Back to Journals » Cancer Management and Research » Volume 13
Inhibition of Orthotopic Genital Cancer Induced by Subcutaneous Administration of Human Papillomavirus Peptide Vaccine with CpG Oligodeoxynucleotides as an Adjuvant in Mice
Authors Wang H, Che Y, Yang Y, Suo J, Wang X
Received 5 March 2021
Accepted for publication 7 June 2021
Published 12 July 2021 Volume 2021:13 Pages 5559—5572
DOI https://doi.org/10.2147/CMAR.S309226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Huan Wang,1,2 Yuxin Che,1 Yang Yang,1 Jinguo Suo,1 Xuelian Wang1
1Department of Microbiology and Parasitology, College of Basic Medical Science, China Medical University, Shenyang, Liaoning, People’s Republic of China; 2Nursing College, Jinzhou Medical University, Jinzhou, Liaoning, People’s Republic of China
Correspondence: Xuelian Wang
Department of Microbiology and Parasitology, College of Basic Medical Science, China Medical University, No. 77 Puhe Road, Shenyang North New Area, Shenyang, 110122, Liaoning, People’s Republic of China
Tel/Fax +86-24-31939077
Email [email protected]
Purpose: Persistent high-risk human papillomavirus (HPV) infection is the most common cause of cervical cancer and its precursor lesions. Although prophylactic HPV vaccines have been applied in the general population for the prevention of HPV infections, no licensed therapeutic HPV vaccine is currently available to treat preexisting HPV infections or HPV-associated diseases, including cervical cancer.
Materials and Methods: The most common murine cervical cancer model used for the evaluation of the efficacy of a therapeutic HPV vaccine in preclinical studies is the ectopic model, which is established by the subcutaneous inoculation of tumor cells, such as TC-1 cells, into the flank of an animal. We have previously demonstrated the efficacy of a therapeutic HPV peptide vaccine adjuvanted with unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide in the clearance of ectopic subcutaneous tumors in C57BL/6 mice after vaccination. In the current study, we established orthotopic genital tumors by injecting TC-1 cells into the vaginal submucosa close to the cervix and assessed whether the subcutaneous administration of the therapeutic vaccine could inhibit the growth of genital tumors. Additionally, we evaluated the effect of the vaccination on the tumor microenvironment.
Results: The results showed that the vaccination induced an increase in infiltrating CD4+ and CD8+ T cells, a decrease in myeloid-derived suppressor cells and cancer-associated fibroblasts, as well as the differential expression of a panel of cytokines, chemokines, and matrix metalloproteinases within the tumor microenvironment.
Conclusion: The administration of the vaccine resulted in the inhibition of established implanted orthotopic genital tumors by inducing strong antitumor immune responses and reversed tolerogenic local immunosuppression in a mouse model of orthotopic genital cancer.
Keywords: orthotopic genital cancer, human papillomavirus, therapeutic vaccine, tumor microenvironment, immunosuppression
Introduction
Cervical cancer is the fourth most frequently diagnosed cancer and also the fourth leading cause of cancer-related death among females globally.1 Persistent high-risk human papillomavirus (HPV) infection is closely associated with the occurrence of cervical cancer and its precursor lesions.2 HPV 16 is the most common high-risk type and is responsible for almost 60% of all cervical cancers.3 Because HPV is the etiological factor for HPV-associated diseases, including cervical cancer, it is possible to control these diseases through vaccination and other therapeutic strategies. Although licensed prophylactic HPV vaccines have been available since 2006,4 they are ineffective at clearing preexisting infections and associated preinvasive lesions. To address this, a therapeutic vaccine that can treat established HPV infections and prevent HPV-associated malignancies via the induction of a strong T cell-mediated immune response is urgently required.
The E6 and E7 oncoproteins of high-risk HPV types are required for the initiation and subsequent progression of HPV-associated cell transformation and immortalization. Because E7 is highly conserved and better characterized immunologically than the E6 antigen in preclinical models,5 HPV E7 serves as an important target for the development of therapeutic HPV vaccines to treat cervical cancer and its precursor lesions.
