Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Inhibition of Yes-Associated Protein-1 (YAP1) Enhances the Response of Invasive Breast Cancer Cells to the Standard Therapy
Authors Guimei M , Alrouh S , Saber-Ayad M , Hafezi SA, Vinod A, Rawat S, Wardeh Y, Bakkour TM, El-Serafi AT
Received 25 June 2020
Accepted for publication 15 October 2020
Published 2 November 2020 Volume 2020:12 Pages 189—199
DOI https://doi.org/10.2147/BCTT.S268926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Maha Guimei,1,2 Sana Alrouh,3 Maha Saber-Ayad,2– 4 Shirin A Hafezi,3 Arya Vinod,3 Surendra Rawat,3 Yazan Wardeh,5 Tala Mohamad Bakkour,5 Ahmed Taher El-Serafi3,6,7
1Department of Pathology, Faculty of Medicine, Alexandria University, Alexandria, Egypt; 2Clinical Sciences Department, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates; 3Sharjah Institute of Medical Research, University of Sharjah, Sharjah, United Arab Emirates; 4Department of Pharmacology, College of Medicine, Cairo University, Cairo, Egypt; 5College of Medicine, University of Sharjah, Sharjah, United Arab Emirates; 6Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Suez Canal University, Ismailia, Egypt; 7Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
Correspondence: Maha Guimei
Department of Pathology, Faculty of Medicine, Alexandria University, 17 Champollion Street, Alexandria, Egypt
Tel +20 1005384268
Email [email protected]
Purpose: The deregulation of the Hippo pathway results in translocation ofYes-associated protein-1 (YAP1) to the nucleus to exert an oncogenic effect. This effect has been demonstrated in several malignancies, yet, in breast cancer (BC), it remains controversial. The present study aimed to investigate the significance of YAP1 expression in BC, its relation to cancer stem cells (CSCs), and the effect of its inhibition on tumor cell survival.
Patients and Methods: We evaluated the expression of YAP1 protein and gene using immunohistochemistry (IHC) and RT-qPCR in FFPE tissue from normal and breast cancer cases. We also studied its association with CSC expression (OCT4, NANOG, and SOX2) and with different clinicopathologic characteristics. Two BC cell lines (MCF7 and MDA-MB-231) were exposed to different concentrations of YAP1 inhibitor “verteporfin” and cell viability was subsequently assessed.
Results: YAP1 mRNA was higher in BC compared to the normal breast tissue (p-value=0.040) and was higher in luminal tumors compared to triple-negative breast cancer (TNBC) (p-value= 0.017). Its expression in tumors was significantly associated with the expression of pluripotency markers (OCT4 and NANOG) (p-value= 0.030 and 0.035, respectively) and its inhibition resulted in a significant reduction of CSC expression in both MCF-7 and MDA-MB-231 cells. YAP1 nuclear expression by IHC, which signifies its activation, was more evident in invasive carcinomas compared to normal breast tissue and in-situ foci where the expression was limited to the cytoplasm. The pretreatment of BC cells (MCF7 and MDA-MB-231) with YAP1 inhibitor “verteporfin” resulted in their sensitization to the effect of tamoxifen and doxorubicin, respectively, and significantly decreased tumor cell proliferation and survival.
Conclusion: Our results imply that YAP1 is highly expressed and activated in BC and its inhibition could represent a possible novel therapeutic strategy that should be further explored and investigated to improve the outcome of breast cancer patients.
Keywords: breast cancer, YAP1, Hippo pathway, cancer stem cells, verteporfin
Introduction
Breast cancer (BC) remains a leading cause of cancer-related deaths among females.1 The identification of the exact molecular subtype is crucial for prognostic purposes as well as for planning the appropriate therapeutic strategy.2 Despite the great advances achieved in the field of BC therapy, more personalized therapeutic regimens are still needed in order to improve the outcome and minimize the undesired side effects of currently used medications.
