Back to Journals » Drug Design, Development and Therapy » Volume 16
Inhibition of CXCR4 in Spinal Cord and DRG with AMD3100 Attenuates Colon-Bladder Cross-Organ Sensitization
Authors Zhang H , Dong X, Yang Z, Zhao J, Lu Q, Zhu J, Li L, Yi S, Xu J
Received 26 August 2021
Accepted for publication 18 December 2021
Published 6 January 2022 Volume 2022:16 Pages 67—81
DOI https://doi.org/10.2147/DDDT.S336242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Hengshuai Zhang, Xingyou Dong, Zhenxing Yang, Jiang Zhao, Qudong Lu, Jingzhen Zhu, Longkun Li, Shanhong Yi, Jie Xu
Department of Urology, Second Affiliated Hospital, Army Medical University, Chongqing, 400037, People’s Republic of China
Correspondence: Jie Xu; Shanhong Yi
Department of Urology, Second Affiliated Hospital, Army Medical University, Chongqing, 400037, People’s Republic of China
Email [email protected]; [email protected]
Background: Cross-sensitization of pelvic organs is one theory for why symptoms of gut sickness and interstitial cystitis/bladder pain syndrome overlap. Experimental colitis has been shown to trigger bladder hyperactivity and hyperalgesia in rats. The chemokine receptor CXCR4 plays a key role in bladder function and central sensitization. We aim to study the role of CXCR4 and its inhibitor AMD3100 in colon-bladder cross-organ sensitization.
Methods: The colitis model was established by rectal infusion of trinitrobenzene sulfonic acid. Western blot and immunofluorescence were used to assess the expression and distribution of CXCR4. Intrathecal injection of AMD3100 (a CXCR4 inhibitor) and PD98059 (an ERK inhibitor) were used to inhibit CXCR4 and downstream extracellular signal-regulated kinase (ERK) in the spinal cord and dorsal root ganglion (DRG). Intravesical perfusion of resiniferatoxin was performed to measure the pain behavior counts of rats, and continuous cystometry was performed to evaluate bladder voiding function.
Results: Compared to the control group, CXCR4 was expressed more in bladder mucosa and colon mucosa, L6-S1 dorsal root ganglion (DRG), and the corresponding segment of the spinal dorsal horn (SDH) in rats with colitis. Moreover, intrathecal injection of the AMD3100 suppressed bladder overactivity, bladder hyperalgesia, and mastocytosis symptoms caused by colitis. Furthermore, AMD3100 effectively inhibited ERK activation in the spinal cord induced by experimental colitis. Finally, treatment with PD98059 alleviated bladder overactivity and hyperalgesia caused by colitis.
Conclusion: Increased CXCR4 in the DRG and SDH contributes to colon inflammation-induced bladder overactivity and hyperalgesia partly via the phosphorylation of spinal ERK. Treatment targeting the CXCR4/ERK pathway might provide a potential new approach for the comorbidity between the digestive system and the urinary system.
Keywords: pain, visceral hypersensitivity, sensory afferents, urgency, colitis, ERK
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is an etiologically unclear chronic pain condition characterized by bladder-related pain and discomfort, troubled frequent urination, and nocturia.1 Growing evidence shows that chronic bladder dysfunction represented by IC/BPS is closely related to gut diseases.2,3 Gut diseases are ten to one hundred times more frequent in IC/BPS patients compared to the general population.4 Similarly, as reported in a recent long-term cohort study, patients diagnosed with irritable bowel syndrome (IBS) are more likely to have IC/BPS.5
The “cross-organ sensitization” hypothesis is currently a widely accepted hypothesis to describe and explain the common pathogenesis of abnormal urination diseases and intestinal diseases.2 The part of neurons in DRG and spinal cord that can receive afferents from the bladder and colon at the same time is the anatomical basis of this hypothesis, and peripheral and central sensitization are the key events.6,7 In rats with colitis, the excitability of sensory afferent fibers and corresponding dorsal root ganglion (DRG) neurons in the bladder increased significantly, and the bladder was more sensitive to chemical stimuli such as capsaicin, carbachol, and bradykinin.8,9 At the spinal cord level, evidence suggests that the activation of microglia promotes bladder hypersensitivity caused by colitis.10 These studies indicate the important role of central and peripheral sensitization in bladder hypersensitivity after colitis. However, the mechanism leading to sensitization of the common neural pathways of the bladder and colon remains unclear.
