Back to Journals » OncoTargets and Therapy » Volume 13
Inhibition of CSRP2 Promotes Leukemia Cell Proliferation and Correlates with Relapse in Adults with Acute Myeloid Leukemia
Authors Wang S, Zhang Y, Liu Y, Zheng R , Wu Z, Fan Y, Li M, Li M, Li T, Li Y, Jiang Z, Wang C, Liu Y
Received 14 September 2020
Accepted for publication 25 November 2020
Published 7 December 2020 Volume 2020:13 Pages 12549—12560
DOI https://doi.org/10.2147/OTT.S281802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Shujuan Wang,1,* Yu Zhang,1,* Yajun Liu,2 Ruyue Zheng,1 Zhenzhen Wu,1 Yi Fan,1 Mengya Li,1 Menglin Li,1 Tao Li,1 Yafei Li,1 Zhongxing Jiang,1 Chong Wang,1 Yanfang Liu1
1Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Orthopaedics, Brown University, Warren Alpert Medical School/Rhode Island Hospital, Rhode Island, RI, USA
*These authors contributed equally to this work
Correspondence: Yanfang Liu; Chong Wang
Department of Hematology, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, Erqi District, Zhengzhou 450052, People’s Republic of China
Email [email protected]; [email protected]
Background: Relapse is a major obstacle in the treatment of acute myeloid leukemia (AML). Refinement of risk stratification may aid the identification of patients who are likely to relapse. Abnormal cysteine and glycine-rich protein 2 (CSRP2) has been implicated in various cancers, but its function remains unclear. The purpose of this study was to explore the role of CSRP2 in predicting adult AML recurrence.
Methods: RT-PCR was used to detect the expression of CSRP2 in 193 newly diagnosed adult AML patients and 44 healthy controls. The competitive risk model was used to calculate the cumulative incidence of relapse rate (CIR), Kaplan–Meier to calculate the relapse-free survival rate (RFS), and the Cox regression model to perform multivariate analysis. Viral transfection was used to construct AML cell lines with stable knockdown of CSRP2, CCK8 to detect proliferation and drug resistance, flow cytometry to detect cell cycle and apoptosis, and Western blot to detect key molecules in signaling pathways.
Results: CSRP2 transcript levels were higher in 193 adult AML compared with 44 healthy controls. In 149 patients who achieved complete remission, those with high CSRP2 transcript levels displayed a lower 2-year CIR and higher 2-year RFS, especially when receiving only chemotherapy. In multivariate analysis, a high CSRP2 transcript level was independently associated with a better RFS. Knockdown of CSRP2 promoted proliferation and cell cycle progression, and reduced chemosensitivity. Western blot analysis showed upregulation of p-AKT and p-CREB in CSRP2-knockdown AML cell lines. Inhibition assays suggested these two signaling pathways participated in the CSRP2-mediated proliferation effects in AML cell lines.
Conclusion: In summary, CSRP2 correlates with relapse in adult AML. Down-regulation of CSRP2 could promote the proliferation of AML cell lines by regulating the AKT and CREB signaling pathways. Therefore, CSRP2 may provide prognostic significance and potential therapeutic targets in the management of AML.
Keywords: acute myeloid leukemia, relapse, cysteine and glycine-rich protein 2, proliferation, cAMP-regulatory element-binding protein
Introduction
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults.1 Although the majority of patients with AML enter complete remission after induction chemotherapy, the recurrence rate is still high, which is the main factor affecting the long-term survival of patients.2 Transplantation may be an optional treatment, but it is challenging to identify high-risk patients suitable for early transplantation. Current risk stratification classifies patients according to age, genetic mutations, and chromosomal changes. It is worth noting that many relapses occur in patients who present favorable characteristics at the time of initial diagnosis.2,3 The new prognostic biomarkers may further improve the current risk stratification of adult AML, therefore optimizing treatment. There is an urgent need to improve the ability to identify patients with a high risk of recurrence, especially when they are currently stratified as patients with favorable risk. For these patients, intensive treatment may improve their prognosis.
