Back to Journals » Journal of Pain Research » Volume 12
Inhibition Of Monocarboxylate Transporter 1 In Spinal Cord Horn Significantly Reverses Chronic Inflammatory Pain
Authors He J, Yu L, Wang Z, Wang Q, Cao JL, Gu L
Received 17 June 2019
Accepted for publication 19 October 2019
Published 5 November 2019 Volume 2019:12 Pages 2981—2990
DOI https://doi.org/10.2147/JPR.S219359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Jian-hua He,1,* Ling Yu,2,* Zhi-yong Wang,1 Qiang Wang,3 Jun-Li Cao,4 Lian-bing Gu1
1Department of Anesthesiology, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research and The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Ultrasound, Affiliated Hospital of Integrate Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing,People’s Republic of China; 3Department of Anesthesiology, Nanjing Meishan Hospital, Nanjing, People’s Republic of China; 4Jiangsu Key Laboratory of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lian-bing Gu
Department of Anesthesiology, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research and The Affiliated Cancer Hospital of Nanjing Medical University, Baiziting 42#, Nanjing, Jiangsu 210009, People’s Republic of China
Tel/fax +86 25 8328 4765
Email [email protected]
Purpose: Chronic inflammatory pain is a common condition in the clinic, and the underlying mechanism is not being completely understood. Various studies have demonstrated that central and peripheral sensitization and synaptic plasticity could play crucial functions in chronic inflammatory pain. Moreover, families of monocarboxylate transporters (MCTs) are closely related to cellular metabolism and synaptic plasticity, and it is also reported that MCTs participate in chronic inflammatory pain. Nevertheless, there is a probability of the engaging role of MCT 1 is in chronic inflammatory pain, but its specific cellular level mechanism is yet to be investigated. In our study, we hypothesized that MCT 1 in the spinal dorsal horn plays an important part in chronic inflammatory pain.
Methods: In experiment A, rats were gone through nociceptive behavioral testing at 1 d day before and 1 d, 3 d, and 7 d after completing complete Freund’s adjuvant (CFA) injection. The specimens collected for detecting MCT 1 by Western blotting. In experiment B, rats were randomly divided into four groups. Intrathecal injection of MCT 1 inhibitor and nociceptive behavioral tests were performed 1 d day before and 1 d, 3 d, 7 d, 14 d, and 21 d after CFA injection. MCT 1 and p-ERK levels in spinal dorsal horn were measured by Western blotting, and GFAP in spinal dorsal horn was detected by immunofluorescence.
Results: The expression of MCT 1 in the spinal dorsal horn was increased during chronic inflammatory pain in rats. The intrathecal injection of an MCT 1 inhibitor evidently diminished the expression of MCT 1 and GFAP in the spinal dorsal horn, and the behavioral nociceptive responses were also attenuated. Meanwhile, the expression of p-ERK was also decreased by the intrathecal injection of an MCT 1 inhibitor.
Conclusion: Our results indicate that MCT 1 very likely play a critical role in regulating chronic inflammatory pain and may influence the regulation of synaptic plasticity via ERK in the spinal dorsal horn of rats.
Keywords: chronic inflammatory pain, monocarboxylate transporter 1, spinal dorsal horn, astrocyte, synaptic plasticity
Introduction
Chronicity of inflammatory pain is one of the common symptoms in the clinical settings and the symptomatic treatment is very complexed due to the lack of knowledge; therefore it is essential to study the mechanism of inflammatory pain.1–5 The peripheral and central hypersensitization are the main mechanisms of chronic pain. The chronic pain animal models exhibit synaptic plasticity in the amygdala, anterior cingulate cortex, spinal cord and other sites in the central nervous system (CNS).6–14 Over the past few years, studies have confirmed the phenomenon that the interaction among neurons, microglia, and astrocyte cells in the CNS demonstrates a crucial part in the development and sustenance of synaptic plasticity during pain hypersensitivity after external inflammation.3,15,16
Monocarboxylate transporters (MCTs), which are the proton-linked plasma membrane conveyors that carry molecules through the cell membrane,were studied in numerous cancer cells. Increasing evidence indicates that MCTs are essential for cell metabolism.17 MCT 2 in CNS is mainly expressed by neurons, whereas MCT 4 is expressed in astrocytes. MCT 1 is primarily expressed in the astrocytes, microvascular endothelial cells, and oligodendrocytes.18–20
Some studies reported that MCTs-mediated lactate transport between astrocyte and neuron is vital for synaptic plasticity and the establishment of long-term memory in the hippocampus. Astrocytes are involved in memory formation through the supply of lactate to regulate the functions of neurons.21–23 The expression of MCT 1 on astrocytes was attenuated or disrupting the neuronal lactate transporter MCT 2 causes amnesia. These studies also indicate that the MCTs-mediated astrocyte–neuron lactate transport in the CNS plays an essential role in remodeling of the synaptic.