CXCR4 is a key receptor for the chemokine CXCL12 and is widely involved in inflammation and neuroregulation. Evidence shows that CXCR4 participates in the induction of central and peripheral sensitization.11 It is widely distributed in neurons and glial cells of DRG and spinal cord, and intrathecal injection of CXCL12 to activate CXCR4 contributes to mechanical hyperalgesia.12 CXCR4 knockdown results in significantly higher nociceptive thresholds than wild-type rats in response to intraplantar complete Freund’s adjuvant injection.13 In addition, AMD3100 prevents bladder hyperalgesia induced by PAR activating peptides.14 The significant increase in voiding interval caused by the CXCR4 receptor antagonist AMD3100 indicates that the CXCR4 receptor has a promoting effect on the voiding reflex.15 Together, these findings suggest that CXCR4 is critical for central and peripheral sensitization and is involved in overactive bladder and bladder hyperalgesia. A previous study found that the number of bladder sensory-related neurons sensitive to CXCR4 signals in the DRG significantly increased after sciatic nerve injury,16 suggesting a possible link between CXCR4 and mutual interference involving different diseases. However, it is less clear whether CXCR4 contributes to colon-bladder cross-organ sensitization.
Thus, in the present study, we established a trinitrobenzene sulfonic acid (TNBS)-induced chronic colitis model in rats and investigated whether CXCR4 is altered in the lumbosacral spinal cord and corresponding ganglia. Additionally, we examined the impact of AMD3100 on bladder hyperactivity and hyperalgesia, and whether ERK signals in the lumbosacral spinal cord and corresponding ganglia are involved as possible downstream.
Materials and Methods
Animal
Female Sprague Dawley rats (200‐250g) were obtained from the Laboratory Animal Center of the Army Medical University. Animals were raised between 22–24°C and given unrestricted access to food and water. All animal tests were conducted according to the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee and approved by the Animal Care and Use Committee of the Army Military Medical University (approval No. AMUWEC20212093).
Induction of Experimental Colitis by TNBS
As previously described,17 isoflurane was used to anesthetize the rats following a 24-hour fast. Next, a PE-50 catheter was introduced into the colon from the anus and the end was 8cm deep. Then an equal volume of mixed TNBS solution and absolute ethanol was injected into the colon at a final concentration of 80 mg/kg. The rats were then positioned supine with the lower body elevated to 60° to reduce drug leakage. Correspondingly, the same volume of 50% ethanol was administered to the control (CON) group.
Drugs and Intrathecal Injection
Intrathecal administration was similar to those reported before.18 CXCR4 inhibitor AMD3100 and ERK inhibitor PD98059 were purchased from MCE (MCE, China). Following intraperitoneal anesthesia of rats with 100 mg/kg pentobarbital, the skin at the level of the fourth through fifth lumbar vertebrae on the back of the mouse was incised. A partial laminectomy at L4/L5 was performed to expose the dura and a 30-gauge needle was placed in the midline into the L4/5 intervertebral region. A catheter with an external diameter of 0.2mm was inserted into the needle hole, and its end was pushed into and terminated at the L6/S1 vertebral level. Subsequently, a 6–0 silk thread was used to secure the catheter’s insertion end to the surrounding tissue. To facilitate administration, the free end of the catheter is sent subcutaneously to the back of the neck to be exposed and fixed. After the catheter implantation, all rats recovered for 2 days prior to experiments. AMD3100 (10ug/20ul), PD98059 (5ug/20ul), or an equivalent amount of solvent were delivered to rats through the intrathecal catheter for 7 consecutive days in separate groups.
Tissue Sections and Staining
One week after the colitis model was established, the rats were slaughtered and tissues including the bladder, distal colon, DRG, and spinal cord were taken. A part of these tissues used for tissue staining was fixed in 4% paraformaldehyde and embedded in paraffin. The fixed bladder, colon, and spinal cord tissues were cut into 5μm thick sections, and DRG tissue was cut into 20μm thick sections. The histomorphological changes of the colon and bladder were analyzed by hematoxylin-eosin (HE) staining and mast cell enumeration was performed by toluidine blue staining. The above staining was carried out following the standard procedures.
MPO Activity Measurement in the Bladder and Colon
The activity of myeloperoxidase (MPO) in bladder and colon tissues was measured using a kit purchased from Nanjing Jiancheng Institute of Bioengineering. In brief, the harvested bladder and colon were homogenized with the MPO assay buffer and centrifuged at 12000g for 5 minutes, and then the supernatant was used to determine MPO activity according to the kit instructions.
Immunohistochemistry
Immunohistochemistry was performed as previously described.19 The rat bladder section was deparaffinized in a gradient concentration of ethanol solution, and then heated for antigen retrieval. At room temperature, 3% hydrogen peroxide was used to block endogenous peroxidase. After blocking with 5% BSA for 1 hour, the sections were incubated with 1:100 PBS containing anti-cytokeratin 20 antibody (Abcam, UK) and kept at 4°C overnight. On the second day, the sections were incubated with HRP-labeled anti-rabbit secondary antibody. After washing the section again, DAB chromogen was used to mark target proteins, and the nucleus was counterstained with haematoxylin.