Cysteine and glycine-rich protein 2 (CSRP2), which encodes the LIM domain protein CSRP2, participates in the regulatory process of organ development and cell differentiation.4 Studies show that CSRP2 is related to the dedifferentiation of hepatocellular carcinoma.5 As one of the components of the pseudopod actin bundle in breast cancer cells, it promotes the invasion and metastasis of breast cancer cells.6,7 Moreover, CSRP2 is a downstream target of miR-27a. Down-regulation of CSRP2 can promote the proliferation and movement of gastric cancer cells.8 Our previous study suggests that CSRP2 is highly expressed in the blasts from patients with adult B cell acute lymphoblastic leukemia (B-ALL); and high expression of CSRP2 is an independent adverse prognostic factor for normal karyotype adult B-ALL.9 To date, there is no study on the function and the prognostic relevance of CSRP2 in adult AML patients. Here, we examined the levels of CSRP2 transcripts for an association with relapse probability in adults with AML. We found that high CSRP2 levels correlated with lower cumulative incidence of relapse and better relapse-free survival in adults with AML, especially for those who only received chemotherapy.
Materials and Methods
Subjects
Bone marrow samples were obtained from adults with AML (N=193) and normal individuals (N=44) recruited at the Department of Hematology of the First Affiliated Hospital of Zhengzhou University between February 2017 and June 2019. Complete clinical and laboratory data were available for 193 subjects. A total of 149 subjects achieved complete remission and were included in the relapse analysis. Subjects were followed until death, loss to follow-up or June 2020. Induction chemotherapy regimens include IA and DA regimens: standard-dose cytarabine (Ara-C) 100–200 mg·m−2·d−1×7 d combined with idarubicin 10–12 mg·m−2·d−1×3d or daunorubicin (DNR) 60 mg·m−2·d−1×3d. The treatment plan after remission is high-dose Ara-C (3g/m,2 once every 12 h, 3d). Those who have not undergone hematopoietic stem cell transplantation (HSCT) share four courses; those who receive HSCT share two courses, followed by HSCT. In total, 41 subjects (28%) received an allogeneic HSCT. Complete remission (CR), relapse and risk-stratification were defined as previously described.10 Cumulative incidence of relapse (CIR) was determined from the date of first CR to the date of first relapse or death in complete remission. Relapse-free survival (RFS) was determined from the date of first CR to the date of first relapse. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, and informed consent was obtained according to the Declaration of Helsinki.
Cell Lines and Agents
Human leukemia cell line HL60 was purchased from GeneChem (Shanghai, China, authenticated by STR profiling) and was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% FBS, 1% penicillin and streptomycin (all from Gibco, Billings, MT, USA) at 37°C with 5% CO2 in a humidified incubator. Small-molecular inhibitor KG501 was purchased from Sigma-Aldrich.
Lentiviral Transduction
HL60 cells were transfected with human CSRP2 shRNA lentiviral particles or empty control lentiviral particles (GeneChem) at a multiplicity of infection (MOI) of 50. Medium containing lentiviral particles was replaced with complete medium 12 h post-infection. Stably transfected HL60 cells were selected with 2μM puromycin dihydrochloride (GeneChem) at 96 h post-infection. The efficiency of genetic modification for the inhibition of CSRP2 was confirmed by both RT-qPCR and Western blot analysis.
RNA Extraction, cDNA Preparation and RT-qPCR
Mononuclear cells were isolated from bone marrow samples by density gradient centrifugation. RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized as described.11 Gene transcript levels were determined according to the Taqman method.12 CSRP2 transcript levels were normalized to ABL1 expression as recommended by the Europe Against Cancer group.13 Copy numbers of CSRP2 and ABL1 were calculated from standard curves using Ct values. Serial dilutions of plasmids expressing ABL1 and CSRP2 were amplified to construct standard quantification curves. Samples were assayed in duplicate to evaluate data reproducibility and average threshold Ct values calculated for expression analyses. Primers and probe sequences are as previously described.9,12
Cell Proliferation and Viability Assay
Cell proliferation was analyzed by the Cell Counting Kit-8 (CCK8, Dojin Laboratories, Kumamoto, Japan) assay. A total of 1×104 cells in 100μL were seeded into each well of 96-well plates. 0, 24, 48 or 72 hours later 10 μL of the kit reagent was added to each well and 3 h later all plates were scanned by a microplate reader at 450 nm. The CCK8 test was also used to evaluate cell viability after drug exposure. Briefly, the cells were seeded at a density of 1×105 cells/mL, and daunorubicin (Solarbio) was added at concentrations of 62.5nM. After 24 h, 10 μL of the kit reagent was added to each well and 3 h later all plates were scanned according to the manufacturer’s instructions. All experiments were performed 3 times independently.