The most common cervical cancer model used for evaluation of the efficacy of the therapeutic HPV vaccine in preclinical studies is the ectopic model, which is established by the subcutaneous inoculation of tumor cells, such as TC-1 cells, into the flank of a rodent.6 Although promising results were reported for a series of candidate vaccines generating to inhibit or clear subcutaneous tumors in preclinical studies,7 these did not translate into satisfactory clinical outcomes for patients with HPV-associated genital neoplasias.5 This may be due to the immunosuppressive tumor microenvironment (TME) associated with tumor progression and the inhibition of the function of vaccine-induced effector cells in the tumor. Because of the constant immunological pressure resulting from the persistent expression of HPV proteins, cervical cancers evolve several immune evasion mechanisms, including the attraction of immune cells with immunosuppressive properties such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), which have direct effects on tumor growth, vascularization, and the modulation of the tumor stroma. These immunosuppressive cells also produce a wide array of cytokines and chemokines, resulting in an immunosuppressive TME in cervical cancer.8 Alternatively, ectopic cervical cancer models may not accurately mimic the site of disease in patients with cervical cancer because HPV-associated neoplasias in patients commonly occur in the genital mucosa where a relatively immunosuppressed environment may exist.9 Nevertheless, an orthotopic cervical cancer model may be suitable for determining the efficacy of a therapeutic vaccine in inhibiting or eliminating tumors located in the genital tract. In this study, we established an orthotopic genital cancer model by implanting tumor cells into the vaginal submucosa close to the cervix to mimic the genital mucosa and provide, as much as possible, a clinically relevant setting.
We have previously demonstrated the efficacy of a therapeutic vaccine containing the HPV16 E7 43–77 peptide, which contains both a cytotoxic T lymphocyte (CTL) epitope (E7 49–57) and two T-helper (Th) epitopes (E7 50–62 and E7 43–77), as well as the adjuvant unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide (CpG ODN) in clearing subcutaneous tumors in C57BL/6 mice 24 days after vaccination.10 In the current study, we assessed whether the subcutaneous administration of an HPV E7 peptide-targeting therapeutic vaccine could inhibit the growth of orthotopic genital tumors and determined the effect of the vaccination on the tumor microenvironment. Our results showed that the vaccination induced a local increase in CD4+ and CD8+ T cells, a decrease in cancer-associated fibroblasts (CAFs), and the differential expression of a panel of cytokines, chemokines, and matrix metalloproteinases (MMPs) in the TME, which resulted in the inhibition of established implanted orthotopic genital tumors. Peptide-based therapeutic vaccines have the advantages of stability, safety, and accessibility, and are amenable to large-scale production, which is especially relevant for low-to-middle income countries where the disease burden is greatest. The vaccine strategy used in this study offers significant therapeutic effects, these data provide a solid foundation for future clinical trials.
Materials and Methods
Mice and Cell Lines
Female C57BL/6 mice, aged 6–8 weeks, were purchased from Liaoning Changsheng Biotechnology Co. Ltd (Benxi, China). The mice were housed in a specific-pathogen-free environment and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of the China Medical University. All experimental procedures were approved by the Institutional Animal Care and Use Committee of China Medical University (IACUC Issue No. CMU2019293).
TC-1 cells were purchased from Beijing Beina Chuanglian Biotechnology Institute (Beijing, China). The cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Biological Industries, Kibbutz Beit Haemek, Israel) supplemented with 10% fetal bovine serum (Biological Industries), 2 mM G418 (Genview, Tallahassee, FL, USA), and penicillin–streptomycin (Biological Industries) at 37 °C and with 5% carbon dioxide.
Peptides and Adjuvant
The HPV16 E7 peptide (E7 43–77) was synthesized by GL Biochem (Shanghai) Ltd (Shanghai, China). CpG ODN 1826 was synthesized by Sangon Biotech (Shanghai) Co. Ltd (Shanghai, China). The sequences of the peptide and CpG ODN were as follows:
E7 43–77: GQAEPDRAHYNIVTFCCKCDSTLRLCVQSTHVDIR; CpG-ODN1826: 5′-TCCATGACGTTCCTGACGTT-3′.
In vivo Tumor Growth Experiments
On day 0, all the mice were injected with ~5×105 TC-1 cells in PBS (25 µL) into the vaginal submucosa near the cervix, and were then divided into four groups. On day 4, one group of mice received a subcutaneous injection of 20 μg of CpG ODN (CpG ODN group); one group received 50 μg of E7 peptide (E7 group); mice in the third group were administered a vaccine containing 20 μg of CpG ODN mixed with 50 μg of E7 peptide (vaccine group); finally, mice in the control group received 100 μL of PBS in the right flank.
Mouse and Tumor Weight
The bodyweight of the mice was measured on days 0, 2, 4, 6, 8, 11, and 13. The mice were euthanized and the tumors were resected on day 14 after vaccination. After weighing, the tumors were preserved at −80 °C until use.