Immunofluorescence Staining
The tissue sections were washed in PBS and blocked with 5% BSA in PBS for 100 minutes. The sections were washed three times and incubated with the primary antibody CXCR4 (Abcam, UK) or p-ERK1/2 (Abcam, UK) at 4°C for 12 hours. Then the sections were washed again in PBS and treated with the secondary antibody diluted in PBS for 100 minutes at room temperature. Goat anti-mouse Alexa Fluor 555 secondary antibody (Thermo Fisher, USA) was used at 1:1000 dilution. After that, the sections were rinsed with PBS and counterstained with DAPI, then analyzed using an inverted fluorescence microscope (Olympus, Denmark).
Western Blot
RIPA buffer containing proteinase inhibitors (Beyotime, China) was used to create tissue extracts, which were then used to perform the analyses., and the concentration of protein was measured by BCA Protein Assay Reagent (Beyotime, China). The tissue lysate containing 40μg of total protein was loaded onto the SDS-PAGE gel and electrophoresed at 120V for 90 minutes, and then the protein to be tested in the gel was transferred to a 0.45m polyvinylidene fluoride membrane (Merck Millipore, Germany). After blocking the membrane in TBST buffer containing 5% skimmed milk powder for 30 minutes, the membranes were incubated for 12 hours at 4 °C with the following antibodies: CXCR4 (Abcam, UK), GAPDH (Proteintech, China), β-tubulin (Proteintech, China), ERK1/2 (Abcam, UK), p-ERK1/2 (Abcam, UK). The membranes were rinsed three times with TBST (5 minutes/wash) the next day before being incubated with goat anti-rabbit secondary antibody (Thermo Fisher, USA) or goat anti-mouse secondary antibody (Thermo Fisher, USA) in 5% skimmed milk powder /TBST, and then rinsed 3 times. Membrane development was carried out using ECL Substrate (Thermo Fisher, USA), and the chemiluminescence was captured using the bioanalytical imaging system C300 (Azure Biosystems, USA) and analyzed using ImageJ software.
Continuous Cystometry
Cystometry was performed on unconscious rats 7 days after induction of experimental colitis to evaluate the urodynamic contraction pressure and time as previously described.18 Briefly, rat bladders were exposed through a 2cm suprapubic incision after they were anesthetized with urethane. Then, through a minimally invasive incision on the top of the bladder, a PE-50 catheter was connected to the bladder lumen and the connection was fixed with surgical sutures. At the end of the operation, the PE-50 tube was attached via a three-way connector to a data acquisition system (Chengdu Instrument Factory, China) for recording voiding frequency and pressure, and to a microperfusion pump (Smiths Medical, USA) for infusing saline (10 mL/h, room temperature). After 20–30 minutes of continuous perfusion, at least 8 stable urination waveforms for each animal were observed and recorded. The maximum bladder pressure (MBP) and inter contractile interval (ICI) were measured and compared between different groups.
Nociceptive Behavior Observation
Assessment of bladder nociceptive behavior was performed as described previously,20 with slight modification. Rats were adapted to the experimental environment 7 days after receiving TNBS or vehicle treatment, and they were placed in a clear plastic box for at least 2 hours before testing. Thereafter, rats were restrained on the rat fixator and urethral catheterization was performed. After confirming that the bladder is empty, resiniferatoxin (RTX, 0.3μM, 0.3mL, dissolved in 80% physiological saline, 10% ethanol, and 10% Tween 80, Cfwlabs, USA) was then instilled into the bladder through the PE-10 tube. The transurethral catheter remained in place for 1 min before removal. Following this, the rats were placed back in the plastic box and their behavior was recorded. During this time, an observer counted the number of harmful behaviors. The number of licking behaviors of licking the lower abdomen and freezing behavior of turning the head to the abdomen was recorded. According to 5 seconds as a time unit, the number of time units in which the above behavior occurs in 15 consecutive minutes was recorded separately.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0. Results were expressed as median (min-max). Pairwise comparisons were performed using a non-parametric Mann–Whitney test. A Kruskal–Wallis test was used in comparisons between four groups and Dunn's post hoc test was used following the Kruskal–Wallis test. P < 0.05 was indicated statistically significant (NS: not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
Results
Histopathological Features of the Colon and Bladder
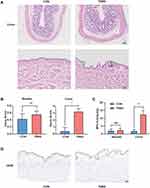
Compared with the CON group, rats after one week of TNBS administration had hypomotility, debilitation and their body weight decreased. The colon of the TNBS group presented with ulceration, mucosal erosion, interstitial congestion, and focal infiltration of inflammatory cells. However, the colon tissues of the control group showed complete epithelial surfaces. The structure of bladder tissues in both groups was intact. The overall structure of the bladder tissue in both groups appeared normal, and the cells were tight and tidy without inflammatory cell infiltration (Figure 1A). TNBS significantly increased the destructive inflammation and tissue damage of the colon but not the bladder as measured by histological score and tissue myeloperoxidase (MPO) levels (Figure 1B and C). The umbrella cell layer is the cell layer in the urothelium that is in direct contact with urine.21 We labelled the umbrella cell with cytokeratin 20 (CK20) by immunohistochemistry and found that the umbrella cell layer in the TNBS group was continuous and complete, which was comparable to the CON group (Figure 1D).