Analysis of Cell Cycle and Apoptosis
The cell cycle analysis was performed using a Cell Cycle Staining Kit (Beyotime Biotechnology, Shanghai, China), and cell apoptosis was assessed using an Annexin V/propidium iodide (PI) apoptosis kit (Beyotime Biotechnology). The cells were starved in RPMI 1640 without FBS for 24 h for synchronization and then seeded at a density of 105 cells/mL in six-well plates with complete medium. After 48 h, the cell cycle and apoptosis were determined by flow cytometry according to the manufacturer’s instructions.
Western Blot Analyses
Western blotting was performed as described previously.14,15 The primary antibodies used were rabbit anti-CSRP2 (Sigma, 1:500), rabbit anti-p21 (Cell Signaling Technology [CST], Beverly, MA, USA; 1:1000), rabbit anti-p27 (CST, 1:1000), rabbit anti-CDK4 (CST, 1:1000), rabbit anti-p-CREB (CST, 1:1000), rabbit anti-p-ERK (CST, 1:1000), rabbit anti p-P38 (CST, 1:1000), rabbit anti-p-SMAD3 (CST, 1:1000), rabbit anti p-AKT (CST, 1:1000), rabbit anti p53 (CST, 1:1000) and mouse anti-GAPDH (Solarbio, Beijing, China, 1:1000). The secondary antibody was goat anti-mouse IgG (Solarbio, 1:1000) and goat anti-rabbit IgG (Solarbio, 1:1000). The protein bands were determined using the ultra ECL kit (Biomed, Beijing, China) according to the manufacturer’s protocol.
Statistical Analyses
Clinical characteristics were compared between subjects with low and high transcript levels of CSRP2 using Pearson’s Chi-square analysis or Fisher exact test for categorical variables; Student’s t-test or Mann–Whitney U-test was used for continuous variables. Receiver operating characteristic (ROC) curves were constructed to evaluate the sensitivity and specificity of CSRP2 transcript levels to estimate RFS. Youden Index was used to calculate the optimal cutoff for high and low transcript levels. Survival functions were estimated by the Kaplan–Meier method and compared by the Log rank test. Cumulative incidences were estimated for relapse to accommodate competing risks. A Cox proportional hazard regression model was used to determine associations between CSRP2 transcript levels and RFS. Variables with P<0.1 in univariable analyses were entered into the multivariable analyses. P<0.05 was considered significant. Analyses were performed by SPSS software version 20.0 (Chicago, IL, USA), Graphpad Prism™ 5.01 (San Diego, California, USA) and R software package (version 3.5.0; http://www.r-project.org).
Results
CSRP2 is Highly Expressed in Bone Marrow Blasts from Subjects with Acute Myeloid Leukemia
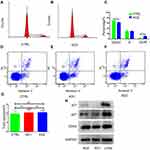
To explore the role of CSRP2 in AML, we first examined CSRP2 levels in AML bone marrow blasts. Transcript levels of CSRP2 were higher in the AML subjects compared with healthy donors (median 3.76%, range [0–280.88%] vs 0.44% [0–1.78%]; P<0.0001; Figure 1A). According to French-America-British (FAB) classification of AML, subjects with FAB M0 and M2 subtypes had relatively higher transcript levels of CSRP2 compared with FAB M1, M4 and M5 subtypes (Figure 1B). Subjects with different cytogenetic risk stratifications also have different transcript levels of CSRP2. Low-risk subjects have significantly higher transcript levels of CSRP2 than high-risk subjects (20.09% [0.09%-280.88%] vs 2.15% [0.08%-177.86%]; P=0.005; Figure 1C).
CSRP2≥8.26% and<8.26% were defined as high transcript levels and low transcript levels, respectively. In the entire cohort, 39.4% (n = 76) patients had high CSRP2 transcript levels. Subjects with low CSRP2 transcript levels showed higher white blood cell (WBC) levels, higher platelets (PLT) levels and higher risk groups compared to subjects with high CSRP2 transcript levels (Table 1). Mutations in gene FLT3-ITD and NPM1 and CBFβ-MYH11 fusion gene were more frequently found in subjects with low CSRP2 transcript levels. The biallelic mutation in gene CEBPA and AML1-ETO fusion gene were more frequently found in subjects with high CSRP2 transcript levels. There were no significant differences between CSRP2 transcript levels and other clinical features such as gender, age, hemoglobin level, CR rate and transplant (Table 1).