Yes-associated protein-1 (YAP1) is the main transcriptional regulator in the Hippo-signaling pathway. This pathway’s main function is to regulate organ size by restricting cell proliferation and enhancing apoptosis.3 YAP1shuttles between the cytoplasm and the nucleus. When Hippo pathway is active, LATS1/2 kinases phosphorylate and sequester YAP1 in the cytoplasm and prevent its translocation to the nucleus to promote the transcription of Hippo pathway downstream genes that are mostly responsible for cellular proliferation and migration.4
The deregulation of the Hippo pathway was reported in several solid malignancies and YAP1 was shown to play an oncogenic role in tumors like lung, colon, ovary, liver, and prostate cancers.5,6
To date, data concerning the exact role of YAP1 in breast cancer remain largely inconsistent. Conflicting results onto whether YAP1 acts as an oncogene or as a tumor suppressor gene have been reported in the literature. Studies suggesting an oncogenic effect have demonstrated that YAP1 overexpression in cell lines was associated with enhanced proliferation,7 whereas those proposing a tumor suppressor effect observed an increased cell migration in the YAP1-downregulated BC cells.8 This controversy between reported results suggests that the role of YAP1 in breast cancer may be contextual and may differ according to specific molecular characteristics of the studied cohort. It could also be related to the wide variability in the methods used to detect YAP1 in these studies.
YAP1 signaling is known to play an important role in promoting embryonic stem cells (ES) and tissue-specific stem cell self-renewal. Recent studies have shown that YAP1 signaling activated cancer stem cells (CSCs) in the liver leading to enhanced tumor propagation.9 Cancer stem cells in the breast regulate epithelial–mesenchymal transition (EMT) and their expression is associated with an aggressive tumor pathology and an enhanced metastatic potential.10 Yet, little is known about the exact relation between of YAP1 expression and CSCs in Breast cancer.
Due to its proposed oncogenic effect, YAP1 has been regarded as a potential target for therapy. Verteporfin (VP) is an FDA-approved photosensitizer that is used in the treatment of macular degeneration.11 It has recently been recognized as a YAP1 antagonist, capable without light activation, of disrupting YAP1–TEAD interaction and thus downregulating the transcription of downstream proto-oncogenes such as c-myc, Axl, and survivin.12,13 The effect of YAP1 inhibition using VP has been investigated in hepatocellular carcinoma, retinoblastoma as well as in ovarian tumors where it resulted in inhibition of tumor cell proliferation by suppressing YAP1 activity.12–14 However, this effect has not been investigated in BC.
In this study, we investigated the significance of YAP1 expression in breast cancer and its relation to CSC expression and other clinicopathological parameters of the tumors. Furthermore, we explored the effect of YAP1 inhibition using “verteporfin” on breast cancer tumor cell survival and proliferation.
Patients and Methods
Study Population
The present study was conducted on 23 formalin-fixed paraffin-embedded (FFPE) specimens from patients diagnosed with Invasive ductal carcinoma, NST. The study comprised five cases of luminal A, 12 cases of luminal B cases and 6 cases triple-negative breast cancer (TNBC). Five samples of normal breast tissues were used as control. Invasive lobular carcinomas and other special type breast carcinomas were not included in the current study. Specimens were obtained from the archives of the pathology department, Alexandria University. All patients had undergone surgical tumor resections whether total mastectomies or local conservative resections followed by chemotherapy ± hormonal therapy and were followed up for a mean period of 5 years after completion of treatment. Patient clinicopathological characteristics including patient age, tumor grade, TNM stage and lymphovascular (LV) invasion as well as follow-up data were retrieved from the records of the Pathology and Oncology departments. Data concerning estrogen and progesterone receptor expression (ER and PR), human epidermal growth receptor-2 (HER2), and Ki67 expression were also retrieved from patient records (Table 1).
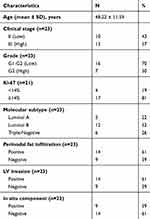 |
Table 1 Clinicopathologic Characteristics of the Studied Tumors |
The study was approved by the Research Ethics Committee of the Faculty of Medicine, Alexandria University (Alexandria, Egypt). Written informed consent was obtained from the patients according to the Helsinki declaration.