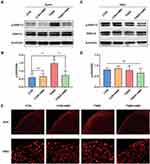
Colon Inflammation Increases CXCR4 Expression in the Distal Colon and Bladder
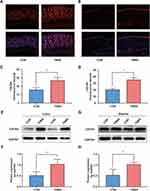
Immunofluorescence and Western blotting techniques were used to examine the differences in the expression of CXCR4 in the distal colon and bladder under normal and colitis conditions. By immunofluorescence in colon sections from CON and TNBS rats, we observed enhanced immunoreactivity of CXCR4 in the mucous layer from TNBS compared to CON rats (Figure 2A and C). Mucosal CXCR4 immunoreactivity was also increased in the bladder after colonic inflammation (Figure 2B and D). The Western blot results indicate that colonic inflammation upregulated CXCR4 expression in the colon and bladder by 1.90-fold and 1.89-fold, respectively (Figure 2E–H).
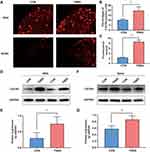
TNBS Induces CXCR4 Upregulation in the Dorsal Root Ganglion and Spinal Cord
Due to the neural crosstalk at the level of DRG and spinal cord is the pathophysiological basis of colon‐to‐bladder crosstalk, we investigated whether experimental colitis upregulates the expression of CXCR4 in the common afferent pathway of the bladder and colon. Immunofluorescence results showed that DRG sections from TNBS treated rats had enhanced immunoreactivity of CXCR4, and a higher number of CXCR4 positive neurons as compared to CON rats (Figure 3A and B). In the SDH of the CON group, there was a low-level expression of CXCR4, which was very prominent by the 7th day after TNBS induction (Figure 3A and C). To further support this conclusion, Western blot analysis confirmed that experimental colitis upregulated the expression of CXCR4 in the lumbosacral spinal cord and corresponding ganglia (Figure 3D–G).
Intrathecal Injection of AMD3100 Improves Colon Inflammation-Induced Bladder Overactivity
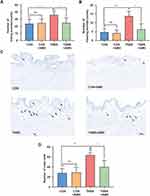
Owing to the expression of CXCR4 was increased in the primary afferent pathway following inflammation of the colon, we hypothesized that it may be related to colon-to-bladder neural crosstalk. We detected the effect of CXCR4 antagonist on bladder overactivity and hyperalgesia induced by TNBS. Cystometry was performed on conscious rats after colitis induction for 7 days. As shown in Figure 4A, the urodynamic curves from the four separate groups were recorded. Similar to previous reports,17 the TNBS group exhibited marked symptoms of bladder overactivity when compared with the CON group.
The ICI in the TNBS group was substantially shorter than that in the CON and CON+AMD groups. AMD3100 treatment significantly shortened the ICI of colitis rats (Figure 4B). Furthermore, AMD3100 can weakly decrease MBP, but differences in MBP between these four groups were less evident (Figure 4C).
AMD3100 Reversed Colon Inflammation-Induced Bladder Hyperalgesia and Mastocytosis
Two kinds of nociceptive behaviors caused by RTX in the bladder, freezing, and licking events were counted to evaluate bladder nociception (Figure 5A and B). In comparison to the CON group, the TNBS group had more freezing and licking events. There were no obvious differences in RTX-induced nociceptive behaviors between the CON and CON+AMD groups. However, intrathecal injection of AMD3100 treatment improved bladder hyperalgesia in rats induced by colon inflammation, with fewer licking and freezing events than the TNBS group. Because the activation of bladder mast cells after colitis promotes abnormal bladder function,22 we also assessed the effects of AMD3100 therapy on the number of bladder mast cells (Figure 5C). Toluidine blue staining indicates that colon inflammation caused bladder mast cells to increase by 2–3 times, which was reversed by blocking CXCR4 (Figure 5D).
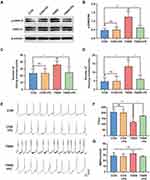
Inhibition of TNBS-Mediated Spinal Cord ERK Activation by AMD3100
CXCR4 activation stimulated by ligands initiates multiple signal pathways, including the ERK pathway.23 To further investigate the possible molecular mechanisms of the anti-bladder-colon cross-organ sensitization effect of CXCR4, we used Western blot to check the protein level of ERK1/2 and p-ERK1/2 in the spinal cord with or without AMD3100 treatment. The phosphorylation of spinal cord ERK1/2 in the TNBS group was 3 times that of the CON group, and this change was attenuated by AMD3100 (Figure 6A and B). However, differences in p-ERK1/2 of DRG among these groups were less evident (Figure 6C and D). To examine this further, the protein expressions of p-ERK1/2 were determined using Immunofluorescence. Seven days after colon inflammation, a significant increase in p-ERK1/2 immunostaining was observed in the dorsal horn. The intraperitoneal administration of AMD3100 inhibited the expression of p-ERK1/2 in SDH. We also found that the number of CXCR4-positive neurons in DRG showed no difference between these groups (Figure 6E), which was consistent with the Western blot data.