 |
Table 1 Association of CSRP2 Transcript Levels with the Clinical Characteristics of Adults with AML |
CSRP2 Expression Levels are Independently Associated with Relapse in Subjects with AML
To assess the clinical significance of CSRP2 in AML, we then analyzed the relation of CSRP2 levels in AML samples and the survival of these patients. Among the 193 subjects, 149 patients achieved CR and were used for CIR and RFS analysis. The median follow-up of these 149 patients was 460 days (range 73–1104 days). Among subjects with high and low CSRP2 transcript levels, the CR rates after one cycle of induction therapy did not differ significantly (77.6% vs 76.9%, P=0.909). The Kaplan–Meier analysis indicated that the cohorts (n = 90 patients) with low transcript levels of CSRP2 displayed a relatively lower 2-year RFS and worse 2-year CIR than the cohorts (n = 59 patients) with high transcript levels of CSRP2 (RFS: 45% [30–60%] vs 75% [58–92%], P=0.004; CIR: 51% [37–65%] vs 22% [7–37%], P=0.002; Figure 2A and B). In multivariate analysis, a high transcript level of CSRP2, a lower risk group and allo-HSCT were independently associated with a better RFS (Table 2). Other factors, such as gender, age and WBC count at diagnosis, did not show significant correlations with RFS (Table 2).
 |
Table 2 Univariate and Multivariate Analysis of RFS in the Whole Cohort and Chemotherapy Only |
Because the prognosis of AML varied among individuals due to different post-remission therapies, we next analyzed the prognostic value of CSRP2 transcript levels in subjects receiving chemotherapy alone and those who underwent allo-HSCT. Our data indicated that in the 108 subjects who only received chemotherapy, low CSRP2 transcript levels were associated with a lower 2-year RFS and worse 2-year CIR than subjects with high CSRP2 transcript levels (RFS: 30% [12–48%] vs 71% [47–94%], P=0.001; CIR: 65% [47–83%] vs 25% [4–46%], P=0.001; Figure 2C and D). In multivariate analyses, low CSRP2 transcript levels were independently associated with a worse RFS (Table 2). Nevertheless, the above correlations were not statistically significant in 41 subjects receiving allo-HSCT post-CR (RFS: 77% [61–94%] vs 83% [62–100%], P=0.648; CIR: 22% [6–37%] vs 17% [0–39%], P=0.700; Figure 2E and F).
CSRP2 Knockdown Promotes AML Cell Proliferation and Drug Resistance
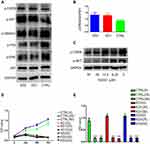
To explore the biological role of CSRP2 in AML, we constructed three stably transfected AML cell lines: the HL60-control cell line (CTRL), the HL60-knockdown cell line 1(KD1) and the HL60-knockdown cell line 2 (KD2). The knockdown of CSRP2 was verified by RT-qPCR and Western blot (Figure 3A and B). The CCK-8 test showed that cell proliferation was significantly increased in both KD1 cells and KD2 cells compared to that in cells transfected with control lentiviral particles (Figure 3C).
Drug resistance is the primary reason for treatment-failure and relapse in AML. So we studied the relationship between CSRP2 and its sensitivity to daunorubicin. CSRP2 KD2 cells showed significantly increased proliferation, indicating more daunorubicin resistance compared with that of the control cells (Figure 3D).
Knockdown of CSRP2 Promotes Cell Cycle Progression in AML Cells
To elucidate how CSRP2 exhibits its antiproliferative effect on AML cells, we analyzed the role of CSRP2 in cell cycle progression and apoptosis. Flow cytometry results indicated that compared to the control group, knockdown of CSRP2 significantly decreased cell counts in the G0/G1 phase and increased cell counts in the G2/M phase (Figure 4A-C). Apoptosis analysis showed that knockdown of CSRP2 had no significant effect on total apoptosis in HL60 cells (Figure 4D-G). To further explore the effect of CSRP2 knockdown during the cell cycle, we assessed cell cycle-related proteins p21 and p27 by Western blot and found that both p21 and p27 were decreased while CDK4 showed no change (Figure 4H).
