Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Inhibition of SK Channels in VTA Affects Dopaminergic Neurons to Improve the Depression-Like Behaviors of Post-Stroke Depression Rats
Authors Wang A , Zhou Y, Chen H , Jin J , Mao Y , Tao S , Qiu T
Received 9 July 2023
Accepted for publication 22 September 2023
Published 9 October 2023 Volume 2023:19 Pages 2127—2139
DOI https://doi.org/10.2147/NDT.S426091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Anqi Wang,1,* Yujia Zhou,2,* Huangying Chen,1 Jiawei Jin,1 Yingqi Mao,3 Shuiliang Tao,4 Tao Qiu5
1First Clinical Medical College, Zhejiang Chinese Medical University, Zhejiang, People’s Republic of China; 2Second Clinical Medical College, Zhejiang Chinese Medical University, Zhejiang, People’s Republic of China; 3Faculty of Chinese Medicine, Macau University of Science and Technology, Macau, People’s Republic of China; 4Basic Medicine College, Zhejiang Chinese Medical University, Zhejiang, People’s Republic of China; 5Department of Neurology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuiliang Tao, Basic Medicine College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310000, People’s Republic of China, Email [email protected] Tao Qiu, Department of Neurology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310000, People’s Republic of China, Email [email protected]
Purpose: This study aimed to investigate the effect of small-conductance calcium-activated potassium channels (SK channels) on the dopaminergic (DA) neuron pathways in the ventral tegmental area (VTA) during the pathogenesis of post-stroke depression (PSD) and explore the improvement of PSD by inhibiting the SK channels.
Patients and Methods: Four groups of Sprague-Dawley rats were randomly divided: Control, PSD, SK channel inhibitor (apamin) and SK channel activator (CyPPA) groups. In both control and CyPPA groups, sham surgery was performed. In the other two groups, middle cerebral arteries were occluded. The behavioral indicators related to depression in different groups were compared. Immunofluorescence was used to measure the activity of DA neurons in the VTA, while qRT-PCR was used to assess the expression of SK channel genes.
Results: The results showed that apamin treatment improved behavioral indicators related to depression compared to the PSD group. Furthermore, the qRT-PCR analysis revealed differential expression of the KCNN1 and KCNN3 subgenes of the SK channels in each group. Immunofluorescence analysis revealed an increase in the expression of DA neurons in the VTA of the PSD group, which was subsequently reduced upon apamin intervention.
Conclusion: This study suggests that SK channel activation following stroke contributes to depression-related behaviors in PSD rats through increased expression of DA neurons in the VTA. And depression-related behavior is improved in PSD rats by inhibiting the SK channels. The results of this study provide a new understanding of PSD pathogenesis and the possibility of developing new strategies to prevent PSD by targeting SK channels.
Keywords: small conductance calcium-activated potassium channel, ventral tegmental area, dopamine, gating modulation, post-stroke depression
Introduction
As many as one-third of stroke patients experience post-stroke depression (PSD), significantly impacting their rehabilitation and daily life.1 The pathogenesis of PSD is multifaceted and not fully understood.2,3 However, recent studies have found that the dysregulation of potassium channels in central neurons may play a significant role in the development of depression.4–6 Calcium-activated potassium (KCa) channels, which are a crucial subset of the potassium channel superfamily, are present in multiple regions of the central nervous system. They play a significant role in regulating the firing patterns of neurons and controlling the release of neurotransmitters.7 Out of the three types of KCa channels identified, the small conductance calcium-activated potassium channels (SK channels) have received more attention due to their high Ca2+ binding affinity and drug-ability.8,9 Neurons express SK channels, which are thought to regulate the firing patterns of dopaminergic (DA) neurons to provide neuroprotective effects.10 In additional, studies have demonstrated that DA neurons located in the ventral tegmental area (VTA) of the midbrain are crucial in the processing of rewards and motivation.11,12 Furthermore, their dysregulation is strongly associated with the symptoms of depression.13,14 The firing patterns, functions, and related behaviors of DA neurons in the VTA are strongly regulated by the SK channels. These channels are strongly associated with being linked to depression, although the exact mechanism remains unclear.15 SK channels are activated upon Ca2+/CaM binding, and their physiological roles and functions are affected by fluctuations in the intracellular Ca2+ concentration. After the blood supply is restored in ischemic stroke, Ca2+ content significantly increases, leading to calcium overload and subsequent cell damage.16 Few studies have analyzed the mechanism of PSD directly through the connection between the SK channels and DA neurons in the VTA. Therefore, we explored the link between increased SK channel changes due to calcium overload after stroke and the pathogenesis of PSD through DA neurons in the VTA, as shown in Figure 1. To explore this relationship, we built a rat model of PSD and examined depression-related behavioral changes and the activity of DA neurons in the VTA. We aimed to investigate the mechanism of targeting DA neurons in the VTA by intervening SK channels as a potential treatment for PSD. It is noteworthy that the heterogeneity of stroke types observed in clinical practice gives rise to distinct pathological mechanisms, which in turn have varying impacts on the development and prognosis of PSD.17 It is necessary to distinguish between stroke types. Because of the animal model used in the experiment, the main simulated PSD is due to macrovascular stroke.