Attenuation of Colitis-Induced Bladder Overactivity and Hyperalgesia by Intrathecal Injection of PD98059
To further investigate whether spinal p-ERK1/2 up-regulation leads to bladder overactivity and hyperalgesia, we directly intrathecally injected PD98059, an ERK inhibitor, 7 consecutive days after the injection of TNBS. Consistent with the expected results, treatment with PD98059 resulted in downregulation of spinal p-ERK1/2 in rats with colitis, but not normal rats (Figure 7A and B). We then determined the effect of PD98059 on bladder hyperalgesia. The Injection of PD98059 significantly reduced the number of two nociceptive responses in TNBS rats. However, PD98059 has minimal impact on the nociceptive behavior of healthy rats (Figure 7C and D). In addition, the TNBS+PD group showed a consistently longer mean ICI compared with the TNBS groups. MBP tended to decrease after PD98059 treatment, although the effect was not significant (Figure 7E–G).
Discussion
In this study, intracolonic administration of TNBS increased the expression of CXCR4 in the colon and bladder and their common afferent pathways. Intrathecal injection of AMD3100 reversed bladder overactivity and hyperalgesia during colonic inflammation. Further, AMD3100 significantly reduced p-ERK1/2 in the SDH but not in the DRG, and treatment with PD98059 alleviated overactive bladder and hyperalgesia caused by colitis. These results suggest that upregulation of CXCR4 in the lumbosacral spinal cord and corresponding DRG promotes bladder dysfunction induced by colitis, and that CXCR4 was partially involved in colon-bladder cross-talk via the activation of spinal cord ERK signals.
There is a bidirectional relationship between IC/BPS and gut diseases, one of which leads to a high risk of another. Central sensitization is considered to be the pathophysiological basis of bladder hypersensitivity after colitis.7 Under normal conditions, the bladder is mainly dominated by Aδ fibers that are responsible for sensing the pressure and volume of the bladder. Unmyelinated C fibers do not participate in normal bladder function and are only activated by high-intensity noxious and chemical stimuli.24 When the colon is damaged or inflamed, injury stimulation leads to repeated activation of C fibers, which induces changes in the excitability and plasticity of neurons in the common afferent nerve pathway of the spinal cord and colon. The excitability threshold of these hypersensitive neurons is reduced, leading to subliminal stimulation under normal conditions that can also activate Aδ and C fibers, and further cause bladder overactivity and hyperalgesia.7
The massive activation of spinal microglia induced by colitis exacerbates central sensitization and leads to overactive bladder and hyperalgesia.10 At the level of the peripheral nervous system, colitis leads to increased expression of brain-derived neurotrophic factors, TRPV1, substance P, and other noxious substances and receptors in DRG neurons, and these noxious substances and receptors promote the occurrence of peripheral sensitization and cause bladder functional abnormalities.17,25,26 These results indicate that central and peripheral sensitization is related to overactive bladder and hyperalgesia induced by colitis.
CXCR4 participates in inflammation of the bladder and colon, and inhibitors of CXCR4 can improve organ function and reduce inflammation.15,27–29 Inflammatory bowel disease leads to higher expression of CXCR4 in the colon, as observed in human patients,27,30 animal models,31 and in the present study. Several studies have revealed that CXCR4 affects intestinal physiology by regulating the intestinal epithelial barrier and the release of inflammatory factors.28,29 Furthermore, CXCR4 expression rose significantly in the bladder mucosa of rats with chemical cystitis.15 The use of CXCR4 inhibitors can improve voiding dysfunction caused by bladder inflammation and obesity.15,32
CXCR4 is an important modulator of nociception and pain sensitivity beyond its involvement in inflammation. Studies on rodents have shown that CXCR4 is expressed in DRG and spinal cord.12 CXCL12 can induce the firing frequency of nociceptive neurons in DRG of normal mice,33 and blocking CXCR4 with drugs can reverse the excessive excitement of nociceptive neurons.34 Peripheral stimulation can also activate CXCR4 in the spinal cord, and further reduce the perception threshold of noxious stimuli.23 Genetic deletion of CXCR4 in rats supports its role in central and peripheral sensitization. Previous studies have revealed that the knockout of CXCR4 prevents mechanical allodynia induced by painful diabetic neuropathy.33 In our research, CXCR4 in L6-S1DRG and corresponding SDH increased significantly following 7 days of colitis. We also observed that preferential expression of CXCR4 in nociceptive small or medium DRG neurons and SDH under colitis conditions, consistent with that of CXCR4 in neuropathic and inflammatory pain.35 Intrathecal administration of AMD3100 significantly ameliorated TNBS colitis-induced bladder overactivity and bladder hyperalgesia but did not yield the same result in untreated rats. Therefore, the abnormal excitability of bladder afferent signals during colitis may be caused by CXCR4-mediated peripheral and central sensitization. Pharmacological intervention on CXCR4 is effective in reversing colon-bladder cross-organ sensitization and normalizing bladder function.