Activation of p-AKT and p-CREB Pathways is Responsible for CSRP2-Mediated Proliferation in AML Cell Lines
To understand the mechanisms behind the increased proliferation from CSRP2 down-regulation, we examined the changes in phosphorylation of leukemia cell growth-related proteins in HL60 cells. Levels of p-SMAD3 were largely unaffected, and phosphorylation of ERK and P38 were downregulated in CSRP2-KD cells (Figure 5A). Proliferation-related molecules, p-AKT and p-CREB, were upregulated with CSRP2 knockdown (Figure 5A and B). The level of proapoptotic p53 was unaffected after CSRP2 knockdown (Figure 5A). To confirm the role of p-CREB in CSRP2-mediated effects, we used a p-CREB inhibitor KG-501 in CSPR2-KD1 cells. Both 25 µM and 50 µM KG-501 could significantly inhibit the expression of p-CREB and p-AKT (Figure 5C). Inhibition of p-CREB abolished the increased proliferation of CSRP2-KD1 and CSRP2-KD2 cells (Figure 5D and E). These data suggest that phosphorylation of CREB and AKT by down-regulated expression of CSRP2 is responsible for the changes in the growth of CSRP2-knockdown HL60 cell lines.
Discussion
In this study, we evaluated the role of CSRP2 in predicting relapse of adult AML patients and explored its mechanism. The expression of CSRP2 in adult AML is significantly higher than that of normal controls, while the low expression of CSRP2 is an independent adverse prognostic factor for relapse in adult AML patients. Knockdown of CSRP2 can promote AML cell line proliferation and cell cycle progression, and increase drug resistance. This effect may be carried out through the AKT and CREB signaling pathway.
It has been found that CSRP2 is abnormally expressed in several tumors, but its role remains controversial. For example, high expression of CSRP2 was significantly associated with the risk of metastasis in patients with breast cancer and poor prognosis in adult B-ALL patients with normal karyotype.6,9 However, CSRP2 downregulation increases the proliferation and motility of gastric cancer cells.8 In this study, we found that higher CSRP2 expression in AML was associated with relatively better RFS and lower CIR. The different role of CSRP2 in malignant diseases may partly contribute to the companying mutations of other genes. For example, CSRP2 high expression was seen in patients with KMT2A rearrangement,9 a poor prognosis BCP ALL subtype. In contrary, AML cases with low CSRP2 transcript levels are more frequent companied by mutations in gene FLT3-ITD, which may contribute to the adverse outcomes. The genetic alterations correlate with differential expression levels of CSRP2 and may explain the poor outcome related to low CSRP2. In addition, the prognosis of AML varies according to the treatment after remission, we further analyzed the prognostic significance of CSRP2 in subjects who received only chemotherapy and those who received allo-HSCT. It is worth noting that the above correlation between the level of CSRP2 transcription and RFS and CIR can still be detected in subjects receiving chemotherapy only, while the prognosis of patients receiving allo-HSCT was not affected by the expression level of CSRP2. Because the prognostic factors of AML are complicated, we performed a multivariate analysis to resolve this complexity. In particular, the low transcription level of CSRP2 at the time of diagnosis was an independent adverse risk factor for relapse in adults with AML, especially for patients who received chemotherapy alone. These data suggest that CSRP2 can be a potential target for predicting the recurrence of AML patients, especially for patients who only receive chemotherapy after CR.
CSRP2 participates in multiple processes both inside and outside of the cells through interactions with a diverse set of cellular targets.4 Hoffmann et al identified CSRP2 as a new cytoskeletal component of invadopodia that critically promoted breast cancer cell invasion and metastasis.6,16 Wang et al reported that CSRP2 was a downstream target of miR-27a in gastric cells by luciferase-reporter assay.8 They further investigated the role of CSRP2 in gastric cancer. They tested the proliferation and motility of HSF-1 cells by downregulation of CSRP2 expression. They found that the downregulation of CSRP2 increased the proliferation and motility of gastric cancer cells.8 Our previous study showed that CSRP2 promoted proliferation in B-ALL cell lines.9 However, the data in this study indicated that knockdown of CSRP2 promotes proliferation and cell cycle progression as well as drug resistance in AML cell lines. It might reflect various functions of CSRP2 in different types of cancers.
