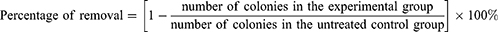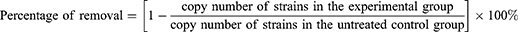Back to Journals » Infection and Drug Resistance » Volume 15
Inhibition and Removal of Mature Mixed-Bacteria Biofilms on Voice Prostheses by Sodium Selenite
Authors Zhang Y , Niu Y , Huo H, Wang J, Jin X, Yang H
Received 18 October 2022
Accepted for publication 20 December 2022
Published 29 December 2022 Volume 2022:15 Pages 7799—7810
DOI https://doi.org/10.2147/IDR.S393434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yongli Zhang,1,2 Yanyan Niu,1,2 Hong Huo,1,2 Jian Wang,1,2 Xiaofeng Jin,1,2 Hua Yang1,2
1Peking Union Medical College, Chinese Academy of Medical Sciences, Peking Union Medical College Hospital, Department of Otolaryngology, Beijing, People’s Republic of China; 2Translational Medicine Center, Peking Union Medical College Hospital, Beijing, People’s Republic of China
Correspondence: Jian Wang; Hua Yang, Department of Otolaryngology, Peking Union Medical College Hospital, Beijing, People’s Republic of China, 100730, Tel +13673164261 ; +13701127757, Fax +86-10-69156311, Email [email protected]; [email protected]
Purpose: Biofilms on voice prostheses are important factors shortening their service life. Sodium selenite has been used to prevent and treat various diseases. Whether sodium selenite can inhibit and remove mature biofilms on voice prostheses is still unknown.
Methods: To verify the effects of sodium selenite on mature mixed-bacteria biofilms (Staphylococcus aureus, Candida albicans, and Streptococcus faecalis) on voice prostheses, we used quantitative and qualitative methods, eg, real-time fluorescence quantitative PCR, crystal violet staining, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) (XTT) reduction assays, and scanning electron microscopy, to measure the effects of sodium selenite on the number of bacterial colonies, biofilm formation ability, metabolic activity, and ultrastructure in a model of mature mixed-bacteria biofilms on voice prostheses and validated the effects in vitro on mature biofilms on voice prostheses from patients.
Results: When exploring the possible mechanism of biofilm inhibition and removal by sodium selenite, we found that it significantly inhibited and removed biofilms on voice prostheses and effectively destroyed the spatial structure of the biofilms. The inhibition and removal effects became more significant with increasing sodium selenite concentrations.
Conclusion: We demonstrated that sodium selenite can inhibit and remove biofilms of mature mixed strains on voice prostheses, providing a novel basis for treating patients’ voice prosthesis biofilms.
Keywords: sodium selenite, voice prostheses, mature biofilms, mixed strains on voice prostheses, remove, inhibit
Corrigendum for this paper has been published.
Introduction
Total laryngectomy is an important method for the treatment of intermediate and advanced laryngeal cancer. However, patients lose their speaking ability after total laryngectomy, which seriously affects the quality of life of patients.1–4 Voice prostheses have become an important method for facilitating patients’ vocalization after total laryngectomy due to advantages such as simple operation, smooth and clear pronunciation, and the ease of mastering pronunciation skills.5,6 The formation of biofilm on a voice prosthesis can cause it to malfunction, which is an important reason for a shortened service life. Patients must have the voice prosthesis replaced with a new one every 3–4 months, which imposes considerable economic and psychological pressure on them. Although some studies have explored the inhibition of biofilm formation on voice prostheses, biofilms on voice prostheses cannot be effectively removed and inhibited.7,8 Therefore, new methods to inhibit and remove biofilms on voice prostheses are needed to prolong their service life.