Materials and Methods
Animals and Treatments
Twenty-four male Sprague-Dawley (SD) rats (grade SPF, weighing 250–300 g) were purchased from Shanghai Xipuer-Beikai Experimental Animal Co., Ltd. A 12-hour light/dark cycle and temperature- and humidity-controlled (23±1°C, 40%) animal room were used to house the rats in groups of less than five in standard cages. The Animal experiment protocol has been reviewed and approved by the Laboratory Animal Management and Ethics Committee of Zhejiang Chinese Medical University (Approval No. IACUC-20201012-06). And the welfare and testing practices of the Experimental Animal Center strictly adhered to the Laboratory Animal Care and Ethics Guidelines of Zhejiang University of Chinese Medicine.
Grouping and Drug Administration
Rats were randomly allocated into 4 groups (n=6/group): Control group (normal saline solution was injected into the VTA of the sham-operated model group without middle cerebral artery occlusion model induction), PSD group (normal saline solution was injected into the VTA of the PSD model group), PSD_Apamin group (SK channel inhibitor Apamin was injected into the VTA of the PSD model group) and Sham_CyPPA group (SK channel activator CyPPA was injected into the VTA of the sham-operated model group without middle cerebral artery occlusion model induction). According to Stereotaxic atlas of the rat brain, the microinjector was inserted into the skull after drilling (VTA: ML±1.0mm, AP-5.2mm, DV-8.0mm),18 and 0.1ng Apamin/0.5μg CyPPA were injected into each side for 5min. Both sides of the brain had injection sites for the VTA. After each injection, the needle was retained for 10min to prevent fluid backflow. The specific injection parts and experimental flow are shown in Figure 2.
The Middle Cerebral Artery Occlusion Model
The middle cerebral artery occlusion (MCAO) model rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital (0.15mL/100g), and the midline neck incision was performed. A mid-neck incision was made, and the vessels were separated. With an arterial clamp, the internal carotid artery (ICA) was clamped and the common carotid artery (CCA) and external carotid artery (ECA) are ligated. In the ECA where the ICA and ECA separate, a silicon-coated monofilament suture was inserted. The thread plug was then inserted from the ECA, along the ICA, and up to the beginning of the middle cerebral artery. The length of the monofilament suture inserted into the ICA was approximately 18mm from the bifurcation. Two hours after suture removal and vessel clip removal, the ECA was closed. The incision was continuously sutured after ligation. For sham-operation group, the median neck incision was made, and the CCA, ECA, and ICA were separated and sutured immediately.19,20 Post-operation, penicillin 40,000 units were injected intraperitoneally daily for three days to prevent infection. The Bederson score was used to determine postischemic neurologic deficit measurement in the rat. Neurologic function was evaluated in a blinded manner, with a score of 0 being excluded from the groups. The Bederson scores for day 1, 3, 5, 7, 14 and 21 differ significantly between the MCAO model and sham-operated group, as shown in Table 1. At 24 hours after completion of the MCAO model, one rat from each group was selected for TTC staining of rat brain tissue. As shown in Figure 3, PSD group and PSD_Apamin group on right side of brain exhibited pale ischemia areas.
 |
Table 1 Bederson Scores of the Rats in the 4 Groups at Different Time Points (n = 6, Mean ± SD) |
PSD Animal Modeling
In the PSD group, the rats were isolated after MCAO model and subsequently subjected to 14 consecutive days of different chronic unpredictable mild stress (CUMS), whereby they were fixed in a special cage for 2 hours per day.21,22 Other CUMS stimulus blocks included 12 h of water deprivation, 24 h of food deprivation, 45°Cage tilt, 1 minute of tail clamping, 5 minutes of swimming in 4°C water, and 36 hours of overnight illumination.21,23,24 Rats were exposed to two of the aforementioned stimuli unpredictably alternately each day to avoid reduced sensitivity to a single stress modality of the same intensity. The timing of the stress was not fixed, but the normal circadian rhythm of the rats was not affected. The sham-operation group was not subjected to isolation and restraint stress, while all other conditions were maintained constant.