A recent study showed that the specific stimulation of spinal dorsal horn astrocytes on neuropathic pain by nerve ligation generates mechanical hyperalgesia, while reducing the expression of MCTs by α-cyano-4-hydroxycinnamate(4-CIN); mechanical allodynia was evidently alleviated in experimental mice. Then, the mechanical hyperalgesia can be reproduced by the intrathecal injection of excessive L-lactate.24,25 We previously reported that the expression of MCT 2 in the spinal dorsal horn is significantly related to pain behaviors in a chronic inflammatory pain model induced by complete Freund’s adjuvant (CFA).26
However, the specific mechanisms about the character of MCT 1 in the spinal cord during chronic inflammatory pain are still not clear. The purpose of this study was to investigate whether MCT 1 plays roles in chronic inflammatory pain induced by CFA in rats.
Methods
Experimental Animals
Male Sprague Dawley rats weighing 200–250 g were purchased from Weitong Lihua Laboratory Animal Technology Co Ltd. (Beijing, China; SCXK-JING2000–0009). The rats were accommodated in a room which were temperature-controlled at 24±1°C (mean±SEM) and with a 12-h light/dark cycle, animals were given food and water ad libitum. The current experiments were admitted by the Animal Care and Use Committee of Nanjing Medical University (Nanjing, China). The whole experiment process was according to the Guide of the Care and Use of Laboratory Animals by National Institutions of Health.27
In order to reduce the disturbance of animal life rhythm, all behavioral testing was performed during the light cycle (between 9:00 a.m. and 5:00 p.m.). The rats were allowed to adapt to experiment’s environmental conditions at least 30 min before the experiments were started.
Chronic Inflammatory Pain Model And Experimental Groups
A rat model of chronic inflammatory pain was established by subcutaneous injection of CFA (100 μL, Sigma-Aldrich, USA) into the left hind paw of the rats. A 25-gauge needle glass syringe was used for injecting into the rats.
In experiment A, 24 rats were used. Behavioral testing was performed 1 d day before and 1 d, 3 d, and 7 d after completing CFA injection. The time point of specimens was collected as described in the Western blotting procedure (n=4).
In experiment B, 56 rats were randomly divided into four groups: (1) the control group, which received an injection of saline (100 μL) in the left hind paw of rats; (2) the CFA group, which received an injection of CFA (100 μL) and intrathecal injection of 10 μL of artificial cerebrospinal fluid; (3) the CFA+CHC (a-cyano-4-hydroxycinnamic acid, MCT 1 inhibitor, Sigma-Aldrich) group, which received an injection of 100 μL CFA and an intrathecal injection of 100 μmol CHC (in 10 μL of 10% dimethyl sulfoxide (DMSO)); and (4) the CFA+DMSO group, which received an injection of 100 μL of CFA and DMSO. The time point of behavioral testing as described in Figure 2A, specimens for Western blotting and immunofluorescencewere collected 7 d after CFA injection.