Intracellular Cytokine Staining
The tumors were cut into small pieces using a blade and digested in 2 mg/mL collagenase D (Roche Diagnostics, Indianapolis, IN, USA) and 0.4 mg/mL DNase (Sigma–Aldrich, St. Louis, MO, USA) at 37 °C for 90 min, and then filtered through a 100-μm strainer to obtain a single-cell suspension. For analysis of intracellular interferon-gamma (IFN-γ) levels, the tumor cells were incubated with 1 μg/mL PMA (Sigma–Aldrich) and 50 μg/mL ionomycin (Sigma–Aldrich) for 5 h. GolgiPlug (BD Biosciences, San Diego, CA, USA) was added to the cultures to inhibit protein secretion for the final 4 h. After stimulation, the tumor cells were stained with PE-conjugated, anti-mouse IFN-γ antibody and analyzed on a BD LSRFortessa flow cytometer (BD Biosciences). Data analysis was performed using FlowJo X software (Treestar, Ashland, OR, USA). All the antibodies were obtained from eBioscience (San Diego, CA, USA).
Intranuclear Transcription Factor Staining
A tumor single-cell suspension was prepared as described above. For analysis of Tregs, single cells were surface-stained with FITC-conjugated anti-mouse CD4 antibody; for intranuclear staining, after fixation and membrane permeabilization, the cells were incubated with PE-conjugated anti-mouse Foxp3 antibody using the Foxp3/Transcription Factor Staining Buffer Set (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Flow cytometric evaluation was performed on a BD LSRFortessa instrument and data were analyzed using FlowJo X software.
Surface Molecule Staining
For analysis of surface molecules associated with T cells, MDSCs, and macrophages, the cells were stained with the following antibodies: PerCP-conjugated anti-mouse CD8a, FITC-conjugated anti-mouse CD4, FITC-conjugated anti-mouse Gr-1, APC-conjugated anti-mouse CD11b, and PE-conjugated anti-mouse F4/80. All the antibodies were obtained from eBioscience (San Diego, CA, USA). Analysis was performed as described above.
Quantitative Real-Time PCR
Total RNA was extracted from tumor tissue samples using Trizol reagent (Takara, Dalian, China) and was reverse transcribed into cDNA using a Reverse Transcription Kit (Takara). A SYBR Green qPCR Master Mix (Vazyme, Nanjing, China) was used for the qPCR. The RT-qPCR was performed in triplicate for each group. The sequences of the primers used are shown in Table 1. Target gene expression was normalized to that of β-actin and relative mRNA expression was quantified using the 2−ΔΔCt method.
 |
Table 1 Summary of Primer Sequences Used for PCR Amplifications |
Immunohistochemistry (IHC)
Immunohistochemical staining for α-SMA, p53, MMP-2, and Ki67 was performed following standard IHC procedures. In brief, 5-µm paraffin sections were deparaffinized and rehydrated via an alcohol gradient, blocked using the UltraSensitive S-P Kit (Maixin Biotechnology, Fuzhou, Fujian, China), and incubated overnight at 4 °C with primary antibodies (rabbit polyclonal) against α-SMA, p53, MMP-2, and Ki67 (ABclonal Biotech Co, Ltd, Wuhan, China; 1:200 dilution). This step was followed by incubation with biotinylated secondary antibodies and streptavidin–horseradish peroxidase (Maixin Biotechnology, Fuzhou, Fujian, China). After incubation, 3,3′-diaminobenzidine tetrahydrochloride (Maixin Biotechnology) was applied as the chromogen. The sections were then counterstained with hematoxylin and coverslipped. The IHC scoring were analyzed as previously described.11 The staining intensity was scored as follows: no staining (0), weak staining (1), moderate staining (2) and strong staining (3). The positive proportion of stained tumor cells was defined as follows: 0 (0%), 1 (1%-10%), 2 (11%-50%), 3 (51%-80%), and 4 (>80%). The staining results were semiquantitatively assessed by multiplying the staining intensity by the percentage of positively stained tumor cells.
Statistical Analysis
All data were analyzed and graphed using GraphPad Prism v.5.0 (GraphPad Software Inc., San Diego, CA, USA). Data are expressed as means ± standard deviation. The significance of differences was determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. A P-value <0.05 was considered statistically significant.