YAP1 Immunohistochemistry (IHC)
Representative 4 µm thick tumor sections were immunohistochemically stained according to previously described protocol.15 Antigen retrieval was done by boiling in a Tris/EDTA (pH 9.0) for 20 minutes. Anti-active YAP1 Rabbit monoclonal antibody was used at a dilution of 1:2000 (ab205270, Abcam, Cambridge, UK). Rabbit-specific HRP/DAB (ABC) IHC Detection Kit (ab64261, Abcam, Cambridge, UK) was used following the manufacture’s protocol. Positive and negative control slides were included in all the runs. Prostate tissue was used as a positive control for YAP1. The immunohistochemical interpretation of YAP1 expression was performed by a research associate (SR) and a pathologist (MG). Because activation of YAP1 leads to its translocation to the nucleus, positive staining was considered only when YAP1 was strongly expressed in more than 20% of the tumor cell nuclei.16 All cytoplasmic staining or minimal nuclear staining were considered negative.
RNA Extraction and RT-qPCR
Total RNA was extracted from FFPE specimens using FFPE RNA purification kit (cat. 25300, NORGEN BIOTEK, Thorold, ON, Canada) following the manufacture’s protocol. This was followed by Reverse transcription into complementary DNA (cDNA) using TruScript First Strand cDNA Synthesis Kit (cat. 54,420, NORGEN BIOTEK, Thorold, ON, Canada).
The reaction mix was based on GoTaq qPCR Master Mix (cat. A6002, Promega, Madison, Wisconsin, Unites States) in a final volume of 20μL. The thermal cycler Rotor-Gene Q (QIAGEN, Hilden, Germany) was used according to the following conditions: 95°C for 15 minutes followed by 40 cycles at 94°C for 15 seconds, 55°C for 30 seconds, 70°C for 30 seconds. The threshold cycle value (CT) of each gene was normalized against the CT value of the housekeeping gene (GAPDH). The fold change was determined as 2−ΔΔCt. Fold change was calculated with reference to control samples. Each sample was tested in triplicates. The primers used are listed in (Table 2).
 |
Table 2 Primer Sequence for YAP1, NANOG, OCT4and SOX-2 |
Cell Culture
MDA-MB-231 and MCF-7 breast cancer cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, St. Louis, Missouri, Unites States) supplemented with 10% Fetal Bovine Serum (FBS, Sigma-Aldrich) and 1% Penicillin/Streptomycin (P/S) (Sigma-Aldrich, St. Louis, Missouri, Unites States) at 37°C in a humidified chamber with 5% CO2.
Paraffin Embedding of Breast Cancer Cell Lines
One million of MCF-7 and MDA-MB-231 cells were collected in a microcentrifuge tube and allowed for centrifugation at 500 g for 6 minutes. Then, 30μL of thrombin and 50μL of plasma were added to the cell pellet. Cells were briefly vortexed, then incubated at 37°C for 15 min to allow for clot formation. Cell clots were then wrapped in a filter paper and transferred to a cassette for processing using Excelsior™ AS Tissue Processor (Thermo Fisher Scientific) that uses the following reagents: 10% Formalin, 15 minutes for fixation, then the following each for 1 hour: 10% Formalin, 75% Alcohol, 90% Alcohol, 95% Alcohol, three changes of 100% Alcohol, three changes 100% Xylene, heated paraffin wax, and then another two changes of paraffin wax each for 30 minutes. Tissues were then embedded in paraffin and FFPE blocks were prepared and stored in room temperature.