Selenium is an indispensable trace element in the human body with antioxidant, antiviral, antitumor, and immune-enhancing effects. Sodium selenite is an important inorganic form of selenium that has been used in the treatment and prevention of a variety of clinical diseases.9 Sodium selenite can induce cancer cell death and significantly improve the therapeutic effect of chemotherapy and radiotherapy in patients with advanced cancer.10,11 It can fight infection in patients with sepsis, improve their organ dysfunction, and reduce their mortality.12,13 Sodium selenite also has a significant inhibitory effect on various bacteria in the human body.14 However, no study has investigated whether sodium selenite can inhibit and remove biofilms on voice prostheses. Based on the strong potential of sodium selenite to treat and prevent a variety of clinical diseases and its ability to effectively inhibit various human bacteria, we speculated that sodium selenite could significantly inhibit and remove mature mixed-bacteria biofilms on voice prostheses, which would provide a novel strategy to treat biofilms on voice prostheses.
Materials and Methods
Bacterial Strains and Materials
We used three common strains of biofilm bacteria—Staphylococcus aureus (ATCC 25923), Candida albicans (SC 5314), and Streptococcus faecalis (ATCC 13419)15–18 to construct an in vitro model of voice prosthesis biofilms. These three strains were cultured at 37°C in 70% yeast extract/peptone/dextrose medium (yeast extract 1% (Sigma‒Aldrich), glucose 2% (Sigma‒Aldrich), and peptone water 2%) + 30% fetal bovine serum (Gibco) (YPDF medium).18,19 A medical grade silicone membrane (thickness: 1 mm) was purchased from Suzhou Shoucheng Electronics Co., Ltd., China, and sterilized at 121°C under autoclave conditions before use. Platelets measuring 4 mm in diameter for 96-well plates and 10 mm in diameter for 24-well plates were punched out of the medical silicone membrane.
Construction of Mature Biofilms on Medical Silicone Membranes
The mature biofilm on the medical silicone membrane plates was constructed based on a previously reported method.19–21 After the above three strains were diluted to an optical density at 600 nm of 0.010, they were mixed in equal volumes, and 200 µL of the mixed-bacteria solution was placed in a 96-well plate (Corning, 3599) with a medical silicone membrane. The cells were cultured in YPDF medium at 37°C for 48 hours.
Determination of the Minimum Mature Biofilm Eradication Concentration (BEC) on Voice Prostheses
We determined the minimum mature BEC on voice prostheses of sodium selenite (Sigma‒Aldrich, S5261) as described previously.22 Briefly, the medical silicone membrane was placed in a 24-well plate, and 1 mL of mixed-bacteria solution was added to each well and cultured at 37°C for 24 hours. Then, different concentrations of sodium selenite (0, 0.50, 0.55, 0.60, 0.65, 0.70, 0.75, 0.80, 0.85, 0.90, 0.95, 1.00 mg/mL) were added to the various wells, and the plate was cultured at 37°C for 24 hours. The number of colonies in each well was evaluated by the plate counting method. By comparison with the negative control (sodium selenite concentration: 0 mg/mL), the lowest sodium selenite concentration resulting in a 50% reduction in the number of colonies on the voice prosthesis biofilms was taken as the BEC50, the lowest sodium selenite concentration resulting in a 70% reduction in the number of colonies was the BEC70, and the lowest sodium selenite concentration resulting in a 90% reduction in the number of colonies was the BEC90.