Drugs And Intrathecal Injection
CHC was used in this study and was dissolved in 10% DMSO. The intrathecal injections were accomplished as described earlier. In brief, after the rats were subjected to inhalational anesthesia with sevoflurane which delivered by a plastic nose mask, the spines were palpated, and the needle was advanced manually through the interspinous space between L5 and L6. The successful puncture was determined by an obvious sense of breakthrough and a typical tail flick. ASCF, CHC or DMSO (10 μL) was intrathecally injected slowly. After the completion of the liquid injection, the syringe needle was kept in a position of intrathecal space for 2 min before withdrawal.28
Nociceptive Behavioral Tests
Thermal Hyperalgesia Test
Paw withdrawal thermal latencies (PWTLs) were evaluated to thermal hyperalgesia according to the previous study.29 Briefly, the rats were placed into testing cages on a glass platform, maintaining the room temperature at 25°C and allowed the rats to acclimatize for at least 30 min. A thermal stimulus (Ugo Basile 37,370, Comerio, Italy) was used. The radiant thermal source was focused on the center of the hind paw. When the rats show the typical sign of lifting of licking of the left hind paw, the radiant heat test was ended, and the duration to the endpoint was regard as PWTL. Before formal testing, a basal PWTL was obtained by adjusting the radiant heat strength. To prevent potential tissue damage, a cutoff of test was automatically set up at 20 s. Three measurements were taken with intervals at least 5 min. The PWTLs were recorded and the mean value was calculated for analysis.
Mechanical Hyperalgesia Test
Paw withdrawal mechanical thresholds (PWMTs) were measured by von Frey filament (Woodland Hills, LA, USA). Before the test, the rats were allowed to place in plastic champers set on an elevated metal mesh floor to habituate for 30 min. A series of von Frey filaments were used to stimulate the plantar surface of each left hind paw, the lifting, shaking, or licking of the rat paw by stimulation, which was considered a positive response. The up-down method was performed as previously described. Briefly, stimuli were presented in a consecutive style and testing was started from the 2.0-g hair. The von Frey hair was penetrated to the plantar surface vertically, maintained for 5 s approximately, and the interval time of stimuli was kept above 30 s. If the initially selected hair cannot trigger a paw withdrawal response, a stronger stimulus was submitted; supposing that a positive response was presented, the weaker stimulus was chosen in the next test. When first crossed of the response threshold was emerged, four additional tests of stimuli that were also followed up or down sequentially. The 50% response threshold was calculated using the formula and results for analysis.30
Western Blotting
On d 7 after CFA injection, the rats were deeply anesthetized by pentobarbital sodium (50 mg/kg, i.p.) and then sacrificed by a decapitation (n=4 in each group). The spinal cord horn from segments L4–L6 was removed and frozen in liquid nitrogen, and then transferred for storage at −70°C for analysis. The tissues were homogenized with lysis buffer at 0°C in tissue blenders. The homogenates were centrifuged (1000 rpm, 4°C) for 10 min to remove all cell debris. The supernatants including the protein lysates were gathered and stored at −70°C for analysis. The concentration of protein in each soluble fraction was measured using the BCA kit. Subsequently, the proteins (50 μg/20 μL) were electrophoresed on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes. Then, the membranes were blocked with 5% skim milk in TBST (tris-buffered saline and 0.1% Tween 20) for 2 hrs at room temperature. When finished, the membranes were washed and then incubated in sealed plastic bags with primary antibodies MCT 1 (1:2000, Abcam, Cambridge, UK) or a rabbit antibody p-ERK (1:1000, Cell Signaling Technology, Danvers, MA, USA) isoforms at 4°C in the refrigerator overnight. Because the molecular weights of p-ERK44, p-ERK42 and β-actin were very close, we had to transfer the same weight proteins onto another membrane for detected β-actin as a control. The membranes were then washed thrice with TBST for 5 min the next day, and then incubated in a secondary antibody (1:500, Sigma-Aldrich) for 2 h at room temperature. After the membranes were washed by TBST, the protein bands were detected by an enhanced chemiluminescence assay (Millipore, USA). Analysis of the results was performed by a gel scanning densitometer (Molecular Dynamics)
Immunofluorescence Analysis
On d 7 after CFA injection, behavioral testing was performed, and then the animals were deeply anesthetized with pentobarbital sodium overdose and perfused intracardially with normal saline followed by 4% cold phosphate-buffered paraformaldehyde (n=4 in each group). Spinal dorsal tissues (L4–L6) were collected and post-fixed in 4% paraformaldehyde for 8 hrs at 4°C. Next, the embedded spinal cords were transversely sectioned at a thickness of 25 μm. After washing in PBS, the tissue sections were incubated with a goat antibody against GFAP (1:200, Santa Cruz Biotechnology, USA) at 4°C for 24 hrs. Then, the tissue sections were washed thrice by PBS and incubated with a secondary antibody for 2 hrs in a dark area at room temperature. Then, the specimens were examined under an immunofluorescence microscope (Zeiss AX10, Germany). The negative controls were served as same procedure but the primary antibody was omitted. The mean fluorescence intensity and positively stained elements were analyzed using Image-Pro Plus 6.0. To obtain the raw data of GFAP immunofluorescence for quantitative measurements, 15 fields covering the dorsal horn of spinal cord in each group were evaluated; each pixel’s mean green fluorescence intensity was normalized to the background intensity in the same image.