Cell Viability MTT Assay
Cells were seeded in 96-well microtiter plates (104 cells/well) overnight. Five micrometers of verteporfin was tested alone and/or in combination with different doses of tamoxifen and doxorubicin for 24 hours. Then, cells were incubated with either tamoxifen alone and/or doxorubicin alone (0.01, 0.05, 0.1, 0.5, 1, 5 and 10 uM) or in combination with 5uM of verteporfin for 24 hours. For viability, a volume 200 μL of Methylthiazolyldiphenyl-tetrazolium bromide, MTT (CAT #M5655) was added to each well to detect cell viability. Then, cells were incubated in a humidified incubator at 5% CO2 at 37°C for 2 hours. Two hundred-microliter Dimethyl sulfoxide (CAT #276855) (DMSO) well was used to dissolve MTT product and the absorbance was read at 570 nm using a plate reader. All concentrations were tested in triplicates. Experiments were carried out 3 times.
Western Blotting
The cell culture dishes were placed on ice. Cell lysates were made using 1% triton Lysis buffer (TLB) cocktail supplemented with PMSF, 100mM NaVo3, 1M NaF, and Protease inhibitor cocktail. Subsequently, the lysates were centrifuged at 14000g for 20 min at 4°C. Then, protein concentration was determined using the Thermo Scientific Pierce BCA Protein Assay Kit. The cell lysates (10µg of protein per lane) were diluted in 1X Laemelli’s buffer solution, at 95°C for 5 min. The proteins were then loaded into SDS-PAGE (10% resolving gel, and 3% stacking gel). Immunoblotting was performed by probing proteins transferred onto nitrocellulose membranes using a Trans-Blot® TurboTM Blotting system (Bio-Rad) according to the standard transfer protocol. The membrane was blocked for 1hr at room temperature with 5% of Bovine Serum Albumin (BSA) in Tween Tris-Buffered saline (TTBS). Followed by incubation with the primary antibody overnight at 4°C on a shaker and then with secondary anti-mouse IgG (1/1000) antibody for 1 hour at room temperature. The blots were then visualized with enhanced chemiluminescence (ECL) Kit and imaged using ChemidocTM Touch Imaging System (Bio-Rad). The housekeeping protein β –actin was used to normalize the levels of protein detected by confirming that protein loading is the same across the gel. A loading control, β –actin (Primary antibody, rabbit) level was detected using anti-rabbit secondary antibody. The following primary antibodies from the R&D system and Cell signaling were used: Anti-YAP1 1;1000 mouse monoclonal antibody (Cat. # MAB8094, R&D system), B-actin 1;1000 Rabbit antibody (cell signaling). Secondary antibodies: Anti-rabbit (1/1000) for B-actin, Anti-mouse for YAP1 (1/1000) were used.
Statistical Analysis
Statistical analyses were done using SPSS version 23. Student’s t and Chi-Square tests were used for continuous and categorical variables and the Mann–Whitney U-test for non-normally distributed samples. Correlation between two quantitative continuous variables was estimated by Spearman’s rho. Values of P < 0.05 were considered statistically significant.
CompuSyn software was used to detect drug interaction. It is based on the Chou-Talalay method for quantitative drug combination applying the median-effect equation. The output is expressed as a combination index (CI), where CI<1, =1, and >1 indicate synergism, additive effect, and antagonism, respectively.
Results
YAP1 Nuclear Expression in Invasive Tumors of Luminal Type
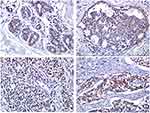
YAP1 cytoplasmic expression was noted in 92% of tumors whereas nuclear expression, which theoretically signifies YAP1 activation, was only demonstrated in 67% of the cases. All cases showing positive nuclear expression consistently showed high mRNA levels by qPCR. We also noted marked heterogeneity in YAP1 protein expression within tumors, with areas showing strong nuclear staining and other areas showing total negativity, tumors were considered positive when more than 20% of the cells showed strong nuclear YAP1 expression.
Out of all the cases showing YAP1 nuclear positivity, 86.6% were of luminal type expressing estrogen and/or progesterone receptors and only 13.3% of the cases belonged to the TNBC category. Yet, YAP1 nuclear expression did not show any statistically significant correlation with independent expression of ER, PR, HER2, or Ki67% or with tumor molecular subtype (Table 3).