Effect of Sodium Selenite on Mature Mixed-Bacteria Biofilms on Voice Prostheses in vitro
In this experiment, five groups were set up: a blank control group (only medium, no biofilms, no sodium selenite), an untreated control group (sodium selenite concentration: 0 mg/mL), and experimental groups (selenite concentrations: BEC30, BEC50, and BEC90). According to the method described above, mature biofilms on voice prostheses were constructed. According to the experimental group, medium and a certain concentration of sodium selenite were added to 96-well plates and cultured at 37°C for 24 hours. We used quantitative and qualitative methods to detect the inhibitory and removal effects of sodium selenite on mature mixed-bacteria biofilms on voice prostheses. We used the real-time fluorescence quantitative PCR method to quantify the DNA contents of the three strains in each group of biofilms to determine the inhibitory effect of sodium selenite on the three strains in the mixed-bacteria biofilms. The crystal violet staining method was used to measure the effect of sodium selenite on the amount of biofilm on voice prostheses to determine the effect of sodium selenite on biofilm formation ability. The effect of sodium selenite on the metabolic activity of the voice prosthesis biofilms was measured by XTT reduction assay. To observe the inhibitory and removal effects of sodium selenite more intuitively on voice prosthesis biofilms, we used scanning electron microscopy (SEM) to observe changes in the ultrastructure of the voice prosthesis biofilms in each group, and laser confocal microscopy was used to observe the distribution of live and dead bacteria on the biofilms. To explore the possible mechanism of action of sodium selenite in the inhibition and removal of mature mixed-bacteria biofilms on voice prostheses, we used the polysaccharide–phenol‒sulfuric acid method and the bicinchoninic acid (BCA) method to quantify the polysaccharides and proteins in each group of biofilms.
Effect of Sodium Selenite on Mature Biofilm on Patient Voice Prostheses
To further verify the inhibitory and removal effects of sodium selenite on mature biofilms on voice prostheses, we performed further in vitro tests on voice prostheses from patients. We collected isolated, nonfunctional voice prostheses from three patients. The biofilms on the voice prostheses were eluted by ultrasonication at room temperature for 30 minutes, and the bacterial strains in the biofilm were collected. The sterilized medical silicone membrane and 2 mL of the collected bacterial solution were placed in a 96-well plate and cultured at 37°C for 48 h. Different concentrations of sodium selenite were added to 96-well plates according to experimental grouping and incubated at 37°C for 24 hours. We used the plate counting method to detect the effect of sodium selenite on the number of bacterial colonies in the mature biofilms on voice prostheses of patients to determine the inhibitory and removal effects of sodium selenite on the bacterial flora in the mature biofilms on voice prostheses. The XTT method was used to verify the inhibitory effect of sodium selenite on the metabolic activity of mature biofilms on voice prostheses.
Plate Counting of Biofilms
The biofilm on the medical silicone membrane was eluted by ultrasonication. The bacterial solution was diluted with phosphate-buffered saline (PBS) (Gibco™, 70011044), and 100 μL of the diluted solution was spread on a YPDF plate, followed by incubation at 37°C for approximately 18 h, imaging, and counting using an automatic colony counter (Interscience, SCAN1200). The percentage of removed colonies in the mature biofilms on voice prostheses by sodium selenite was calculated using Formula (1):
Real-Time Fluorescence Quantitative PCR
The standard plasmids of S. aureus, C. albicans, and S. faecalis were constructed. The standard plasmid solutions were diluted five times with a starting concentration of 109 copies/µL, and the standard curve was plotted. A bacterial genome extraction kit (Vazyme, DC103) was used to extract the DNA of the bacterial strains from the biofilms according to the kit manual. The SYBR Green Master Mix (VAZYME, Q111-02) kit was used to perform qPCR on the obtained strain DNA and plasmids. The qPCR conditions were as follows: predenaturation at 95°C for 5 min, then 40 cycles of denaturation at 95°C for 15 sec, annealing at 56°C for 20 sec, and extension at 72°C for 40 sec. The primers for real-time fluorescence quantitative PCR are listed in Table 1. The percentage of removed strain copies in the biofilms by sodium selenite was calculated using Formula (2).
