higher in the serum and BALF of COPD model rats compared with controls, suggesting that IL-18 and IL-1β are involved in COPD disease progression.
To further demonstrate the involvement of NLRP3 in the development of COPD, we examined the expression of several proteins involved in the NLRP3 inflammatory vesicle pathway, such as the junction protein ASC, the effector protein caspase-1, and the receptor protein NLRP3, in lung tissue. These proteins were significantly increased in the lung tissue of COPD rats, suggesting that the NLRP3 inflammatory vesicle signaling pathway is activated during COPD disease progression. The assembly of the NLRP3 inflammatory vesicle complex leads to the cleavage and activation of caspase-1, which further triggers the processing and release of IL-1β and IL-18, and ultimately leads to cell death through a pyrolytic process. Nachmias et al15 used LPS to stimulate isolated A549 cells to create an in vitro model of acute exacerbation of COPD and also confirmed that NLRP3 inflammatory vesicle activity was upregulated in acute exacerbation of COPD. Our study also confirmed that NLRP3 related protein content was significantly increased in the COPD model group. After treatment with P22077, the contents of ASC, caspase-1 and NLRP3 were decreased.
We found that the NLRP3 inflammatory vesicle pathway can be activated in rats in response to smoke and infection, but the cellular mechanisms regulating its activation are not fully understood. Multiple post-translational modifications and interactions of NLRP3 have been identified that regulate the activation of NLRP3 inflammatory vesicles. Lopez-Castejon et al16 first suggested that DUB inhibitors could inhibit NLRP3 ubiquitination and thus block NLRP3 inflammatory vesicle activation. Since then, ubiquitination was identified an important regulator of inflammatory vesicle assembly. Song et al17 found that the ubiquitin-specific peptidase 1 (USP1)-associated factor 1 (UAF1) deubiquitinase complex promoted NLRP3 inflammatory vesicle activation by targeting NLRP3 and p65, inhibiting their degradation, and enhancing NLRP3 and pro-IL-1β expression. USP7, which stabilizes p53, was first identified in vivo as a tumor suppressor and later found to regulate inflammation by modulating NF-κB signaling pathway proteins.18 USP7 is thus now attracting increasing attention as a potential inflammatory target. P22077, a USP7 inhibitor, has also been shown to inhibit the NF-κB and MAPKs pathways and exert important anti-inflammatory effects in vitro and in vivo by promoting K48-linked ubiquitination and TRAF6 degradation.19 Palazon-Riquelme et al9 found that in macrophages, P22077 prevents the formation and activation of inflammatory vesicles by inhibiting USP7 and USP47 and reducing ASC oligomerization and speckling. Liu et al20 also demonstrated that USP7 inhibitors can reduce the inflammatory response in osteoarthritis by inhibiting the NLRP3 inflammatory pathway. In this study we further showed that the protein expression of NLRP3 inflammatory vesicle complex (ASC, caspase-1, and NLRP3) in lung tissues of rats was significantly reduced by treating rats with the USP7 inhibitor P22077, and the levels of IL-18 and IL-1β, the downstream inflammatory mediators of NLRP3, in BALF and serum of rats were also significantly reduced. This suggests that P22077 may inhibit the formation of the NLRP3 inflammatory vesicle complex in COPD rats and affect the activation of inflammatory vesicle pathway, thus reducing the release of downstream inflammatory mediators and ultimately controlling the disease progression of COPD.
Conclusion
The NLRP3 inflammatory vesicle pathway is activated during the development of COPD in rats in response to microbial infection and smoke, resulting in caspase-1 activation and secretion of the pro-inflammatory cytokines IL-1β and IL-18. P22077, an inhibitor of the deubiquitinating enzyme USP7 and USP47, reduced the airway inflammatory response and airway inflammatory cell production in COPD rats by inhibiting the activation of the NLRP3 inflammatory vesicle pathway and reducing the production and release of downstream inflammatory factors. These results suggest new directions for the future clinical treatment of COPD.
Abbreviations
IL-18, interleukin-18; IL-1β, interleukin-1β; COPD, Chronic obstructive pulmonary disease; AMC, macrophages; NEU, neutrophils; LYM, lymphocytes; HE, hematoxylin-eosin; PAS, Schiff’s periodate stain; USP1, ubiquitin-specific peptidase 1; UAF1, associated factor 1.