Noxious stimulation from the colon causes the bladder sensory neurons to be cross-activated and induces the release of neuropeptides from the axons of these neurons.25 Neuropeptides stimulate mast cells, causing them to release harmful and pro-inflammatory substances such as neurotrophic factors, inflammatory factors, histamine, and serotonin,36 which stimulate hypersensitivity of the bladder afferent nerves adjacent to the mast cells.22,37,38 As a key participant in central sensitization, spinal cord microglia has been reported to activate during the above process and promote the occurrence of overactive bladder and mastocytosis.10 There is direct evidence that CXCR4 is expressed in microglia and blockade of CXCR4 can inhibit the activation of spinal cord microglia in painful conditions.39,40 In this study, AMD3100 treatment reduced the number of mast cells in the bladder. Thus, bladder hypersensitivity caused by colitis is partly attributable to the increase in bladder mast cells caused by the upregulation of CXCR4 in the bladder afferent pathway.
Many previous studies have shown that the permeability of the bladder epithelium increases significantly after colon inflammation.41,42 Mast cell activation has been proved to be a key factor, and mast cell stabilizers can reverse the increase in epithelial permeability.22 The permeability change of the urothelium is an important cause of bladder pain and urination symptoms.43 The predisposing factors for increased epithelial permeability are complex, such as the damage of the epithelial barrier and the increase of paraepithelial transport.44 Interestingly, we found that the expression of CK20 in the TNBS group and the CON group was similar, which indicates that the integrity of the bladder epithelial umbrella cell layer did not change after colitis. The study by Towner et al also proved this.45 These results indicate that the neuropeptides released by mast cells are involved in regulating the permeability of the bladder epithelium through a neuromodulation mechanism,46 rather than directly destroying the epithelial barrier like acrolein and other toxins. In addition, neuropeptide-induced enhanced expression of macrophage migration inhibitory factor, a non-homologous ligand of CXCR4,47 might explain the enhanced expression of CXCR4 in the bladder epithelium.48
The spinal cord serves as the end of a shared pathway that accepts afferents from the colon and bladder. The central terminals of colon and bladder converge and entangle in SDH.9 Previous studies have shown that ERK activation in the SDH leads to hyperfunction of the bladder in cyclophosphamide cystitis and spinal cord injury models49,50 and bladder visceral hyperalgesia after cystitis.51 In our current study, TNBS was shown to induce activation of ERK1/2 in L6-S1 SDH. In pain models that include plantar incisions and chronic ischemia-induced pain, activation of ERK1/2 has been shown to be a critical downstream pathway for CXCR4 to central sensitization.23 In this study, selective CXCR4 blockade inhibited SDH ERK1/2 activation in response to TNBS. This indicates that the activation of CXCR4 in the colitis model promotes the phosphorylation of ERK in SDH. We found that overactive bladder and hyperalgesia after colitis were inhibited by chronic intrathecal injection of PD98059. These results indicate that the activation of CXCR4 in the spinal cord after colitis caused an increase in ERK phosphorylation, thereby inducing high excitability in the bladder afferent pathway in the spinal cord. However, the expression of p-ERK1/2 in DRG was not affected by chronic colitis and AMD3100 treatment. The same results were previously found in rat models of cystitis and colitis.52,53 The ERK in DRG may be activated in the early stage of colon inflammation and return to normal after the activation of ERK in the spinal cord. That is, harmful stimuli induce peripheral sensitization and then central sensitization.
Conclusion
In summary, current research demonstrates that upregulation of CXCR4 contributes to colon-bladder cross-organ sensitization. Moreover, blocking CXCR4 with AMD3100 attenuated the bladder overactivity and hyperalgesia induced by colitis via lowering the activation of spinal ERK. Therefore, our findings help elucidate the pathophysiology of cross-organ sensitization and visceral hypersensitivity induced by colitis in rats, and the CXCR4/ERK signal may be a potential therapeutic target for comorbidity between digestive and genitourinary tracts.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number: 81974101) and grant from Clinical Research Talent Training Plan of Army Medical University (2018XLC3033).
Disclosure
The authors declare that they have no conflicts of interest regarding this work nor the publication of this paper.