Results
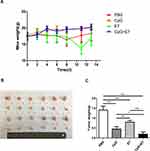
Vaccination Induced Tumor Inhibition
The bodyweight of the mice in the four groups was measured seven times within 14 days. Mice in the PBS and E7 groups were lighter than those in the CpG and CpG+E7 groups; however, the differences were not significant (Figure 1A). Images of tumors excised from the mice on day 14 are shown in Figure 1B. The administration of CpG, E7, and the vaccine resulted in a significant reduction in tumor growth (CpG: 68.09%, 0.45±0.11 g, P<0.001; E7: 43.26%, 0.80±0.09 g, P<0.001; vaccine: 86.52%, 0.20±0.10 g, P<0.001) when compared with that of the control group (1.41±0.20 g) (Figure 1C). Moreover, tumor growth was significantly inhibited in the vaccine group compared with that in either the CpG ODN or E7 peptide group (P<0.001) (Figure 1B and C).
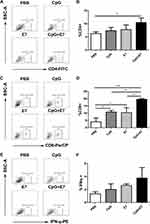
Vaccination Induced Cellular Immune Responses
IFN-γ is a key cytokine in vaccination-induced cellular immune responses and is primarily produced by CD4+ and CD8+ T cells.12 We determined the percentages of total CD4+ T cells, total CD8+ T cells, and total IFN-γ-producing cells in the tumor tissue. A representative dot plot of the percentage of total CD4+ T cells from one mouse from each group is shown in Figure 2A. The results showed that the percentage of total CD4+ T cells was significantly higher in the CpG+E7 group (10.49±1.77%, n=3) than in the PBS group (6.26±0.69%, n=3) (P<0.05, Figure 2B). The percentage of CD4+ T cells was also higher in the CpG (7.38±1.26%, n=3) and E7 (7.81±1.77%, n=3) groups compared with that in the PBS group (6.26±0.69%, n=3); however, the difference was not significant. A representative dot plot of the percentage of total CD8+ T cells from one mouse from each group is shown in Figure 2C. Compared with the PBS group, the percentage of total CD8+ T cells was significantly greater in the CpG+E7 (P<0.001), E7 (P<0.05), and CpG (P<0.05) groups (Figure 2D). Furthermore, the percentage of total CD8+ T cells was significantly higher in the vaccine group (19.6±0.62%) than in the CpG (10.81±1.14%) or the E7 (10.42±3.38%) group. A representative dot plot of the percentage of total IFN-γ-producing cells from one mouse from each group is shown in Figure 2E. The percentage of total IFN-γ-producing cells was also greater in the CpG+E7 (3.76±1.62%, n=3), E7 (2.62±0.25%, n=3), and CpG (2.01±0.88%, n=3) groups compared with that in the PBS group (1.33±0.31%, n=3); however, the differences were not significant (Figure 2F).
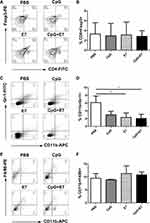
Vaccine Administration Led to a Reduction in the Numbers of Immunosuppressive Cells
Tregs, MDSCs, and TAMs are immunosuppressive cells, as well as a component of the immune system with essential roles in the maintenance of self-tolerance. We determined the percentage of Tregs (CD4+Foxp3+ T cells), TAMs (CD11b+F4/80+ cells), and MDSCs (CD11b+Gr-1+ cells) in the tumor tissue. The results showed that the administration of the vaccine led to a significant reduction in the percentage of MDSCs in the tumor tissue compared with that of the control group (2.05±1.07% vs 6.13±1.70%, respectively; P<0.05) (Figure 3D). No significant differences in the percentages of Tregs and TAMs (Figure 3B, F) were found among the groups. Representative dot plots of the percentages of Tregs, MDSCs, and TAMs from one mouse from each group are shown in Figure 3A, C, and E, respectively.
Vaccination Upregulated the Levels of Cellular Immune Factors and Downregulated Those of Immunosuppressive Factors
T helper type 1 (Th1) cells produce interleukin (IL)-2, thereby increasing the cytolytic activity of tumor-infiltrating lymphocytes. Our RT-qPCR results indicated that the relative mRNA expression levels of IL-2 were significantly increased in the vaccine group (16-fold; P<0.05) (Figure 4A) compared with that in the control group. IL-4 is a cytokine secreted primarily by immune cells, including mast cells, Th2 cells, eosinophils, and basophils, and functions as a potent regulator of immunity.13 The mRNA expression level of IL-4 was significantly decreased in the tumor tissues of the CpG+E7 (0.28±0.12, n=3), E7 (0.13±0.03, n=3), and CpG (0.22±0.06, n=3) groups compared with that of the PBS group (P<0.001, Figure 4B). In addition, the relative mRNA expression level of transforming growth factor beta (TGF-β) was significantly decreased in the vaccine group compared with that of the PBS group (P<0.05, Figure 4C). Vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis under both physiological and pathological conditions.14 The relative mRNA expression level of VEGF in the CpG+E7 (0.18±0.001, P<0.01) and CpG (0.42±0.04, P<0.05) groups was significantly decreased compared with that in the PBS group (Figure 4D). Chemokines and chemokine receptors mediate immune cell trafficking into the tumour micro environment.15 Here, we found that the mRNA expression levels of CCL1 (P<0.001), CCL2 (P<0.001), CCL3 (P<0.001), CCL5 (P<0.001), CCL12 (P<0.001), and CCL21 (P<0.001) were significantly decreased in the CpG+E7 group compared with that in the PBS group (Figure 4E–J). Dysregulated MMP activity is associated with prognosis in cervical cancer.16 The qPCR results showed that the mRNA expression levels of MMP-2 (P<0.001), MMP-3 (P<0.001), and MMP-9 (P<0.001) were significantly decreased in mice administered the vaccine compared with those of mice administered PBS (Figure 4K–M).