Sucrose Preference Test
Before the text, rats were trained to drink sugar water. There were four sections: adaptation, baseline measurement, deprivation, and preference test.25,26 For the first time, a set of two bottles of 1% sugar water were placed simultaneously inside each cage for 24 hours. For the second time, two bottles of 1% sugar water or pure water were randomly placed inside each cage for 24 hours. A 24-hour fast was performed on each group of rats prior to the formal experiment. Each bottle of pure water and sugar water was weighed before being given to the rats in each group to ensure there were no leaks. After 24 hours, the bottles were weighed again, and sugar water consumption and pure water consumption were investigated. The preference for sucrose water was calculated as follows: Preference for sucrose water = (sucrose water consumption/total liquid consumption) × 100%.
Open-Field Test
The open field test box was 100cmX100cm, surrounded by 30cm high, located in a dark soundproof room, and the box was equally divided into 16 compartments. The rats were placed from the central area, and the visual tracking system was used to track the activity path of the rats within 5 minutes. The number of independent support (helping the wall), the number of grooming, the number of feces (requiring manual work), the total distance, the residence time in the central area and the movement distance, the residence time in the four corners, and the number of crossing the grid were recorded.
Elevated Plus-Maze Test
There were two open arms and two closed arms in the elevated plus maze, and the rats were free to explore the device for five minutes before the test. Afterwards, they were placed on the elevated plus maze’s central platform, facing any open arms they saw.27 The number of times that rats entered the open arm, the residence time in the open arm, and the movement distance were recorded. The ratio of the rats’ time, distance, and frequency in the open arm was calculated by dividing it with the total time, distance, and frequency of all four arms.28
Quantitative Real-Time PCR
Using RNA extraction kits, RNA was extracted, and the resulting RNA concentration was added to all components following the reagent instructions to create a 20μL system. After setting the reverse transcription program, 180μL of DEPC water was added to each tube and diluted 10 times to create a total volume of 200μL CDNA sample, which was then stored at −20°C. The PCR primer sequences for the relevant genes are shown in Table 2, according to the purchased fluorescent dye, 5μL CNDA samples were added to make 20μL system. In the fluorescence quantitative PCR instrument, the following parameters were set for 40 cycles. In each sample, the 2−ΔΔCt method was employed based on the Ct values to calculate the relative expression of the target gene. Data reporting for PCR was in accordance with MIQE guidelines.29
 |
Table 2 PCR Primer Sequences |
Immunofluorescence Staining
In deeply anesthetized animals, ice-cold PBS was infused along with paraformaldehyde 4% through the cardiac veins. The brains were cryoprotected in 30% sucrose solution until they sank to the bottom. The sample was embedded in OCT and cut into a coronal section of 10 μm thickness using a freezing microtome (Thermo). The frozen sections underwent antigen repair and were then permeabilized using 0.5% TritonX-100. The sections were then blocked for an hour at room temperature with PBS containing 10% normal goat serum. The primary antibody: TH Rabbit Polyclonal Antibody (Proteintech, No. CL488-25859, 1:400) was then added. As a negative control, PBS was used in place of the primary antibody and incubated overnight at −4◦C. Fluorescently labeled secondary antibody: CoraLite 594-conjugated Goat Anti-Rabbit IgG (Proteintech, No. SA00013-4, 1:100) was incubated in an oven at 37◦C for 30min. The coated tablet containing Dapi was added, and then the results were observed using a digital fluorescence scanning analyzer. Fluorescence images were captured with a VS200 (Olympus) 20 × objective. The results were quantified using ImageJ Pro Plus software.
Statistical Analysis
Statistical Methods SPSS 26.0 statistical software was applied. GraphPad Prism 9.50 was used for graphs. At least three independent experiments were performed, and the mean/SD was calculated. A one-way ANOVA was used to compare the statistical differences, and a level of significance of P < 0.05 was considered significant.
Results
SK Channel Inhibition Ameliorates Depression-Like Behavior Induced by PSD
Based on the sucrose preference test, the control group had a statistically significant higher sucrose preference than the other three groups. The PSD and sham_CyPPA groups had significantly decreased sucrose preference (P < 0.001) compared to the control group, with average reductions of 58.67% and 56.27%. The sucrose preference was found to be higher in PSD_apamin group compared to the PSD group but lower than the control group (P < 0.05), increased by 32.07% and decreased by 26.60%, respectively. In addition, the sucrose preference of PSD_apamin group was 29.67% higher than that of sham_CyPPA group (P < 0.05), as presented in Figure 4A.