Statistical Analysis
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc. San Diego, CA) were used to perform statistical analysis. Data are expressed as mean±SD. The data of PWTLs and PWMTs after inoculation were normally distributed and compared with baseline values and between groups using two-way ANOVA for repeated measures followed by Bonferroni correction. Analysis of the Western blot data and the quantification of immunofluorescence using one-way ANOVA followed by post hoc Bonferroni multiple comparisons. p Value less than 0.05 was considered as statistical significance.
Results
Changes Of MCT 1 In The Spinal Dorsal Horn And The Development And Maintaining Of Thermal Hyperalgesia With Mechanical Allodynia Induced By CFA
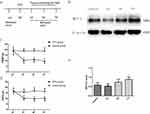
Rapid and long-lasting thermal hyperalgesia and mechanical allodynia appeared within 1 d, 3 d, and 7 d after CFA injection but not in the sham rats simultaneously. This symptom was sustained on d 7 after CFA injection (Figure 1C and D). After the behavioral tests were completed, we obtained segments of the spinal dorsal horn for Western blot analysis (n=4) (see the illustration of experimental protocol in Figure 1A). Compared with the control group, the CFA group exhibited an increase level of MCT 1 protein expression in spinal cord horn on d 7 (Figure 1B and E).
Intrathecal Injection Of CHC Reversed Thermal Hyperalgesia And Mechanical Allodynia Induced By CFA
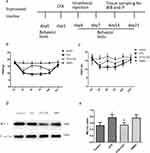
The results of the experiments described above suggest that MCT 1 may be involved in CFA-induced chronic inflammatory pain in the spinal cord. We, therefore, wondered whether an MCT 1 inhibitor can reverse sustained hyperalgesia induced by CFA. To investigate this question, a single dose of CHC (100 μmol, 10 μL) was given in an intrathecal injection to rats 1 d after CFA (n=14, after the completion of the behavioral tests) (see illustration of experimental protocol in Figure 2A). The behavioral tests showed that CHC reversed the hyperalgesia and allodynia that had been established by CFA. On d 1, the PWTLs and PWMTs were obviously reduced in the CFA group compared with the control group. However, 2 d after intrathecal injection, the quantity of PWMTs of the rats in the CHC+CFA group was significantly reversed compared with those in the CFA group and DMOS group. This effect was sustained on d 7 and d 14 after CFA injection. On d 21, no differences of the PWMTs were observed between the CHC+CFA group and the CFA group. Meanwhile, the PWTLs of the rats in the CHC+CFA group were also significantly reversed compared with those in the CFA group and DMOS group, but this effect only was showed on d 3. There were no differences between the CFA group and the DMSO group, indicating that CHC but not DMSO reversed the effect of thermal hyperalgesia and mechanical allodynia induced by CFA (Figure 2B and C).
Intrathecal Injection Of CHC Reversed The CFA-Induced Expression Of Spinal MCT 1
Western blot analyses were used to quantify the expression of MCT 1 in the spinal cord horn. Compared to the control group, MCT 1 protein expression in rat’s spinal dossal horn from the CFA group was much higher. CHC (100 μmol, 10 μL) significantly suppressed the expression of the MCT 1 protein compared with that the levels of protein in the CFA group (p<0.05). In addition, no differences were detected in the expression of the MCT 1 protein between the rats in the CFA and the DMSO group (Figure 2D and E).