References
1. Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553. doi:10.1016/j.juro.2015.01.086
2. Grundy L, Brierley SM. Cross-organ sensitization between the colon and bladder: to pee or not to pee? Am J Physiol Gastrointest Liver Physiol. 2018;314(3):G301–G308. doi:10.1152/ajpgi.00272.2017
3. Kaplan SA, Dmochowski R, Cash BD, Kopp ZS, Berriman SJ, Khullar V. Systematic review of the relationship between bladder and bowel function: implications for patient management. Int J Clin Pract. 2013;67(3):205–216. doi:10.1111/ijcp.12028
4. Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49(5ASuppl):52–57. doi:10.1016/S0090-4295(99)80332-X
5. Chang KM, Lee MH, Lin HH, Wu SL, Wu HC. Does irritable bowel syndrome increase the risk of interstitial cystitis/bladder pain syndrome? A cohort study of long term follow-up. Int Urogynecol J. 2021;32(5):1307–1312. doi:10.1007/s00192-021-04711-3
6. Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci. 2010;153(1–2):106–115. doi:10.1016/j.autneu.2009.07.006
7. Reynolds WS, Dmochowski R, Wein A, Bruehl S. Does central sensitization help explain idiopathic overactive bladder? Nat Rev Urol. 2016;13(8):481–491. doi:10.1038/nrurol.2016.95
8. Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290(6):F1478–1487. doi:10.1152/ajprenal.00395.2005
9. Grundy L, Harrington AM, Castro J, et al. Chronic linaclotide treatment reduces colitis-induced neuroplasticity and reverses persistent bladder dysfunction. JCI Insight. 2018;3(19). doi:10.1172/jci.insight.121841
10. Majima T, Funahashi Y, Kawamorita N, et al. Role of microglia in the spinal cord in colon-to-bladder neural crosstalk in a rat model of colitis. Neurourol Urodyn. 2018;37(4):1320–1328. doi:10.1002/nau.23484
11. Luo X, Wang X, Xia Z, Chung SK, Cheung CW. CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neurosci. 2016;27(1):83–92. doi:10.1515/revneuro-2015-0016
12. Reaux-le Goazigo A, Rivat C, Kitabgi P, Pohl M, Melik Parsadaniantz S. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci. 2012;36(5):2619–2631. doi:10.1111/j.1460-9568.2012.08179.x
13. Yang F, Sun W, Luo WJ, et al. SDF1-CXCR4 signaling contributes to the transition from acute to chronic pain state. Mol Neurobiol. 2017;54(4):2763–2775. doi:10.1007/s12035-016-9875-5
14. Kouzoukas DE, Meyer-Siegler KL, Ma F, Westlund KN, Hunt DE, Vera PL. Macrophage migration inhibitory factor mediates PAR-induced bladder pain. PLoS One. 2015;10(5):e0127628. doi:10.1371/journal.pone.0127628
15. Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298(3):F589–600. doi:10.1152/ajprenal.00628.2009
16. Foster R, Jung J, Farooq A, et al. Sciatic nerve injury induces functional pro-nociceptive chemokine receptors in bladder-associated primary afferent neurons in the rat. Neuroscience. 2011;183:230–237. doi:10.1016/j.neuroscience.2011.03.035
17. Xia CM, Gulick MA, Yu SJ, et al. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation. 2012;9:30. doi:10.1186/1742-2094-9-30
18. Yang Y, Zhang H, Lu Q, et al. Suppression of adenosine A2a receptors alleviates bladder overactivity and hyperalgesia in cyclophosphamide-induced cystitis by inhibiting TRPV1. Biochem Pharmacol. 2021;183:114340. doi:10.1016/j.bcp.2020.114340
19. Luo J, Yang C, Luo X, et al. Chlorogenic acid attenuates cyclophosphamide-induced rat interstitial cystitis. Life Sci. 2020;254:117590. doi:10.1016/j.lfs.2020.117590
20. Kawamorita N, Yoshikawa S, Kashyap M, et al. Liposome based intravesical therapy targeting nerve growth factor ameliorates bladder hypersensitivity in rats with experimental colitis. J Urol. 2016;195(6):1920–1926. doi:10.1016/j.juro.2015.12.090
21. Romih R, Korosec P, de Mello W
22. Fitzgerald JJ, Ustinova E, Koronowski KB, de Groat WC, Pezzone MA. Evidence for the role of mast cells in colon-bladder cross organ sensitization. Auton Neurosci. 2013;173(1–2):6–13. doi:10.1016/j.autneu.2012.09.002
23. Hu Q, Zheng X, Li X, et al. Electroacupuncture alleviates mechanical allodynia in a rat model of complex regional pain syndrome Type-I via suppressing spinal CXCL12/CXCR4 signaling. J Pain. 2020;21(9–10):1060–1074. doi:10.1016/j.jpain.2020.01.007
24. Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29(1):128–139. doi:10.1002/nau.20837
25. Pan XQ, Gonzalez JA, Chang S, Chacko S, Wein AJ, Malykhina AP. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol. 2010;225(2):262–273. doi:10.1016/j.expneurol.2010.05.012
26. Lei Q, Malykhina AP. Colonic inflammation up-regulates voltage-gated sodium channels in bladder sensory neurons via activation of peripheral transient potential vanilloid 1 receptors. Neurogastroenterol Motil. 2012;24(6):575–585, e257. doi:10.1111/j.1365-2982.2012.01910.x
27. Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 interactions in inflammatory bowel disease. Theranostics. 2013;3(1):40–46. doi:10.7150/thno.5135
28. Mikami S, Nakase H, Yamamoto S, et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327(2):383–392. doi:10.1124/jpet.108.141085
29. Xia XM, Wang FY, Zhou J, Hu KF, Li SW, Zou BB. CXCR4 antagonist AMD3100 modulates claudin expression and intestinal barrier function in experimental colitis. PLoS One. 2011;6(11):e27282. doi:10.1371/journal.pone.0027282
30. Dotan I, Werner L, Vigodman S, et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16(4):583–592. doi:10.1002/ibd.21106
31. Lin X, Wang H, Li Y, et al. Functional characterization of CXCR4 in mediating the expression of protein C system in experimental ulcerative colitis. Am J Transl Res. 2017;9(11):4821–4835.