Similar results were found in both the elevated plus-maze test regarding the ratio of time difference between each group entering the closed arm and the sucrose preference test. The PSD group and the sham_CyPPA group both had a significant increase in the time it took to enter the closed arm when compared to controls (P < 0.001). The average ratio increased by 26.56% and 25.77%, respectively. The time difference ratio of entering the closed arm was lower in PSD_apamin group compared to PSD group and sham_CyPPA group (P < 0.05). This decrease was 14.15% and 13.17%, respectively. Furthermore, the ratio of time to enter the closed arm in the PSD_apamin group was 12.40% increased than that in the control group (P < 0.05), as shown in Figure 4B. When exploring the open arm, both PSD and sham_CyPPA groups showed a reduction of distance difference ratios when compared to the control group (P < 0.05), as presented in Figure 4C. Specifically, the PSD group and sham_CyPPA group showed a decrease of 24.34% and 23.18%, respectively. There was no significant difference between PSD group and sham_CyPPA group in sucrose preference test and elevated plus-maze test. The movement trajectories of the four groups of rats in the elevated plus-maze test are depicted in Figure 4D–G.
The results of the open field test showed that the PSD group and the sham_CyPPA group had a significantly reduced time to enter the central area compared to the control group (P < 0.05). The average time for the PSD group and the sham_CyPPA group was reduced by 12.75s and 12.70s, respectively. The difference was statistically significant, as seen in Figure 4H. As compared to the control group, both the PSD group and the sham_CyPPA group showed an increased distance into the corner area (P < 0.05). The average distance was increased by 15.36m and 16.29m, respectively. Furthermore, the distance into the corner area increased by 16.75m and 17.68m in the PSD_apamin group compared to the PSD group and sham_CyPPA group, respectively. This difference was statistically significant (P < 0.05), as illustrated in Figure 4I. The results showed that the sham_CyPPA group had an average of 10.33 fewer standing uprights compared to the control group (P < 0.05), as illustrated in Figure 4J and K indicated that the average grooming frequency in the PSD_apamin group was found to be 2.33 times lower compared to the PSD group. Compared to the PSD group, the sham_CyPPA group showed a decrease of 2.67 times, which was statistically significant (P < 0.05).
Activation and Inhibition of SK Channels Alter the mRNA Expression of KCNN1 and KCNN3
We utilized an apamin inhibitor and CyPPA activator to treat the SK channels of DA neurons in the VTA. Through structural analysis of the SK channels, we were able to differentiate between the SK1, SK2, and SK3 subtypes, which are encoded by the KCNN1, KCNN2, and KCNN3 genes, respectively.30,31 The results presented in Figure 5 indicate that the expression level of KCNN1 was higher in the PSD_apamin group compared to the sham_CyPPA group (P < 0.05), with a statistically significant difference observed. The expression level of KCNN3 in the PSD group was lower than that in the control group (P < 0.05). The KCNN2 expression level was found to be significantly lower (P < 0.05) in the sham_CyPPA group compared to the control group. In addition, there was no significant difference in the expression level of KCNN2 among all groups.
 |
Figure 5 The expression levels of SK channel KCNN1, KCNN2 and KCNN3 gene in each group were analyzed by qRT-PCR (n = 3, per group). *P<0.05. |
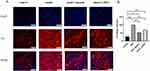
The Immunofluorescence Expression of DA Neurons in the VTA of PSD Rats is Reduced by Inhibiting SK Channels
To further illustrate the activity of DA neurons in the rat VTA, the TH-labeled immune response of these neurons was observed under a 20X fluorescence inverted microscope, as shown in Figure 6A. The rate-limiting enzyme TH primarily regulates DA synthesis. TH average optical density of each group was obtained after analysis, as shown in Figure 6B. In the PSD group, optical density increased by 71.28% over the control group (P<0.001), suggesting significantly higher immunofluorescence expression of DA neurons. The average optical density of the PSD_apamin group and the sham_CyPPA group increased by 24.13% (P<0.05) and 39.18% (P<0.01), respectively, compared to the control group. The average optical density of TH in the PSD_apamin group was significantly decreased by 47.15% (P<0.001) compared to the PSD group. This suggests that the expression of immunofluorescence in DA neurons decreased after the inhibition of the SK channels by apamin. In addition, the average optical density in the sham_CyPPA group decreased by 32.10%, compared to the PSD group (P<0.01). On the one hand, the control group had the lowest average optical density, while the sham_CyPPA group had a lower average optical density than the control group. On the other hand, the PSD group had the highest average optical density value, and the PSD_apamin group had a higher average optical density than the PSD group.
Discussion
The study demonstrated that activation of the SK channels in PSD rats led to increased expression of DA neurons in the VTA, resulting in depression-related behavioral changes. In turn, inhibiting the SK channels with apamin reduced the expression of DA neurons in the VTA and improved depression-related behaviors in PSD rats. The results suggest that PSD is linked to alterations in the expression of DA neurons in the VTA, which are regulated by the SK channels. The inhibition of the SK channels by pharmacological means shows promise in providing effective antidepressant effects.