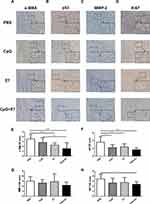
Immunohistochemical Staining for α-SMA, P53, MMP-2, and Ki67 in the Tumor Tissue
CAFs can promote tumor growth by aiding cell proliferation, angiogenesis, metabolism, and epithelial cell migration.17 Here, we evaluated the expression of α-SMA (Figure 5A), p53 (Figure 5B), MMP-2 (Figure 5C), Ki67 (Figure 5D) in the tumor tissues of the four groups by IHC. We found that α-SMA expression in the CpG+E7 group was significantly decreased compared with that in the PBS group (1.55±1.21 vs 3.55±0.93; P<0.001) (Figure 5E), suggesting that vaccine administration may reduce the numbers of CAFs in the TME. P53 has been reported to be highly expressed in cervical squamous cell carcinoma, while high expression of this protein is correlated with poor prognosis in patients with cervical cancer.18,19 The p53 IHC score was significantly lower in the vaccine group than in the PBS group (1.64±0.50 vs 3.55±1.44; P<0.001) (Figure 5F), which suggested that the administration of the vaccine might affect p53 levels. No significant differences were observed in the IHC score of MMP-2, a known risk factor for cervical cancer (Figure 5G).20 Ki67 is an established marker of proliferating cells and has been proposed to have prognostic value in cervical cancer.21 Additionally, Ki67 expression is strongly associated with the severity of cervical lesions. Here, we found that the Ki67 IHC score was significantly lower in the vaccine group than in the PBS group (2.36±0.50 vs 3.46±0.93; P<0.05) (Figure 5H), suggesting that vaccine administration inhibited tumor cell proliferation, in agreement with the reduction in tumor growth observed in this group (Figure 1B, C).
Discussion
No animal model of natural HPV infection-induced orthotopic cervical cancer is currently available. Orthotopic models of cervical cancer can be established through 1) induction using chemical reagents such as methylcholanthrene;22 2) surgical orthotopic implantation of cervical cancer tissue from patients or implanting cells from cervical cancer cell lines into the cervix;23 and 3) injecting cells from the U14 cervical cancer cell line into the vaginal submucosa near the cervix24 or inoculating TC-1 cells by vaginal instillation after gently disrupting the cervicovaginal epithelia using cytobrushes.25 In this study, we established an orthotopic genital cancer model by injecting TC-1 cells into the vaginal submucosa close to the cervix to mimic orthotopic cervical cancer. This animal modeling method is simpler to operate and less time-consuming compared with the in situ-induced model using chemical reagents. Moreover, our method avoids local fibrosis and scar formation due to tissue repair after the complex surgical implanting. The tumor location and tumor size in the negative control group (Figure 1B and C) showed little variation, which suggested that the injection technique applied in this study and the inoculated tumor cell number were stable and consistent even though the cervical area was limited.