32. Macoska JA, Wang Z, Virta J, Zacharias N, Bjorling DE. Inhibition of the CXCL12/CXCR4 axis prevents periurethral collagen accumulation and lower urinary tract dysfunction in vivo. Prostate. 2019;79(7):757–767. doi:10.1002/pros.23781
33. Jayaraj ND, Bhattacharyya BJ, Belmadani AA, et al. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest. 2018;128(6):2205–2225. doi:10.1172/JCI92117
34. Yang F, Sun W, Yang Y, et al. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation. 2015;12:219. doi:10.1186/s12974-015-0441-2
35. Bai L, Wang X, Li Z, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull. 2016;32(1):27–40. doi:10.1007/s12264-015-0007-4
36. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398–410. doi:10.1111/j.1365-2567.2007.02705.x
37. Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol Renal Physiol. 2007;292(1):F123–130. doi:10.1152/ajprenal.00162.2006
38. Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29(1):77–81. doi:10.1002/nau.20817
39. Liu ZY, Song ZW, Guo SW, et al. CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model. CNS Neurosci Ther. 2019;25(9):922–936. doi:10.1111/cns.13128
40. Luo X, Tai WL, Sun L, et al. Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain. 2016;12. doi:10.1177/1744806916636385
41. Greenwood-van Meerveld B, Mohammadi E, Tyler K, et al. Mechanisms of visceral organ crosstalk: importance of alterations in permeability in rodent models. J Urol. 2015;194(3):804–811. doi:10.1016/j.juro.2015.02.2944
42. Towner RA, Smith N, Saunders D, et al. Assessment of colon and bladder crosstalk in an experimental colitis model using contrast-enhanced magnetic resonance imaging. Neurogastroenterol Motil. 2015;27(11):1571–1579. doi:10.1111/nmo.12654
43. Hurst RE, Greenwood-van Meerveld B, Wisniewski AB, et al. Increased bladder permeability in interstitial cystitis/painful bladder syndrome. Transl Androl Urol. 2015;4(5):563–571. doi:10.3978/j.issn.2223-4683.2015.10.03
44. Montalbetti N, Rued AC, Clayton DR, et al. Increased urothelial paracellular transport promotes cystitis. Am J Physiol Renal Physiol. 2015;309(12):F1070–1081. doi:10.1152/ajprenal.00200.2015
45. Towner RA, Smith N, Saunders D, et al. Contrast enhanced magnetic resonance imaging as a diagnostic tool to assess bladder permeability and associated colon cross talk: preclinical studies in a rat model. J Urol. 2015;193(4):1394–1400. doi:10.1016/j.juro.2014.10.120
46. Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69(4 Suppl):34–40. doi:10.1016/j.urology.2006.08.1109
47. Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS One. 2008;3(12):e3898. doi:10.1371/journal.pone.0003898
48. Meyer-Siegler KL, Vera PL. Substance P induced release of macrophage migration inhibitory factor from rat bladder epithelium. J Urol. 2004;171(4):1698–1703. doi:10.1097/01.ju.0000115883.49365.1a
49. Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci. 2005;21(3):773–781. doi:10.1111/j.1460-9568.2005.03893.x
50. Cruz CD, McMahon SB, Cruz F. Spinal ERK activation contributes to the regulation of bladder function in spinal cord injured rats. Exp Neurol. 2006;200(1):66–73. doi:10.1016/j.expneurol.2006.01.016
51. Lai HH, Qiu CS, Crock LW, Morales MEP, Ness TJ, Gereau R. Activation of spinal extracellular signal-regulated kinases (ERK) 1/2 is associated with the development of visceral hyperalgesia of the bladder. Pain. 2011;152(9):2117–2124. doi:10.1016/j.pain.2011.05.017
52. Qiao LY, Gulick MA. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1368–1375. doi:10.1152/ajpregu.00570.2006
53. Yu SJ, Grider JR, Gulick MA, Xia CM, Shen S, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Exp Neurol. 2012;238(2):209–217. doi:10.1016/j.expneurol.2012.08.007
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.