To enhance the reliability of the experimental results in the depression-related behavioral tests, we employed multiple behavioral tests. On the one hand, we found that the PSD rat model exhibited depression-related behavioral changes. Compared to the sham-operated group, PSD group with depression-related behavioral changes indicated the validity of our model. On the other hand, we observed that PSD rats treated with SK channel inhibitors showed a reduction in depression-related behavioral changes, suggesting that inhibiting SK channel activation may improve these changes in PSD rats. In addition, in the sham group, administration of the SK channel activator resulted in depression-related behavioral changes as compared to the control group. This suggests that activation of the SK channel can lead to depression-related behavioral changes. Many studies have shown a strong correlation between the SK channels and the development of depression.32,33 As Andrew34 discovered, the conductance of the SK channels significantly affected DA neurons’ firing properties. They also noted that the conductance of SK is reliant on the levels of intracellular Ca2+. When Ca2+ levels are low, the SK channels remain inactive, while high Ca2+ levels cause them to remain open, resulting in a hyperpolarized state. The strength of the SK channel conductance determines the firing pattern of DA neurons and is closely related to Ca2+ concentrations. As a result, an increase in Ca2+/CaM after a stroke can activate the SK channels, leading to an effect on DA neurons in the VTA and ultimately causing PSD. Etchepare15 and Ping35 utilized apamin to investigate the effect of the SK channels on DA neuron firing. It is possible that the ultimate pharmacological pathway of antidepressants is to regulate neuronal excitability by inhibiting the K+ channels. They also found that decreased SK channel function increased DA neuron firing irregularity. In addition, research has shown that apamin has the potential to decrease immobility time in forced swimming tests (FST) in mice. Conversely, the K+ channel openers have been found to increase the immobility time.36 These studies are consistent with our findings that manipulating the activity of SK channel can lead to depression-related behavioral changes. This suggests that the changes in SK channels in the VTA may contribute to the development of PSD.
In our study, we observed statistical differences in the expression of KCNN1 and KCNN3 genes, while KCNN2 gene did not show any significant difference in qRT-PCR detection. Further analysis suggested that this could be due to the varying drug sensitivity of the three SK channel genes in the VTA towards apamin and CyPPA. This notion is supported by different literature. According to Etchepare,15 the decrease in the SK channel function may be due to a decrease in the surface content of the SK channels. It was revealed that SK3 channels were the dominant isoform in the VTA, whereas the membrane content of the SK2 channels was significantly lower and in some cases undetectable. The research has demonstrated the significance of the SK3 channels in monoaminergic neurons. Several studies have suggested that the development of selective blockers or negative gating modulators of KCNN3 could be a significant breakthrough in the development of new medications for psychiatric disorders.8,9 Contrary to our findings, Adelman37 reported that apamin had the strongest effect on the SK2 channels and reduced the affinity of blocking SK1 and SK3 by 10–50 times. However, their study also supported the notion that apamin could play a crucial role in affecting neuronal excitability through the SK channels. According to our research, the KCNN1 gene is more prevalent in the VTA than KCNN2 and KCNN3. This speculates that the pathogenesis of PSD and depression may differ in the SK channel subunits. In recent years, there have been some studies on the different roles of the SK channel subtypes in depression. Nashed6 discovered that mice with partially or completely ablated the SK3 channel expression in the brain exhibited an antidepressant phenotype in both the FST test and the novelty-induced esophagus (NIH) test. Furthermore, a direct administration of compounds that bind to SK3 channel isoforms also induced antidepressant-like effects in the ventricular system, according to the researchers. SK3 channels have been shown to be associated with cognitive and affective symptoms of stress-related disorders among the three SK channel subtypes.38 In a study conducted by Jacobsen,39 SK3 channel knockout mice were examined, and it was found that the absence of this gene resulted in increased 5-HT and DA secretion in the brain. The knockout of this gene resulted in a significant decrease in resting time during the FST test, indicating an obvious antidepressant state. The findings of these studies indicate that the SK1, SK2 and SK3 channels encoded by KCNN1, KCNN2 and KCNN3, respectively, may have distinct functions in the development of PSD. Further investigations are required to fully understand the varying roles of the three SK subunits in the pathogenesis of PSD. In addition, our study revealed that the gene expression of SK channel KCNN1 in the VTA exhibited the highest levels in the model_apamin group, contrasting with the peak expression observed for KCNN2 and KCNNN3. This finding is consistent with the conclusion that KCNN1, KCNN2 and KCNN3 have different roles in PSD. We will continue to explore the positive or reverse roles of the three sub-genes in PSD. The plan is to target inhibition of the three genes separately to identify which one plays the main role in PSD adjustment and improve the experimental metric for measuring activation of single K+ channels by manual voltage-clamp electrophysiological methods.40,41