The immunization route influences the type and strength of vaccine-induced immune responses as well as the efficacy of therapeutic vaccines against cancer. Many studies have shown that the mucosal immunization route has obvious advantages over parenteral vaccination (such as the subcutaneous route) for controlling mucosal tumors.26 Intranasal administration is a common mucosal immunization route. In a preclinical study using an HPV-associated genital tumor model, intranasal administration of a therapeutic HPV peptide vaccine incorporating a combination of α-galactosylceramide and CpG ODN adjuvants was demonstrated to induce the sustained elimination of established genital tumors in over 85% of treated mice.27 Intranasal administration of an HPV peptide vaccine containing only α-galactosylceramide as an adjuvant was also demonstrated to be capable of inhibiting the growth of HPV-related orthotopic head and neck or lung cancers, whereas intramuscular administration of the vaccine was not. Intravaginal administration is another mucosal immunization route for controlling genital cancer and was demonstrated to be more effective than the subcutaneous route at suppressing orthotopic genital tumor growth.28 Lee et al demonstrated that the intravaginal administration, but not subcutaneous immunization, of a vaccine containing HPV peptides and flagellin as an adjuvant could suppress orthotopic genital tumor growth and promote the long-term survival of tumor-bearing mice.29 However, also using an orthotopic genital tumor model, Decrausaz et al reported that subcutaneous immunization of a therapeutic HPV peptide vaccine that included two adjuvants elicited more robust cell-mediated responses compared with mucosal vaccination and promoted the regression of tumors in the genital mucosa.30 In the current study, we administered the vaccine subcutaneously and demonstrated that it exerted an inhibitory effect on orthotopic genital tumor growth. This indicated that subcutaneous-based immunization is also efficient at inhibiting genital tumors in mice. The discrepancy between the results of subcutaneous vaccination and mucosal vaccination may be attributed to differences in vaccine regimen (different vaccine formulations and different types of adjuvants) and experimental conditions.
Cellular immunity is necessary for the elimination of HPV-infected and HPV-transformed tumor cells. HPV-specific CD8+ CTLs are considered critical for the immune defense against cervical cancer. CD4+ T cells provide help in priming the generation, expansion, maintenance, and memory formation of CD8+ T cells by delivering essential activation signals to professional antigen-presenting cells or by producing cytokines. In antitumor immunity, CD4+ T cells can be recruited to the tumor and interact with tumor cells. Activated CD4+ Th1 cells can produce IFN-γ, which mediates the enhancement of MHC expression on tumor cells, as well as the recognition and eradication of tumor cells by T cells. Moreover, CD4+ T cells are required for CD8+ T cell infiltration into the tumor, which is critical for the efficacy of immunotherapy and long-term antitumor effects.31−33 Although CD4+ T cells are not classical cytotoxic cells, some can directly promote antitumor responses by killing tumor cells through FasL and TRAIL interactions, by secreting granzymes, or by inhibiting angiogenesis.34−36 The HPV16 E7 43–77 peptide in the vaccine contains one CD8+ T cell epitope and two CD4+ T cell epitopes. A single administration of the vaccine led to an increase in the simultaneous infiltration of CD4+ and CD8+ T cells (Figure 2A and B) in tumor tissue, which likely represents one of the mechanisms by which the vaccine inhibited the established tumors. In addition, the relative expression of Th2-related cytokines, such as IL-4 (Figure 4B), was significantly lower, whereas that of Th1-related chemokines, such as IL-2 (Figure 4A), was higher, in tumor tissue from vaccine-inoculated mice, indicating that the vaccine may have induced Th1 polarization.
Immunosuppression plays a pivotal role in cancer development. Tregs comprise a subset of T cells that are responsible for the maintenance of immune homeostasis. They also suppress tumor immune responses. High numbers of tumor-infiltrating Foxp3+ Tregs, and a low ratio of CD4+ or CD8+ T-cells to Foxp3+Tregs, have been observed in cervical cancer patients. Tregs within tumors interact with other immunosuppressive cells, including CAFs, type 2 macrophages, and MDSCs, and negatively regulate a variety of cells, including CTLs.37 MDSCs are a heterogeneous population of cells that expand in cancers and directly interact with tumor cells to support their growth and progression in the TME.38 TAM (CD11b+/CD68+/F4/80+ macrophages) infiltration appears to be a significant unfavorable prognostic factor for cancer patients.39 Surprisingly, in our study, we found that the number of MDSCs, but not macrophages or Tregs, was significant decreased in tumor tissues of vaccinated mice. This result is different from that obtained for subcutaneous TC-1 tumors treated by subcutaneous administration of this vaccine, which led to a decrease in the numbers of immunosuppressive cells.11 This may be explained by the variable influence of the vaccination on the TME of the orthotopic and ectopic cervical cancer models.