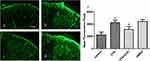
Intrathecal Injection Of CHC Reversed The CFA-Induced Expression Of Spinal GFAP
Previous studies have reported that central sensitization is developed and maintained by the activation of neurons and glial cells. To evaluate whether CHC influences the activation of astrocytes induced by CFA injection, we detected the expression of the astrocyte marker GFAP in the spinal cord by Immunofluorescence analysis. The results showed that CHC significantly suppressed the upregulation of GFAP in the spinal cord after CFA injection. However, DMSO did not show a notable influence on the expression of GFAP (Figure 3A–D). The quantitative immunofluorescence is expressed as the mean fluorescence pixels in the dorsal horn of the spinal cord. A significant difference revealed between the CFA group and the control group by one-way ANOVA statistical analysis followed by post hoc Bonferroni multiple comparisons. (*p<0.05 and **p<0.01) was also shown between the CHC+CFA group and the CFA group (#p<0.05 and ##p<0.01) (Figure 3E).
Intrathecal Injection Of CHC Inhibited The Expression Of P-ERK Induced By CFA In The Spinal Dorsal Horn
To evaluate the mechanism of CHC on the CFA-induced activation of neurons and glia in the dorsal horn of the spinal cord, we also analyzed the expression of p-ERK by Western blot. The results showed that the administration of CHC (100 μmol, 10 μL) significantly suppressed the expression of p-ERK in the spinal cord horn in chronic inflammatory pain model induced by CFA (p<0.05) (Figure 4A and B).
Discussion
Our study showed that MCT 1 exists in the spinal cord horn and increases with the development and maintenance of pain by CFA-induced central sensitization. Also, the intrathecal injection of an inhibitor of MCT 1 reversed thermal hyperalgesia and mechanical allodynia induced by CFA. This confirmed that MCT 1 in the spinal cord participates in chronic inflammatory pain. We also detected the expression of spinal MCT 1 by Western blot, and the results demonstrated the effectiveness of CHC intrathecal injection. To explore the mechanism of MCT 1 involvement in chronic inflammatory pain in the spinal cord, we further detected the expression of p-ERK in the spinal cord, and the results showed that CFA clearly induces the expression of the p-ERK protein in the spinal dorsal horn. We also observed that, when the activation of MCT 1 is attenuated, the expression of p-ERK decreases and it seems that MCT 1 may be involved in chronic inflammatory pain by activating the ERK signaling pathway. This result is in accordance with multiple studies on ERK in different pain states.31–35 MCT 1 may attenuate ERK by influencing the regulation of the energy, abnormally activated neurons and thus exhibit an analgesic effect on pain.
Accumulating studies have demonstrated that MCT 1 is mainly expressed in endothelial cells and astrocytes in the CNS. Due to the importance of astrocytes in chronic pain and the distribution of MCT 1 by astrocytes,36–39 we think that MCT 1 may affect the activation of astrocytes during chronic inflammatory pain. The experimental results confirm our assumption that the activation of the astrocyte marker GFAP is obviously attenuated by CHC. It seems that the analgesic effect of CHC also inhibits the activation of astrocytes. To our knowledge, the present study demonstrates that MCT 1 in the spinal cord participates in chronic inflammatory pain and this new understanding of chronic inflammatory nociceptive mechanisms may provide a progressive approach in finding a new target for the treatment.
Over the last decade, an increasing body of evidence has demonstrated that glia in the CNS plays more important roles in pain processing than previously thought. Neurons, microglia, and astrocytes are interrelated and interact with each other; they also act as elemental mechanisms in acute and chronic pain.38,40,41 The results of this study and previous studies indicate that MCTs transfer lactate from astrocytes to neurons that are involved in the initiation and sustained of inflammatory and neuropathic pain. Furthermore, another study indicated that head and neck squamous cell carcinoma induces an acidic bone microenvironment and leads to bone pain via the lactate transporter MCT 4.42 Thus, the collected evidence reveals that MCTs are involved in pathological processes in some pain states, and it also supports the astrocyte–neurone lactate shuttle hypothesis of pain mechanisms.