The results of the immunofluorescence assay demonstrated that PSD rats exhibited higher expression levels of DA neurons in the VTA, which decreased after the administration of apamin. Moreover, when SK channels are activated with CyPPA, DA neurons are more expressed than in the control group. It appears that inhibiting SK channels reduces DA neuron activity in the VTA, thus ameliorating depression-like behaviors observed in PSD rats. Recent studies have shown an increased focus on changes in dopaminergic circuits in relation to depression and PSD. The VTA, responsible for DA synthesis, is one of the major nuclei being researched in this area.42,43 In addition, we found evidence supporting a relationship between depressive-like behavior and increased brain DA expression in PSD rats. In 2007, Krishnan44 first observed increased activity of VTA DA neurons in social defeat (SD) mice. In a study conducted by Iacono,45 it was found that social failure mice experienced a loss of inhibitory response to DA in their VTA neurons. Accordingly, DA-mediated self-inhibition and DA-induced VTA inside-out currents may both be reduced by social failure stress. Su46 found that the decrease in automatic inhibition, mediated by K+ channels Kv7.4, to DA neurons in the VTA in certain projection pathways, could be a potential factor in the development of depressive-like behaviors. Although the hypothesis that depressed states are mediated by an increased activity of VTA DA neurons has been proposed, related pharmacological treatments have not been used in patients.47 Several studies have also reached the opposite conclusion that low dopaminergic response to direct VTA injury in animal models can lead to depressive behavior. This may be due to the specific vulnerability of cells to hypoxic-ischemic injury. A reduction in DA cell number within the VTA can lead to a decrease in DA concentration, which typically recovers after 2–3 weeks.42,48 However, PSD is yet to be understood in terms of how VTA DA neurons contribute to the process. Differences in animal models may also play a role in the varying results observed.47 It is possible that DA neurons with varying localization, projection targets, and neurochemical characteristics in the VTA may have unique contributions to depression.
PSD is a prevalent clinical complication that can significantly hinder the recovery and quality of life of stroke patients if left untreated. Currently, there is a lack of research on the direct connection between SK channels and DA neurons in the VTA in the mechanism of PSD. Our study offers significant insights into the mechanisms of PSD, which will not only improve the understanding of potential novel therapeutic targets for PSD but also create opportunities for the development of anti-PSD drugs that target SKCa channels. These drugs could potentially exert their effects more accurately and rapidly with fewer side effects. There are some limitations to this research experiment. On the one hand, PSD model of assessment is not comprehensive, and a lack of serology, NE, and 5-HT depression-related indicators of detection are present. On the other hand, the Ca2+ levels in our hypothesized pathways have not been validated further, and a relevant pathway mechanism will be undertaken with a quantitative fluorescence (Fluo-4) assay.
In our follow-up study, we plan to delve deeper into the three subtypes of SK channels. To ensure greater accuracy in our experimental metrics, we will address the issues of unspecificity and other limitations of classical pharmacological tools by utilizing manual voltage-clamp electrophysiological techniques to measure individual K+ channel activity.40,41 In addition, we will investigate whether apamin is capable of causing morphological changes and electrophysiological changes in DA neurons in the VTA, as a means of SK channel intervention in the treatment of PSD. One of our future research directions is investigating how different areas of cerebral infarction affect the occurrence of poststroke depression. Small vessel acute ischemic strokes are different from other stroke subtypes, which have a high incidence and are closely related to small vessel disease (SVD).49 The SVD is closely related to neuropsychiatric disorders,50 as well as having a high risk of PSD, so research on this condition is extremely important.
Conclusion
Our animal studies indicate that the activation of SK channels can increase the expression of DA neurons in the VTA of PSD rats, which results in depression-related behavioral changes. The inhibition of SK channels caused by apamin administration can decrease the expression of DA neurons in the VTA and ameliorate depression-related behaviors. Our study reveals a direct association between the activation of SK channel in the VTA and the exacerbation of depression-related behaviors in PSD rats, thereby offering novel insights into the pathogenesis of PSD. These results suggest that restrained SK channels may be a new choice to treat PSD. Inhibition of SK channels can improve the depression-like behavior of PSD rats.
Abbreviations
SK, small-conductance calcium-activated potassium channel; DA, dopamine; VTA, ventral tegmental area; PSD, post-stroke depression; KCa, calcium-activated potassium channels; MCAO, middle cerebral artery occlusion model; ICA, internal carotid artery; CCA, common carotid artery; ECA, external carotid artery; CUMS, chronic unpredictable mild stress; KCNN1, KCNN mammalian genes of SK1 channel subtypes; KCNN2, KCNN mammalian genes of SK2 channel subtypes; KCNN3, KCNN mammalian genes of SK3 channel subtypes; FST, forced swimming test; NIH, novelty-induced esophagus test.