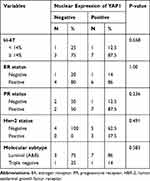 |
Table 3 Nuclear Expression of YAP1 in Relation to Ki67%, ER, PR, Her2 and Tumor Molecular Subtype |
In the foci of Ductal carcinoma in situ (DCIS), YAP1 IHC expression was mainly cytoplasmic with no evidence of nuclear staining. Whereas in normal breast tissue, YAP1 expression was only limited to the myoepithelial cells with faint cytoplasmic staining in luminal epithelial cells (Figure 1A-D).
YAP1 mRNA Expression is Higher in BC and is Associated with Stemness
The expression of YAP1 mRNA was significantly higher in BC compared to the normal breast (p=0.040, Mann–Whitney U-test) (supplementary Figure 1) and was higher in Luminal tumors compared to TNBC (p=0.017, t-test). (Figure 2) There was no statistically significant correlation between YAP1 mRNA expression and patient age, tumor grade, TNM stage, perinodal fat infiltration, lymphovascular invasion nor with patient clinical outcome after the follow-up period.
 |
Figure 2 YAP1 mRNA expression in the different molecular subtypes of breast cancer. qRT-PCR analysis showing significant increase in average YAP1 mRNA in luminal compared to TNBC (*P = 0.017). |
In BC tissues, the expression of CSCs (OCT4, NANOG, SOX2) was significantly higher compared to normal breast tissue (Figure 3A). The expression of OCT4 and NANOG was significantly associated with higher YAP1 expression in tumors (p-value= 0.030 and 0.035, respectively, Spearman’s Rho test). Stem cell markers showed a significant association with features of aggressiveness; OCT4demonstrated a significant association with a high proliferation index (Ki-67% ≥14%) (p=0.010, t-test), and SOX2 was associated with lymphovascular invasion (p=0.005, t-test). (Table 4)
 |
Table 4 Stem Cell Marker Expression in Breast Cancer in Relation to the Different Clinicopathological Characteristics of the Tumors |
Treatment of MCF-7 cells with YAP1 inhibitor “VP”, at a dose of 5uM, resulted in a significant reduction of YAP1 mRNA (p=0.04) and a significant reduction in expression of pluripotency markers; SOX2 and NANOG (p-value= 0.008 and 0.005, respectively). (Figure 3B)
Whereas in MDA-MB-231 cells, a similar reduction in mRNA expression of YAP1 and SOX2 was observed, yet, it did not reach statistical significance. Moreover, the expression of NANOG was increased in VP-treated cells compared to untreated cells (p=0.03). (Figure 3C)
The present study did not demonstrate a significant difference between the mRNA expression of YAP1 in the two studied breast cancer cell lines (MCF-7 and MDA-MB-231) (p=0.09). And on the protein level, the IHC study showed that YAP1 was equally expressed in both nuclei and cytoplasm in both cell lines (Figure 4A-C).
YAP1 Inhibition by Verteporfin Sensitizes BC Cells to Standard Therapy
First, we confirmed the inhibitory effect of verteporfin on YAP1 expression in both MCF7 and MDA-MB-231 cell lines. Then, we explored the effect of verteporfin treatment alone at several doses on tumor cell viability. For MDA-MB231 cells, IC50 was 3.986 µM calculated by GraphPad Prism8. For MCF7 cells, the maximum inhibition of proliferation was only 28% (so no IC50 was calculated). (Figure 5A-C)
After that, we evaluated the effect of pre-treatment of cells with different doses of verteporfin (either 2 or 5 uM) followed after 24 hours by treatment with Tamoxifen or Doxorubicin. We compared the effect of VP pretreatment on cell viability with the effect of using tamoxifen or Doxorubicin as a single treatment. We found that pretreatment with verteporfin at a dose of (5 µM) for 24 hours followed by tamoxifen in the case of MCF7 cells caused the sensitization of the pre-treated cells and resulted in a significant reduction of proliferation at all doses of tamoxifen compared to untreated cells (p <0.05). Similar results were obtained upon pretreatment of MDA-MB231 cells with doxorubicin. The interaction between verteporfin and tamoxifen in MCF7 or verteporfin and doxorubicin in MDA-MB231 was calculated through observing the effect at all tested concentrations of each drug as well as the combinations. Combining verteporfin with either tamoxifen or doxorubicin resulted in a CI <1, indicating synergism (Figure 6A and B).