 |
Table 1 Primers for Real-Time Fluorescence Quantitative PCR |
Crystal Violet Staining Method
The culture medium in the wells was aspirated, and the biofilms were washed with PBS three times and fixed with 0.10 mL of 10% methanol solution (BBI, A601617) for 15 minutes. The methanol solution was aspirated, and 0.1 mL of crystal violet staining solution (KeyGEN BioTech, KGA229) was added. The plates were placed at room temperature for 20 minutes, washed three times with PBS, and dried at 37°C. Then, 0.10 mL of 33% glacial acetic acid (Sangon Biotech (Shanghai) Co., Ltd., A501931) was added to each well, and 80 µL of the solution was taken to measure the absorbance at 590 nm using a photometer (Beckman, AD340). The degree of inhibition of biofilm formation ability by sodium selenite was calculated using Formula (3):
XTT Reduction Assay
The culture medium in the wells was aspirated and washed with PBS three times. Then, 500 μL of XTT assay working solution (KeyGEN BioTech, KGA313) was added. The reaction was carried out at 37°C in the dark for 4 hours, and the absorbance value at 450 nm was measured in a spectrophotometer. The degree of inhibition of the metabolic activity of biofilms by sodium selenite was calculated using Formula (2).
SEM
The culture medium in the well plate was aspirated. The cells were washed with PBS three times, fixed in 2.5% glutaraldehyde phosphate buffer (BBI, A600875) at 4°C overnight, washed twice with 0.15% glutaraldehyde phosphate buffer, and then dehydrated in an ethanol series (40%, 70%, 90%, 100%) for 15 minutes each time. Then, the cells were dried in a critical-point desiccator. After spraying on gold in a vacuum coating device, the cells were observed under SEM (ZEISS, GeminiSEM 360).
Laser Confocal Microscopy
The live and dead bacteria on the biofilms were stained according to the instructions of the LIVE/DEAD BACLIGHT BACTERIAL C 1 KIT (Invitrogen, L7012). The culture medium in the well plate was aspirated, and 0.85% NaCl was added to rinse the medical silicone membrane three times. One microliter of component A (SYTO 9 dye, 3.34 mM) and 0.5 µL of component B (propidium iodide, 30 mM) were added to 1 mL of PBS and mixed thoroughly. Three microliters of the mixed staining solution was added to each well, followed by incubation at room temperature in the dark for 15 minutes. Observation was performed under a laser confocal microscope (Olympus, FV3000).
Polysaccharide–phenol‒sulfuric Acid Method
A 5% phenol solution (Shanghai test, 10015328) and 98% concentrated sulfuric acid (Sinopharm Hushi, 10021608) were mixed at a ratio of 1:5 to prepare the chromogenic solution. A total of 180 µL of the chromogenic solution was added to 60 µL of the biofilm eluate and different concentrations of glucose solution (Amresco, 0188). The mixture was thoroughly mixed and heated in a metal bath at 100°C for 25 min. The absorbance at 490 nm was measured in a spectrophotometer using 100 µL of the solution.
BCA Method
CST RIPA buffer (Beyotime, P0013B) was used to lyse the biofilms of each group, and proteins were collected. Different concentrations of the BSA standard and the BCA working solution were prepared according to the instructions of the BCA protein concentration determination kit (Sangon Biotech, C503021). Fifty microliters of sample lysis buffer or BSA standard was mixed well with 500 µL of BCA working solution, incubated in a 37°C water bath for 30 min, and then cooled to room temperature. The absorbance of each well at 560 nm was measured in a spectrophotometer.
Data Analysis
Each experiment was performed at least three times, and the results of multiple repeated experiments are expressed as the mean ± standard deviation (SD). Differences between the experimental group and the untreated control group were evaluated by one-way analysis of variance. A P value <0.05 indicated that the difference was statistically significant. The data were analyzed with SPSS 26.0 software (IBM).