There are some differences between our study and previous studies. In a previous study, the mRNA expression of MCT 2, MCT 1, and MCT 4 in the spinal cord was decreased in an animal model of neuropathic pain by small interfering RNAs; thus, the experimental results showed that the knockdown of MCT2 attenuated L-lactate-induced mechanical hyperalgesia.25 However, the knockdown of MCT 1 and MCT 4, which are expressed in astrocytes in the spinal cord, did not influence mechanical hyperalgesia induced by L-lactate. There may be several reasons for this. First, the experimental pain model which was being performed in our research was dissimilar with the other studies. Second, the other group used small interfering RNAs to attenuate mRNA expression, and we employed specific drugs for inhibiting protein expression. Another explanation for the inconsistent results may include differences in experimental conditions such as the animal strain and behavioral methods.
There are some limitations to our study. We did not measure MCT 4 in the spinal dorsal horn, so we did not determine whether the expression of MCT 4 changes in the spinal dorsal horn during chronic inflammatory pain. We did not directly measure the CFA-induced lactate concentration in the spinal cord, but a recent study by Keisuke Miyamoto et al25 revealed that spinal astrocytes themselves induce nociceptive hypersensitivity through excessive L-lactate release. Their findings may support our conclusion indirectly.
In the future, synaptic factors directly related to the synaptic plasticity involved in the energy metabolism of brain cells via astrocyte–neuron lactate shuttles representing the critical factors for brain synapses should be investigated. The study of the specific mechanism of the synergistic effect of MCT 1, MCT 2, and MCT 4 in different chronic pain states is also necessary. Moreover, research investigating the activation of astrocytes via aberrant astrocyte–neuron lactate shuttles that are involved in spinal nociceptive sensitization is also required.
Conclusions
In summary, we provide the first evidence that CHC attenuates CFA-induced chronic inflammatory pain by inhibiting MCT 1 activity in the spinal dorsal horn. MCT 1 may influence the p-ERK signaling pathway and the activation of astrocytes. Our study suggests that MCT 1 in the spinal cord may be a potential drug target for chronic inflammatory pain treatment.
Acknowledgments
This study is supported by the open study of Jiangsu Key Laboratory of Anesthesiology, Jiangsu Province, China (KJS1407), and the foundation of the sponsored project of Jiangsu Provincial Six Talent Peaks (WSW-019). We thank Dr Abdul Mannan (Jiangsu Key Laboratory of Anesthesiology, Xuzhou Medical University) for providing assistance in language editing.
Author Contributions
Jian-hua He, Jun-Li Cao, and Lian-bing Gu designed the study. Ling Yu and Zhi-Yong Wang performed the animal experiments. Qiang Wang analyzed the data. Jian-hua He and Ling Yu wrote the manuscript. All authors contributed to data analysis, drafting or revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi:10.1016/j.cell.2009.09.028
2. Grosser T, Woolf CJ, FitzGerald GA. Time for nonaddictive relief of pain. Science. 2017;355:1026–1027. doi:10.1126/science.aan0088
3. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi:10.1038/nrd4334
4. Mansfield KE, Sim J, Jordan JL, et al. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157:55–64. doi:10.1097/j.pain.0000000000000314
5. Yekkirala AS, Roberson DP, Bean BP, et al. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov. 2017;16:810. doi:10.1038/nrd.2017.202
6. Ikeda H, Heinke B, Ruscheweyh R, et al. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi:10.1126/science.1080659
7. Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014;37:343–355. doi:10.1016/j.tins.2014.04.002
8. Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi:10.1097/01.anes.0000264769.87038.55
9. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi:10.1126/science.288.5472.1765
10. Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi:10.1016/j.tins.2008.01.003
11. Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–496. doi:10.1038/nrn.2016.68
12. Koga K, Descalzi G, Chen T, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;86:1109. doi:10.1016/j.neuron.2015.05.016
13. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi:10.1038/nrn1704
14. Wu Y, Yao X, Jiang Y, et al. Pain aversion and anxiety-like behavior occur at different times during the course of chronic inflammatory pain in rats. J Pain Res. 2017;10:2585–2593. doi:10.2147/JPR
15. Fan XC, Fu S, Liu FY, et al. Hypersensitivity of prelimbic cortex neurons contributes to aggravated nociceptive responses in rats with experience of chronic inflammatory pain. Front Mol Neurosci. 2018;11:85. doi:10.3389/fnmol.2018.00085
16. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi:10.1016/j.jpain.2009.06.012
17. Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi:10.1111/jnc.2005.94.issue-1
18. Ideno M, Kobayashi M, Sasaki S, et al. Involvement of monocarboxylate transporter 1 (SLC16A1) in the uptake of L-lactate in human astrocytes. Life Sci. 2018;192:110–114. doi:10.1016/j.lfs.2017.10.022
19. Liu Z, Sneve M, Haroldson TA, et al. Regulation of monocarboxylic acid transporter 1 trafficking by the canonical Wnt/beta-catenin pathway in rat brain endothelial cells requires cross-talk with notch signaling. J Biol Chem. 2016;291:11.