We also studied changes in several growth- and survival-related pathways in association with CSRP2 down-regulation in HL60 cells. The phosphoinositide 3-kinase (PI3K)-Akt pathway (PI3K-Akt pathway) is among one of the intracellular pathways aberrantly upregulated in cancers including AML.17 Activation of this pathway seems important in leukemogenesis. Given the central role of this pathway in metabolism, the bioenergetics of AML cells may depend on downstream signaling within this pathway.17 Our results showed an upregulation of AKT phosphorylation in CSRP2 knockdown leukemia cells, suggesting an involvement of p-AKT in pro-survival mechanisms mediated by CSRP2. Transcription factors are key regulators of the gene expression pattern and directly control central processes such as proliferation, survival and self-renewal.18 cAMP-regulatory element-binding protein (CREB) is a crucial transcriptional factor, which can be activated through phosphorylation by a number of kinases, including Akt.18 CREB is overexpressed and constitutively phosphorylated in a number of human cancer including AML, and appears to play a direct role in disease pathogenesis and prognosis.18 We found that both p-AKT and p-CREB levels were increased in CSRP2 knockdown leukemia cells, indicating that CSRP2 knockdown may increase p-CREB levels through upregulation of p-AKT. Several cell cycle-related genes are regulated by p-CREB and p-AKT.19–22 Knockdown of CREB induces cell cycle arrest and significantly reduces tumor cell proliferation.23–25 Our research found that cyclin-dependent kinase inhibitors p21 and p27 were reduced in CSRP2 knockdown cells. These results indicate that p-CREB and p-AKT may influence proliferation by regulating the expression of cell cycle-related genes. Interestingly, a CREB inhibitor KG501 reduced both p-CREB and p-AKT levels and ameliorated pro-proliferative effects caused by knockdown of CSRP2. These data suggested that CSRP2 could modulate the growth and survival of AML leukemia cells through the regulation of AKT and CREB pathways. In this regard, these abnormal alterations caused by CSRP2 may provide potential therapeutic drug targets in the future. We also observed decreases in the phosphorylation of p38 MAPK and p-ERK in CSRP2-KD leukemia cells. They may be downstream effectors modulated by CSRP2 in leukemia cells, which will require further investigation in the future. Maybe higher pAKT sustains higher proliferation without implying pERK in the pathway. The role of p38 MAPK is more difficult to interpret as it has pro-apoptotic and oncogenic functions depending on cellular context and stimuli.26 The role of p38 MAPK pathway in CSRP2-KD leukemia cells will require further investigation in the future.
There are several limitations to our study. First, it was retrospective and lack of an external validation cohort. Second, patients were not randomized to receive HSCT or chemotherapy due to practical and ethical reasons. Third, there is interaction between CSPR2 transcript levels and risk stratification, gene mutations and karyotypes. Considering these limitations, our conclusions require a randomized, prospective trial in a larger population group to provide more objective results.
In summary, our data indicated that low CSRP2 transcript levels were correlated with a relatively higher CIR and worse RFS in adults with AML, especially for those who received only chemotherapy. In addition, knockdown of CSRP2 promoted proliferation and cell cycle progression of AML cell lines through regulation of the AKT and CREB pathways. Taken together, although further confirmation is needed, our data suggest that CSRP2 may provide prognostic significance and potential therapeutic targets in the management of AML.
Data Sharing Statement
All raw data are accessible by requesting the corresponding author (Yanfang Liu).
Acknowledgments
We thank all the treating physicians for allowing us to enroll their patients and the patients for allowing us to analyze their data.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81800137 and U1804191] and Henan Medical Science and Technology Research Project (grant number 2018020068).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wouters BJ, Delwel R. Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood. 2016;127:42–52. doi:10.1182/blood-2015-07-604512
2. Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127:62–70. doi:10.1182/blood-2015-07-604546
3. Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission. an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–590. doi:10.1038/nrclinonc.2012.150
4. Weiskirchen R, Erdel M, Utermann G, Bister K. Cloning, structural analysis, and chromosomal localization of the human CSRP2 gene encoding the LIM domain protein CRP2. Genomics. 1997;44:83–93. doi:10.1006/geno.1997.4855
5. Midorikawa Y, Tsutsumi S, Taniguchi H, et al. Identification of genes associated with dedifferentiation of hepatocellular carcinoma with expression profiling analysis. Jpn J Cancer Res. 2002;93:636–643. doi:10.1111/j.1349-7006.2002.tb01301.x
6. Hoffmann C, Mao X, Dieterle M, et al. CRP2, a new invadopodia actin bundling factor critically promotes breast cancer cell invasion and metastasis. Oncotarget. 2016;7:13688–13705. doi:10.18632/oncotarget.7327
7. Hoffmann C, Mao X, Brown-Clay J, et al. Hypoxia promotes breast cancer cell invasion through HIF-1alpha-mediated up-regulation of the invadopodial actin bundling protein CSRP2. 2018;8:10191.