Data Sharing Statement
The datasets generated for this study are available on request from the corresponding author.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81774230) and the Department of Education Project of Zhejiang Provincial (No. FX2021031).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Frank D, Gruenbaum BF, Zlotnik A, Semyonov M, Frenkel A, Boyko M. Pathophysiology and current drug treatments for post-stroke depression: a review. Int J Mol Sci. 2022;23:23.
2. Ghaffari A, Akbarfahimi M, Rostami HR. Discriminative factors for post-stroke depression. Asian J Psychiatr. 2020;48:101863. doi:10.1016/j.ajp.2019.101863
3. Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70–80. doi:10.1016/j.genhosppsych.2020.06.011
4. Costi S, Han M-H, Murrough JW. The potential of KCNQ potassium channel openers as novel antidepressants. CNS Drugs. 2022;36(3):207–216. doi:10.1007/s40263-021-00885-y
5. Costi S, Morris LS, Kirkwood KA, et al. Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am J Psychiatry. 2021;178(5):437–446. doi:10.1176/appi.ajp.2020.20050653
6. Nashed MG, Waye S, Hasan SMN, et al. Antidepressant activity of pharmacological and genetic deactivation of the small-conductance calcium-activated potassium channel subtype-3. Psychopharmacology. 2022;239(1):253–266. doi:10.1007/s00213-021-06045-w
7. Shah KR, Guan X, Yan J. Structural and functional coupling of calcium-activated BK channels and calcium-permeable channels within nanodomain signaling complexes. Front Physiol. 2021;12:796540. doi:10.3389/fphys.2021.796540
8. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57(4):463–472. doi:10.1124/pr.57.4.9
9. Brown BM, Shim H, Christophersen P, Wulff H. Pharmacology of small- and intermediate-conductance calcium-activated potassium channels. Annu Rev Pharmacol Toxicol. 2020;60:219–240. doi:10.1146/annurev-pharmtox-010919-023420
10. Dolga AM, de Andrade A, Meissner L, et al. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014;5(1):e999. doi:10.1038/cddis.2013.530
11. Fox ME, Lobo MK. The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry. 2019;24(12):1798–1815. doi:10.1038/s41380-019-0415-3
12. Al-Hasani R, Gowrishankar R, Schmitz GP, et al. Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nat Neurosci. 2021;24(10):1414–1428. doi:10.1038/s41593-021-00898-2
13. Douma EH, de Kloet ER. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev. 2020;108:48–77. doi:10.1016/j.neubiorev.2019.10.015
14. Nagai Y, Takayama K, Nishitani N, et al. The role of dorsal raphe serotonin neurons in the balance between reward and aversion. Int J Mol Sci. 2020;21(6):2160. doi:10.3390/ijms21062160
15. Etchepare L, Gréa H, Durand P, Bouchet D, Groc L. NMDA receptor membrane dynamics tunes the firing pattern of midbrain dopaminergic neurons. J Physiol. 2021;599(11):2933–2951. doi:10.1113/JP281104
16. Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102(2):893–992. doi:10.1152/physrev.00041.2020
17. Gasull T, Arboix A. Molecular mechanisms and pathophysiology of acute stroke: emphasis on biomarkers in the different stroke subtypes. Int J Mol Sci. 2022;23(16):9476. doi:10.3390/ijms23169476
18. Tong Q, Cui X, Xu H, et al. D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum. Mol Psychiatry. 2023;28(2):625–638. doi:10.1038/s41380-022-01809-y
19. Li L, Dong L, Xiao Z, et al. Integrated analysis of the proteome and transcriptome in a MCAO mouse model revealed the molecular landscape during stroke progression. J Adv Res. 2020;24:13–27. doi:10.1016/j.jare.2020.01.005
20. Zhou Z, Xu N, Matei N, et al. Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J Cereb Blood Flow Metab. 2021;41(2):267–281. doi:10.1177/0271678X20910533
21. Fan Q, Liu Y, Sheng L, et al. Chaihu-Shugan-San inhibits neuroinflammation in the treatment of post-stroke depression through the JAK/STAT3-GSK3beta/PTEN/Akt pathway. Biomed Pharmacother. 2023;160:114385. doi:10.1016/j.biopha.2023.114385
22. Liu Y, Peng J, Leng Q, Tian Y, Wu X, Tan R. Effects of aloe-emodin on the expression of brain aquaporins and secretion of neurotrophic factors in a rat model of post-stroke depression. Int J Mol Sci. 2023;24:6.