20. Pandey SK, Yadav S, Goel Y, et al. Cytotoxic action of acetate on tumor cells of thymic origin: role of MCT-1, pH homeostasis and altered cell survival regulation. Biochimie. 2019;157:1–9. doi:10.1016/j.biochi.2018.10.022
21. Erlichman JS, Hewitt A, Damon TL, et al. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats: a test of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28:4888–4896. doi:10.1523/JNEUROSCI.5430-07.2008
22. Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi:10.1371/journal.pone.0028427
23. Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi:10.1016/j.cell.2011.02.018
24. Koyanagi S, Kusunose N, Taniguchi M, et al. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun. 2016;7:13. doi:10.1038/ncomms13102
25. Miyamoto K, Ishikura KI, Kume K, et al. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia. 2019;67:27–36. doi:10.1002/glia.23474
26. He JH, Xu L, Shen Y, et al. The changes of monocarboxylate transporter-2 in spinal cord horn in a rat model of chronic inflammatory pain. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2015;31:19–22.
27. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi:10.1016/0304-3959(83)90201-4
28. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi:10.1016/0014-2999(80)90515-4
29. Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;31:445–451. doi:10.1016/0091-3057(88)90372-3
30. Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi:10.1016/0165-0270(94)90144-9
31. Cui XY, Dai Y, Wang SL, et al. Differential activation of p38 and extracellular signal-regulated kinase in spinal cord in a model of bee venom-induced inflammation and hyperalgesia. Mol Pain. 2008;4:17. doi:10.1186/1744-8069-4-17
32. Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi:10.2174/1876386300902010011
33. Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi:10.1038/16040
34. Song XS, Cao JL, Xu YB, et al. Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol Sin. 2005;26:789–798. doi:10.1111/j.1745-7254.2005.00123.x
35. Zhuang ZY, Gerner P, Woolf CJ, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi:10.1016/j.pain.2004.12.022
36. Blaszczyk L, Maitre M, Leste-Lasserre T, et al. Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation. J Neuroinflammation. 2018;15:349. doi:10.1186/s12974-018-1378-z
37. Chiang CY, Sessle BJ, Dostrovsky JO. Role of astrocytes in pain. Neurochem Res. 2012;37:2419–2431. doi:10.1007/s11064-012-0801-6
38. Kim SK, Nabekura J, Koizumi S. Astrocyte-mediated synapse remodeling in the pathological brain. Glia. 2017;65:9. doi:10.1002/glia.v65.11
39. Tian G, Luo X, Tang C, et al. Astrocyte contributes to pain development via MMP2-JNK1/2 signaling in a mouse model of complex regional pain syndrome. Life Sci. 2017;170:64–71. doi:10.1016/j.lfs.2016.11.030
40. Ikeda H, Mochizuki K, Murase K. Astrocytes are involved in long-term facilitation of neuronal excitation in the anterior cingulate cortex of mice with inflammatory pain. Pain. 2013;154:2836–2843. doi:10.1016/j.pain.2013.08.023
41. Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi:10.1038/nrn2533
42. Hasegawa K, Okui T, Shimo T. Lactate transporter monocarboxylate transporter 4 induces bone pain in head and neck squamous cell carcinoma. Int J Mol Sci. 2018;19:13. doi:10.3390/ijms19113317
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.