Discussion
The inactivation of the Hippo pathway with the resultant YAP1 translocation to the nucleus is known to exert an oncogenic effect in many tumors.4 However, data concerning the exact role of YAP1 in breast cancer are still far from being conclusive. The present study demonstrated a significantly higher mRNA levels as well as predominantly nuclear protein expression of YAP1 in invasive breast cancers compared to normal breast tissue. We also demonstrated a significantly higher YAP1 expression in luminal compared to TNBC.
The observed higher mRNA levels of YAP1 together with the nuclear protein expression in tumors provide good evidence for YAP1 activation in BC compared to normal breast.4 These results are following studies that have previously suggested an oncogenic role for YAP1 in BC; One study has shown that animals transplanted with YAP1-overexpressing cells had an enhanced tumor growth7 and another study demonstrated that YAP1 nuclear expression was higher in brain metastasis and was associated with shorter overall survival in BC patients.17 On the other hand, an opposite effect was suggested by Yuan et al who showed that YAP1 expression was lost in tumors compared to normal tissues, and its knockdown increased invasiveness and reduced tumor response to treatment.8 The observed discrepancy between the results of these studies can be attributed to the different methods used to evaluate YAP1 expression as well as the different types of antibodies being used. Hence, in our study, we opted to use an antibody that is specifically directed against “active YAP1”, we combined the detection of both mRNA and protein to confirm our results and we used both human BC tissue as well as BC cell lines.
In the present study, YAP1 expression was significantly higher in hormone receptor-positive tumors (luminal) compared to TNBC. This was evident both on the mRNA level and less evident on the protein level. Previous studies have also shown reduced YAP1 expression in breast cancers lacking ER and PR expression18 as well as increased nuclear YAP1 expression in PR positive tumors.19 Thus, our data further emphasize the existence of a positive correlation between hormone receptors and YAP1 activity in BC. This relation can be explained by the ability of YAP1 to modulate the ligand-dependent transcriptional activity of ER and PR via its WW domain-binding protein (WBP-2),20 or by the fact that YAP1/TEAD4 act as cofactors that bind to ER-bound enhancers, induce ER target genes and enhance the Estrogen-induced oncogenic growth.21 This interesting observation certainly warrants an in-depth investigation in order to elucidate the exact underlying mechanistic pathways linking YAP1 to hormone receptors and further explore the possibility of using YAP1 as an actionable therapeutic target in luminal tumors.
The ability of malignant cells to initiate tumors is known to be highly dependent on self-renewal and stem cell-like properties. These properties are orchestrated by embryonic antigens like (OCT4, NANOG, and SOX-2), and are controlled by signaling through various pathways including the Hippo pathway.22,23 Chemotherapeutic agents target only the non-CSCs population within tumors and leaves behind a CSC-rich tumor environment responsible for drug resistance, metastasis, and recurrence, which are the major causes of cancer mortality.24,25 This, in turn, has led to a search for novel therapeutic strategies that can target or inhibit the CSC-generating pathways. In the present study, we demonstrated a significantly higher expression of stem cell markers (OCT4 and NANOG) in BC compared to normal breast and a significant association between the expression of those markers and YAP1 was identified. This association between YAP1and stemness has been demonstrated in several tumors. However, the exact CSC-specific regulatory mechanisms have not been fully investigated. In urinary bladder carcinomas, SOX2 was associated with YAP1 expression and contributed to the accumulation of urothelial CSCs.26 In lung cancer cells, NANOG and OCT4 expression were downregulated in spheroids silenced for YAP1/TAZ,27 and in Non-small cell lung cancer cells (NSCLC), YAP1 was found to interact with OCT4 in order to induce self-renewal in vitro.28 As for breast cancer, one recent study has shown that attenuation of YAP1 nuclear accumulation was associated with decreased expression of stemness markers.29
In the present study, we demonstrated that MCF-7 cells expressed high levels of YAP1 mRNA and that inhibition of YAP1 in these cells, using VP, was associated with a significant reduction in the expression stemness markers (SOX2 and NANOG). This, may in turn, suggest that targeting YAP1 could represent a potential novel method for reducing CSCs in hormone-dependent BC cells.