Different lymphocyte subsets are recruited into the TME by distinct chemokine–chemokine receptor signaling pathways, and these immune cells differentially regulate tumor immunity and cancer progression.15 The recruitment of monocytic MDSCs and macrophages into the TME is mediated by CCL2 and CCL5.40−42 Their expression by tumor cells is related to the numbers of MDSCs and TAMs in many tumors and is also correlated with poor prognosis in some cancers, including cervical cancer. CCL2 can also stimulate metastasis-associated macrophages to secrete CCL3, which further promotes macrophage retention in the tumor.41 In addition, CCL12 may promote the recruitment of MDSCs to the TME.43 In our study, analysis of the chemokine profile in the TME revealed that the expression of CCL2, CCL3, CCL5, and CCL12 were significantly decreased in the vaccine group, which may explain the reduced numbers of immunosuppressive MDSCs. Tregs are recruited to the TME via interactions between chemokines and chemokine receptors, including CCL1–CCR844 and CCL21–CCR7.45 Although the expression of CCL1 and CCL21 was decreased in the vaccine group, the recruitment of Tregs into the tumor was not, which indicated that chemokines may not be the only trigger for the recruitment of Tregs into the TME.
Tumor invasion and metastasis involve multistep steps, including the degradation of subepithelial basement membranes and the extracellular matrix (ECM).46 MMPs degrade most ECM components and play a key role in the dissemination of malignant neoplasms.47 MMP-2 can enhance the invasive ability of cervical cancer cells by degrading type IV collagen and fibronectin, which are components of all basement membranes,20 while MMP-3 secretion can serve as a marker of poor prognosis in cervical cancer.48 In addition, the upregulation of MMP-9 expression in both CIN II–III lesions and invasive carcinomas may be suggestive of tumor progression.49 Our results showed that vaccine administration induced a significant decrease in intratumoral MMP-2, MMP-3, and MMP-9 levels compared with those of controls. This suggests that the vaccine may have a marked effect on MMP regulation and thereby determine tumor invasiveness and disease outcome in cervical carcinoma. That the decrease in the number of MMP-2-positive tumors, as assessed by IHC, did not show statistical significance may stem from the large fraction of tumors that were weakly stained.
Conclusion
In the current study, we established an orthotopic genital tumor model and showed that the subcutaneous administration of a therapeutic HPV peptide-based vaccine inhibited the growth of established tumors. This may have resulted from an increase in infiltrating CD4+ and CD8+ T cells, a decrease in MDSC and CAFs, as well as the differential expression of a panel of cytokines, chemokines, and MMPs in the TME.
Abbreviations
HPV, human papillomavirus; CpG ODN, unmethylated cytosine-phosphate-guanosine oligodeoxynucleotide; TME, tumor microenvironment; MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; TAMs, tumor-associated macrophages; MMPs, matrix metalloproteinases; CAFs, cancer-associated fibroblasts; RT-qPCR, quantitative real-time reverse transcription polymerase chain reaction; IHC, immunohistochemistry; SD, standard deviation; ANOVA, analysis of variance; CTLs, cytotoxic T lymphocytes; CCL, CC chemokine ligand; ECM, extracellular matrix; α-SMA, alpha-smooth muscle actin; VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor beta.
Ethical Statement
All experimental procedures were approved by the Institutional Animal Care and Use Committees of China Medical University (IACUC Issue No. CMU2019293).
Funding
This work was supported by the National Natural Science Foundation of China (No. 81472439).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899.
3. Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4–7.
4. Villa LL. HPV prophylactic vaccination: the first years and what to expect from now. Cancer Lett. 2011;305(2):106–112.
5. Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23(1):75.
6. Mohebbi A, Ebrahimzadeh MS, Baghban Rahimi S, et al. Non-replicating Newcastle Disease Virus as an adjuvant for DNA vaccine enhances antitumor efficacy through the induction of TRAIL and granzyme B expression. Virus Res. 2019;261:72–80.
7. van Poelgeest MI, Welters MJ, van Esch EM, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a Phase II trial. J Transl Med. 2013;11:88.
8. Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011;4(3):361–375.
9. Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53(5):208–214.
10. Yang Y, Che Y, Zhao Y, Wang X. Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo. Int Immunopharmacol. 2019;69:279–288.
11. Che Y, Yang Y, Suo J, An Y, Wang X. Induction of systemic immune responses and reversion of immunosuppression in the tumor microenvironment by a therapeutic vaccine for cervical cancer. Cancer Immunol Immunother. 2020.
12. Burke JD, Young HA. IFN-gamma: a cytokine at the right time, is in the right place. Semin Immunol. 2019;43:101280.
13. Ho IC, Miaw SC. Regulation of IL-4 expression in immunity and diseases. Adv Exp Med Biol. 2016;941:31–77.
14. Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–467.
15. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572.
16. Azevedo Martins JM, Rabelo-Santos SH, do Amaral Westin MC, Zeferino LC. Tumoral and stromal expression of MMP-2, MMP-9, MMP-14, TIMP-1, TIMP-2, and VEGF-A in cervical cancer patient survival: a competing risk analysis. BMC Cancer. 2020;20(1):660.
17. Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211(8):1503–1523.
18. Avall-Lundqvist EH, Silfversward C, Aspenblad U, Nilsson BR, Auer GU. The impact of tumour angiogenesis, p53 overexpression and proliferative activity (MIB-1) on survival in squamous cervical carcinoma. Eur J Cancer. 1997;33(11):1799–1804.
19. Huang LW, Chou YY, Chao SL, Chen TJ, Lee TT. p53 and p21 expression in precancerous lesions and carcinomas of the uterine cervix: overexpression of p53 predicts poor disease outcome. Gynecol Oncol. 2001;83(2):348–354.
20. Davidson B, Goldberg I, Kopolovic J, et al. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma--a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;73(3):372–382.
21. Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11(3):1566–1572.
22. Chen B, Hou ZH, Dong Z, Li CD. Crocetin downregulates the proinflammatory cytokines in methylcholanthrene-induced rodent tumor model and inhibits COX-2 expression in cervical cancer cells. Biomed Res Int. 2015;2015:829513.
23. Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic Xenograft (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10(2):e0117417.
24. Zong S, Wang X, Yang Y, et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. Eur J Pharm Biopharm. 2015;93:127–135.
25. Yang M, Yu T, Wang YY, et al. Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv Healthc Mater. 2014;3(7):1044–1052.
26. Nardelli-Haefliger D, Dudda JC, Romero P. Vaccination route matters for mucosal tumors. Sci Transl Med. 2013;5(172):172–174.
27. Sierra G, Dorta-Estremera S, Hegde VL, Nookala SMK, Yanamandra AV, Sastry KJ. Intranasal therapeutic peptide vaccine promotes efficient induction and trafficking of Cytotoxic T cell response for the clearance of HPV vaginal tumors. Vaccines (Basel. 2020;8:2.
28. Li S, Zhu W, Ye C, et al. Local mucosal immunization of self-assembled nanofibers elicits robust antitumor effects in an orthotopic model of mouse genital tumors. Nanoscale. 2020;12(5):3076–3089.
29. Lee SE, Hong SH, Verma V, et al. Flagellin is a strong vaginal adjuvant of a therapeutic vaccine for genital cancer. Oncoimmunology. 2016;5(2):e1081328.
30. Decrausaz L, Domingos-Pereira S, Duc M, et al. Parenteral is more efficient than mucosal immunization to induce regression of human papillomavirus-associated genital tumors. Int J Cancer. 2011;129(3):762–772.
31. Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70(21):8368–8377.
32. Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180(5):3122–3131.
33. Marzo AL, Kinnear BF, Lake RA, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165(11):6047–6055.
34. Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650.
35. Matsuzaki J, Tsuji T, Luescher IF, et al. Direct tumor recognition by a human CD4(+) T-cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci Rep. 2015;5:14896.
36. Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12(6):677–686.
37. Najafi M, Farhood B, Mortezaee K. Contribution of regulatory T cells to cancer: a review. J Cell Physiol. 2019;234(6):7983–7993.
38. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174.
39. Manrique SZ, Correa MA, Hoelzinger DB, et al. Foxp3-positive macrophages display immunosuppressive properties and promote tumor growth. J Exp Med. 2011;208(7):1485–1499.
40. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225.
41. Kitamura T, Qian BZ, Soong D, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212(7):1043–1059.
42. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–220.
43. Shi H, Zhang J, Han X, et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1beta-mediated increase in E-selectin expression. Int J Cancer. 2017;140(6):1370–1383.
44. Xu Y, Dong X, Qi P, et al. Sox2 communicates with tregs through CCL1 to promote the stemness property of breast cancer cells. Stem Cells. 2017;35(12):2351–2365.
45. Chauhan SK, Saban DR, Dohlman TH, Dana RCCL. 21 conditioned regulatory T cells induce allotolerance through enhanced homing to lymphoid tissue. J Immunol. 2014;192(2):817–823.
46. Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10(6):415–433.
47. Crawford HC, Matrisian LM. Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis. 1994;14(1–6):234–245.
48. Arguello-Ramirez J, Perez-Cardenas E, Delgado-Chavez R, Solorza-Luna G, Villa-Trevino S, Arenas-Huertero F. Matrix metalloproteinases-2, −3, and −9 secreted by explants of benign and malignant lesions of the uterine cervix. Int J Gynecol Cancer. 2004;14(2):333–340.
49. Davidson B, Goldberg I, Kopolovic J, et al. Expression of matrix metalloproteinase-9 in squamous cell carcinoma of the uterine cervix-clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999;72(3):380–386.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.