8. Wang J, Guan X, Zhang Y, et al. Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts. Cell Physiol Biochem. 2018;49:869–883. doi:10.1159/000493218
9. Wang SJ, Wang PZ, Gale RP, et al. Cysteine and glycine-rich protein 2 (CSRP2) transcript levels correlate with leukemia relapse and leukemia-free survival in adults with B-cell acute lymphoblastic leukemia and normal cytogenetics. Oncotarget. 2017;8:35984–36000. doi:10.18632/oncotarget.16416
10. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults. 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447.
11. Ruan G-R, Qin Y-Z, Chen -S-S, et al. Abnormal expression of the programmed cell death 5 gene in acute and chronic myeloid leukemia. Leuk Res. 2006;30(9):1159–1165. doi:10.1016/j.leukres.2005.12.028
12. Wang S, Wang C, Wang W, Hao Q, Liu Y. High RASD1 transcript levels at diagnosis predicted poor survival in adult B-cell acute lymphoblastic leukemia patients. Leuk Res. 2019;80:26–32. doi:10.1016/j.leukres.2019.03.005
13. Beillard E, Pallisgaard N, van der Velden VHJ, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi:10.1038/sj.leu.2403136
14. Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GVN, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–2089. doi:10.1038/leu.2014.112
15. Wang S-J, Lu W-Y, Liu K-Y. Adiponectin receptor agonist AdipoRon suppresses adipogenesis in C3H10T1/2 cells through the adenosine monophosphate‑activated protein kinase signaling pathway. Mol Med Rep. 2017;16:7163–7169. doi:10.3892/mmr.2017.7450
16. Hoffmann C, Mao X, Brown-Clay J, et al. Hypoxia promotes breast cancer cell invasion through HIF-1α-mediated up-regulation of the invadopodial actin bundling protein CSRP2. Sci Rep. 2018;8:10191. doi:10.1038/s41598-018-28637-x
17. Nepstad I, Hatfield KJ, Grønningsæter IS, Reikvam H. The PI3K-Akt-mTOR signaling pathway in human Acute Myeloid Leukemia (AML) cells. Int J Mol Sci. 2020;21.
18. Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer. Implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15:2583–2587.
19. Desdouets C, Matesic G, Molina CA, et al. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi:10.1128/MCB.15.6.3301
20. Yoshizumi M, Wang H, Hsieh CM, Sibinga NE, Perrella MA, Lee ME. Down-regulation of the cyclin A promoter by transforming growth factor-beta1 is associated with a reduction in phosphorylated activating transcription factor-1 and cyclic AMP-responsive element-binding protein. J Biol Chem. 1997;272:22259–22264. doi:10.1074/jbc.272.35.22259
21. Lee RJ, Albanese C, Stenger RJ, et al. The pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells.. J Biol Chem. 1999;274:7341–7350.
22. Zhang C, Yu Y, Ma L, Histamine FP. H3 receptor promotes cell survival via regulating PKA/CREB/CDKN1A signal pathway in hepatocellular carcinoma. Onco Targets Ther. 2020;13:3765–3776. doi:10.2147/OTT.S250655
23. Chen P, Li M, Hao Q, Zhao X, Hu T. Targeting the overexpressed CREB inhibits esophageal squamous cell carcinoma cell growth. Oncol Rep. 2018;39:1369–1377.
24. Aggarwal S, Kim S-W, Ryu S-H, Chung W-C, Koo JS. Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein. Cancer Res. 2008;68:981–988. doi:10.1158/0008-5472.CAN-06-0249
25. Linnerth NM, Greenaway JB, Petrik JJ, Moorehead RA. cAMP response element-binding protein is expressed at high levels in human ovarian adenocarcinoma and regulates ovarian tumor cell proliferation. Int J Gynecol Cancer. 2008;18:1248–1257. doi:10.1111/j.1525-1438.2007.01177.x
26. Rahman SMT, Zhou W, Deiters A, Haugh JM. Optical control of MAP kinase kinase 6 (MKK6) reveals that it has divergent roles in pro-apoptotic and anti-proliferative signaling. J Biol Chem. 2020;295:8494–8504. doi:10.1074/jbc.RA119.012079
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.