As for MDA-MB-231 cells, although they expressed high levels of YAP1 mRNA, VP treatment resulted in a reduction of SOX2expression that was not as significant as well as an increased NANOG expression in VP-treated cells compared to untreated cells. This latter unexpected finding together with the less evident reduction in stem cell markers in MDA-MB-231 cells can be attributed to the high PD-L1 expression in these cells. PD-L1 is known to regulate OCT4 and NANOG and has a direct effect on sustaining the stemness of CSCs in a PI3K/AKT‐dependent manner.30
Since the results of the present study mostly favored a tumor-promoting effect for YAP1 in BC, we decided to explore the effect of YAP1 inhibition using verteporfin (VP) on tumor cell survival and proliferation. We observed that adding the YAP1 inhibitor, verteporfin (VP) to Tamoxifen, or Doxorubicin resulted in a significantly reduced survival of both MCF-7 and MDA-MB231 cells, respectively, by exerting a synergistic effect to the anticancer activity of these commonly used drugs. The anticancer effect of VP has been recently explored in many tumors; In bladder cancer cells (UMUC-3 and 5637 cells), VP dramatically inhibited cancer cell invasion properties31 and in retinoblastoma cell lines (Y79 and WERI), it inhibited the growth, proliferation, and viability of the tumor cells in a dose-dependent manner.13 Although not demonstrated in our study, VP is suggested to exert this effect by increasing the levels of a YAP1 chaperon protein, 14-3-3σ thus retaining YAP1 in the cytoplasm and targeting it for degradation in the proteasome.32
Conclusion
Although the findings in our study remain riddled with many open questions, and despite the small number of cases examined in the study, the available data provide sufficient evidence to pinpoint YAP1 as a prime candidate for the development of anti-cancer treatments and suggest that its inhibition using VP, or any other inhibitory technique, could represent a promising strategy that may result in more effective personalization of BC treatment regimens.
Acknowledgment
The authors acknowledge the support offered by the Research Institute of Medical and Health Sciences at the University of Sharjah.
Disclosure
The authors declare no conflicts of interest.
References
1. Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017;151:1–32.
2. Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1848–1855. doi:10.1158/1055-9965.EPI-12-0474
3. Porazinski S, Wang H, Asaoka Y, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521(7551):217–221.
4. Hagenbeek TJ, Webster JD, Kljavin NM, et al. The Hippo pathway effector TAZ induces TEAD-dependent liver inflammation and tumors. Sci Signal. 2018;11(547):547. doi:10.1126/scisignal.aaj1757
5. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. doi:10.1016/j.ccell.2016.05.005
6. Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582–1589. doi:10.1016/j.humpath.2008.04.012
7. Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. Eur J Cancer. 2012;48(8):1227–1234. doi:10.1016/j.ejca.2011.10.001
8. Yuan M, Tomlinson V, Lara R, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008;15(11):1752–1759. doi:10.1038/cdd.2008.108
9. Zhu P, Wang Y, Wu J, et al. LncBRM initiates YAP1 signalling activation to drive self-renewal of liver cancer stem cells. Nat Commun. 2016;7(1):13608. doi:10.1038/ncomms13608
10. Wang D, Lu P, Zhang H, et al. Oct-4 and nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5(21):10803–10815. doi:10.18632/oncotarget.2506
11. Henney JE. From the food and drug administration. JAMA. 2000;283(21):2779. doi:10.1001/jama.283.21.2779-JFD00004-2-1
12. Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi:10.1101/gad.192856.112
13. Brodowska K, Al-Moujahed A, Marmalidou A, et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res. 2014;124:67–73. doi:10.1016/j.exer.2014.04.011
14. Feng J, Gou J, Jia J, Yi T, Cui T, Li Z. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco Targets Ther. 2016;9:5371–5381. doi:10.2147/OTT.S109979
15. Guimei M, Eladl MA, Ranade AV, Manzoor S. Autophagy related markers (Beclin-1 and ATG4B) are strongly expressed in Wilms’ tumor and correlate with favorable histology. Histol Histopathol. 2019;34(1):47–56.