Experimental Results
Determination of the Minimum Mature BEC on Voice Prostheses
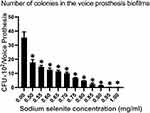
The number of colonies in the biofilms in each group was measured by the plate counting method to determine the minimum mature BEC of sodium selenite on voice prostheses. As shown in Figure 1, the number of colonies in the biofilms in the sodium selenite treatment group was significantly lower than that in the untreated control group. When the concentration of sodium selenite was 0.50 mg/mL, the number of colonies in the biofilms was reduced by 50% from the negative control level, that is, the BEC50 was 0.50 mg/mL, the BEC70 was 0.65 mg/mL, and the BEC90 was 0.80 mg/mL. When the concentration of sodium selenite was 1 mg/mL, no colonies were found in the biofilms; that is, the BEC100 was 1.00 mg/mL. Therefore, sodium selenite can remove colonies from mature biofilms on voice prostheses, and the removal effect is more obvious at higher concentrations.
Effects of Sodium Selenite on Mature Mixed-Bacteria Biofilms on Voice Prostheses in an in vitro Model
RT‒qPCR was used to measure the DNA contents of S. aureus, C. albicans, and S. faecalis of the mature mixed-bacteria biofilms on voice prostheses in vitro to determine the effect of sodium selenite on the removal of each of these three bacterial strains on voice prosthesis biofilms. We found that the DNA contents of S. faecalis, S. aureus, and C. albicans in the biofilms of the sodium selenite groups were significantly lower than those in the untreated control group (sodium selenite concentration: 0 mg/mL) (Figure 2A,B, and C, P < 0.05). Compared with that in the untreated control group, sodium selenite had a significant removal rate for the three strains in the mature mixed-bacteria biofilms on voice prostheses, with higher concentrations of sodium selenite corresponding to a more obvious removal effect (Table 2). The same concentration of sodium selenite showed the most significant degree of removal of C. albicans and weaker removal of S. aureus and S. faecalis (Table 2).
The effect of sodium selenite on the biofilm formation ability of the mature mixed strains on voice prostheses was examined using the crystal violet staining method. Compared with no treatment, sodium selenite significantly reduced the absorbance of the biofilms at 590 nm, with higher concentrations of sodium selenite yielding a more obvious effect (Figure 3 A, P < 0.05). Sodium selenite can significantly inhibit mature biofilm formation on voice prostheses, and the degree of inhibition of sodium selenite on the mature biofilm formation ability of the mixed strains increased with the concentration (Table 3).
 |
Table 3 Percentage Reductions in the Biofilm Formation Ability and Metabolic Activity of the Mature Mixed Strains on Voice Prostheses by Sodium Selenite (Mean ± SD) |
The XTT reduction assay was used to detect the absorbance at 450 nm of mature mixed-bacteria biofilms on voice prostheses to determine the metabolic activity of the biofilms. Sodium selenite significantly inhibited the absorbance of biofilms at 450 nm, with higher concentrations corresponding to a more significant inhibitory effect (Figure 3B, P < 0.05). Compared with observations in the untreated control group, sodium selenite significantly inhibited the metabolic activity of mature biofilms on voice prostheses, and the inhibitory degree of sodium selenite on the metabolic activity of the mature mixed-bacteria biofilms on voice prostheses increased with the concentration (Table 3).
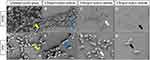
By SEM, we observed the ultrastructure of the mature biofilms on voice prostheses. In the untreated control group, most of the medical silicone membrane was covered by biofilm, and the colonies in the biofilm were numerous and densely arranged, forming a complex spatial structure (shown by the yellow arrow in Figure 4). Compared with that in the untreated control group, when the concentration of sodium selenite was 0.50 mg/mL, the area covered by biofilms on medical silicone membrane and the number of colonies in the biofilms were significantly reduced, and the proximity of the arrangement between strains in the area covered by biofilms decreased (shown by red arrows in Figure 4). When the concentration of sodium selenite was 0.65 mg/mL, the area covered by the biofilms and the number of colonies further decreased, the colonies were scattered, and no biofilm formation with a complex spatial structure was observed, although a small number of strains had formed colonies (white arrow in Figure 4). When the concentration of sodium selenite was 0.80 mg/mL, no obvious biofilm or colony formation was noted on the medical silicone membrane, and the bacterial colonies were mostly sporadically scattered on it (shown by the black arrow in Figure 4). Under SEM, we observed that sodium selenite significantly removed cells and colonies from the biofilms and inhibited the formation of the complex spatial structure of the biofilms. Higher concentrations of sodium selenite produced more obvious inhibition and removal effects.