23. Li Z, Xu H, Xu Y, et al. Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation. CNS Neurosci Ther. 2021;27(12):1570–1586. doi:10.1111/cns.13732
24. Ma W, Song J, Wang H, et al. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019;225:88–97. doi:10.1016/j.lfs.2019.04.006
25. Liu M-Y, Yin C-Y, Zhu L-J, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13(7):1686–1698. doi:10.1038/s41596-018-0011-z
26. Chen S, Chen F, Amin N, et al. Defects of parvalbumin-positive interneurons in the ventral dentate gyrus region are implicated depression-like behavior in mice. Brain Behav Immun. 2022;99:27–42. doi:10.1016/j.bbi.2021.09.013
27. Chen B, Xu J, Chen S, et al. Dysregulation of striatal dopamine D2/D3 receptor-mediated by hypocretin induces depressive behaviors in rats. J Affect Disord. 2023;325:256–263. doi:10.1016/j.jad.2023.01.031
28. Cho W-H, Noh K, Lee BH, et al. Hippocampal astrocytes modulate anxiety-like behavior. Nat Commun. 2022;13(1):6536. doi:10.1038/s41467-022-34201-z
29. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi:10.1373/clinchem.2008.112797
30. Köhler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273(5282):1709–1714. doi:10.1126/science.273.5282.1709
31. Dilly S, Graulich A, Farce A, Seutin V, Liegeois JF, Chavatte P. Identification of a pharmacophore of SKCa channel blockers. J Enzyme Inhib Med Chem. 2005;20(6):517–523. doi:10.1080/14756360500210989
32. O’Donovan B, Adeluyi A, Anderson EL, Cole RD, Turner JR, Ortinski PI. Altered gating of K(v)1.4 in the nucleus accumbens suppresses motivation for reward. Elife. 2019;2019:8.
33. Cadet JL, Brannock C, Krasnova IN, et al. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry. 2017;22(8):1196–1204. doi:10.1038/mp.2016.48
34. Oster A, Faure P, Gutkin BS. Mechanisms for multiple activity modes of VTA dopamine neurons. Front Comput Neurosci. 2015;9:95. doi:10.3389/fncom.2015.00095
35. Ping HX, Shepard PD. Apamin-sensitive Ca(2+)-activated K+ channels regulate pacemaker activity in nigral dopamine neurons. Neuroreport. 1996;7(3):809–814. doi:10.1097/00001756-199602290-00031
36. Kaster MP, Ferreira PK, Santos AR, Rodrigues AL. Effects of potassium channel inhibitors in the forced swimming test: possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res. 2005;165(2):204–209. doi:10.1016/j.bbr.2005.06.031
37. Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi:10.1146/annurev-physiol-020911-153336
38. Smolin B, Karry R, Gal-Ben-Ari S, Ben-Shachar D. Differential expression of genes encoding neuronal ion-channel subunits in major depression, bipolar disorder and schizophrenia: implications for pathophysiology. Int J Neuropsychopharmacol. 2012;15(7):869–882. doi:10.1017/S1461145711001428
39. Jacobsen JPR, Weikop P, Hansen HH, et al. SK3 K+ channel-deficient mice have enhanced dopamine and serotonin release and altered emotional behaviors. Genes Brain Behav. 2008;7(8):836–848. doi:10.1111/j.1601-183X.2008.00416.x
40. Weaver CD, Denton JS. Next-generation inward rectifier potassium channel modulators: discovery and molecular pharmacology. Am J Physiol Cell Physiol. 2021;320(6):C1125–C1140. doi:10.1152/ajpcell.00548.2020
41. Rajabian A, Rajabian F, Babaei F, Mirzababaei M, Nassiri-Asl M, Hosseinzadeh H. Interaction of medicinal plants and their active constituents with potassium ion channels: a systematic review. Front Pharmacol. 2022;13:831963. doi:10.3389/fphar.2022.831963
42. Ma B, Chen J, Mu Y, et al. Proteomic analysis of rat serum revealed the effects of chronic sleep deprivation on metabolic, cardiovascular and nervous system. PLoS One. 2018;13(9):e0199237. doi:10.1371/journal.pone.0199237
43. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. doi:10.1038/nrn.2016.57
44. Krishnan V, Han M-H, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi:10.1016/j.cell.2007.09.018
45. Lo Iacono L, Catale C, Martini A, et al. From traumatic childhood to cocaine abuse: the critical function of the immune system. Biol Psychiatry. 2018;84(12):905–916. doi:10.1016/j.biopsych.2018.05.022
46. Su M, Li L, Wang J, et al. Kv7.4 channel contribute to projection-specific auto-inhibition of dopamine neurons in the ventral tegmental area. Front Cell Neurosci. 2019;13:557. doi:10.3389/fncel.2019.00557
47. Kaufling J. Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression. Cell Tissue Res. 2019;377(1):59–71. doi:10.1007/s00441-019-03007-9
48. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666–679. doi:10.1038/mp.2017.16
49. Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. doi:10.3390/ijms23031497
50. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–696. doi:10.1016/S1474-4422(19)30079-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.