16. Vlug EJ, van de Ven RAH, Vermeulen JF, Bult P, van Diest PJ, Derksen PWB. Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer. Cell Oncol. 2013;36(5):375–384. doi:10.1007/s13402-013-0143-7
17. Kim HM, Jung WH, Koo JS. Expression of Yes-associated protein (YAP) in metastatic breast cancer. Int J Clin Exp Pathol. 2015;8(9):11248–11257.
18. Tufail R, Jorda M, Zhao W, Reis I, Nawaz Z. Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131(3):743–750. doi:10.1007/s10549-011-1435-0
19. Cao L, Sun PL, Yao M, Jia M, Gao H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum Pathol. 2017;68:166–174. doi:10.1016/j.humpath.2017.08.032
20. Dhananjayan SC, Ramamoorthy S, Khan OY, et al. WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol. 2006;20(10):2343–2354. doi:10.1210/me.2005-0533
21. Zhu C, Li L, Zhang Z, et al. A non-canonical role of YAP/TEAD is required for activation of estrogen-regulated enhancers in breast cancer. Mol Cell. 2019;75(4):791–806.e798. doi:10.1016/j.molcel.2019.06.010
22. Ohgushi M, Minaguchi M, Rho-Signaling-Directed SY. YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell. 2015;17(4):448–461. doi:10.1016/j.stem.2015.07.009
23. Park JH, Shin JE, Park HW. The role of Hippo pathway in cancer stem cell biology. Mol Cells. 2018;41(2):83–92.
24. Shibata M, Ham K, Hoque MO. A time for YAP1: tumorigenesis, immunosuppression and targeted therapy. Int J Cancer. 2018;143(9):2133–2144. doi:10.1002/ijc.31561
25. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–629.
26. Ooki A, Del Carmen Rodriguez Pena M, Marchionni L, et al. YAP1 and COX2 coordinately regulate urothelial cancer stem-like cells. Cancer Res. 2018;78(1):168–181. doi:10.1158/0008-5472.CAN-17-0836
27. Noto A, De Vitis C, Pisanu ME, et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36(32):4573–4584. doi:10.1038/onc.2017.75
28. Bora-Singhal N, Nguyen J, Schaal C, et al. YAP1 regulates OCT4 activity and SOX2 expression to facilitate self-renewal and vascular mimicry of stem-like cells. Stem Cells. 2015;33(6):1705–1718. doi:10.1002/stem.1993
29. Zheng L, Xiang C, Li X, et al. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol Oncol. 2018;11(1):72. doi:10.1186/s13045-018-0613-5
30. Almozyan S, Colak D, Mansour F, et al. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer. 2017;141(7):1402–1412. doi:10.1002/ijc.30834
31. Dong L, Lin F, Wu W, Liu Y, Huang W. Verteporfin inhibits YAP-induced bladder cancer cell growth and invasion via Hippo signaling pathway. Int J Med Sci. 2018;15(6):645–652. doi:10.7150/ijms.23460
32. Wang C, Zhu X, Feng W, et al. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J Cancer Res. 2015;6(1):27–37.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.