Data Sharing Statement
The provided data supporting the findings of this study are included within the article.
Ethics Approval
Ethical clearance was sought from the Ethics Committee of the North Sichuan Medical College, and follow the European Union Directive (2010/63/EU) on the protection of laboratory animals used for scientific causes.
Acknowledgments
The authors acknowledge the laboratory provided by North Sichuan Medical College.
Author Contributions
All authors have made significant contributions to the reported work, encompassing conception, study design, execution, data acquisition, analysis and interpretation. They have actively participated in drafting, revising or critically reviewing the article. Furthermore, they have provided their final approval for the version intended for publication and reached a consensus on the journal of submission. Additionally, they acknowledge their responsibility for all aspects of this research endeavor.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. Available from: https://goldcopd.org/2023-gold-report-2/.
2. World Health Organization. Projections of mortality and causes of death, 2017-2060 【EB/OL】 2018.
3. Zhang X, Xu A, Lv J, et al. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur J Med Chem. 2020;185:111822. doi:10.1016/j.ejmech.2019.111822
4. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi:10.1038/nm.3893
5. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi:10.1038/nri3452
6. Yang J, Liu Z, Xiao TS. Post-translational regulation of inflammasomes. Cell Mol Immunol. 2017;14(1):65–79. doi:10.1038/cmi.2016.29
7. Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. doi:10.1074/jbc.M112.407130
8. Zeng M, Zhang X, Xing W, Wang Q, Liang G, He Z. Cigarette smoke extract mediates cell premature senescence in chronic obstructive pulmonary disease patients by up-regulating USP7 to activate p300-p53/p21 pathway. Toxicol Lett. 2022;359:31–45. doi:10.1016/j.toxlet.2022.01.017
9. Palazon-Riquelme P, Worboys JD, Green J, et al. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 2018;19(10). doi:10.15252/embr.201744766
10. Cui Y, Luo L, Zeng Z, et al. MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells. Cell Death Dis. 2023;14(1):2. doi:10.1038/s41419-022-05455-8
11. Juliana C, Fernandes-Alnemri T, Wu J, et al. Anti-inflammatory compounds parthenolide and bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285(13):9792–9802. doi:10.1074/jbc.M109.082305
12. Song X, Wang C, Bai C. Establishment of rat chronic obstructive pulmonary disease model: a comparison between exposure to cigarette smoke alone and in combination with intra-tracheal injection of lipopolysaccharide. Former Acad J Sec Mil Med Univers. 2010;31(03):246–249. doi:10.3724/SP.J.1008.2010.00246
13. Kim RY, Pinkerton JW, Gibson PG, Cooper MA, Horvat JC, Hansbro PM. Inflammasomes in COPD and neutrophilic asthma. Thorax. 2015;70(12):1199–1201. doi:10.1136/thoraxjnl-2014-206736
14. Franklin BS, Bossaller L, De Nardo D, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15(8):727–737. doi:10.1038/ni.2913
15. Nachmias N, Langier S, Brzezinski RY, et al. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS One. 2019;14(5):e214622. doi:10.1371/journal.pone.0214622
16. Faner R, Sobradillo P, Noguera A, et al. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res. 2016;2(3):00002–2016. doi:10.1183/23120541.00002-2016
17. Song H, Zhao C, Yu Z, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11(1):6042. doi:10.1038/s41467-020-19939-8
18. Mitxitorena I, Somma D, Mitchell JP, et al. The deubiquitinase USP7 uses a distinct ubiquitin-like domain to deubiquitinate NF-kB subunits. J Biol Chem. 2020;295(33):11754–11763. doi:10.1074/jbc.RA120.014113
19. Zhao XB, Ji FY, Li HR, et al. P22077 inhibits LPS-induced inflammatory response by promoting K48-linked ubiquitination and degradation of TRAF6. Aging. 2020;12(11):10969–10982. doi:10.18632/aging.103309
20. Liu G, Liu Q, Yan B, Zhu Z, Xu Y. USP7 inhibition alleviates H(2)O(2)-induced injury in chondrocytes via inhibiting NOX4/NLRP3 pathway. Front Pharmacol. 2020;11:617270. doi:10.3389/fphar.2020.617270
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.