We used laser confocal microscopy to observe the distribution of live and dead bacteria in mature mixed-bacteria biofilms on voice prostheses. As shown in Figure 5, many live bacterial colonies were observed in the biofilms of the untreated control group. Comparatively, when the concentration of sodium selenite was 0.50 mg/mL, the number of colonies in the biofilms was significantly reduced, and the strains in the biofilms were mostly live bacteria. When the concentration of sodium selenite was 0.65 mg/mL, the number of bacterial colonies in the biofilms was further reduced, and both live and dead bacteria could be observed in biofilm colonies. When the concentration of sodium selenite was 0.80 mg/mL, a small number of colonies remained in the biofilms, most of which contained dead bacteria. Therefore, sodium selenite can effectively remove and kill the strains of mature mixed-bacteria biofilms on voice prostheses, with a stronger effect at higher concentrations.
 |
Figure 5 Distribution of dead bacteria on the biofilm of mature mixed strains. In Figure 5, green fluorescence indicates live bacteria, and red fluorescence indicates dead bacteria. The untreated control group (A) had many colonies, most of which were live bacteria; the number of colonies in the sodium selenite treatment groups was significantly reduced. The residual colonies in the biofilms at a sodium selenite concentration of 0.5 mg/mL mostly contained live bacteria (B). When the sodium concentration reached 0.65 mg/mL or 0.80 mg/mL, the remaining colonies mostly contained dead bacteria (C and D). |
We used the polysaccharide–phenol‒sulfuric acid method and the BCA method to detect the amounts of polysaccharides and proteins in the biofilms. Compared with those in the untreated control group, when the concentration of sodium selenite was 0.50 mg/mL, the amounts of protein and polysaccharides in the biofilms were significantly reduced (Figure 6, P < 0.05); when the concentration of sodium selenite was 0.65 mg/mL and 0.80 mg/mL, the amounts of protein and polysaccharides in the biofilm were further reduced (Figure 6, P < 0.05). The results suggest that sodium selenite can inhibit the synthesis of polysaccharides and proteins in the extracellular matrix of biofilms.
Effect of Sodium Selenite on Mature Biofilms on Patients’ Voice Prostheses
To verify the inhibitory and removal effects of sodium selenite on the mature biofilms of patient voice prostheses, we used the XTT reduction assay and the plate counting method to detect the metabolic activity and the number of colonies of the biofilms. Compared with that in the untreated control group, the number of colonies in the biofilms and the absorbance of the biofilms in the sodium selenite treatment group were significantly reduced (Figure 7, P < 0.05). Higher concentrations of sodium selenite resulted in more significant reductions in the number of colonies and the absorbance values of biofilms. Compared with those in the untreated control group, the degree of inhibition with respect to the number of colonies in the biofilms and the metabolic activity of the biofilms increased with the concentration (Table 4). Therefore, sodium selenite also had inhibitory and removal effects on the mature biofilms from patients’ voice prostheses, with stronger inhibitory and removal effects at higher concentrations.
 |
Table 4 Percentage Reductions in the Number of Mature Biofilm Colonies and Their Metabolic Activity in Voice Prostheses Biofilms from Patients by Sodium Selenite (Mean ± SD) |
Discussion
In this study, we explored the inhibition and removal of mature mixed-bacteria biofilms on voice prostheses by sodium selenite. To verify the inhibitory and removal effects of sodium selenite on the biofilm of mature mixed strains of voice prostheses, we examined the number of colonies, biofilm formation ability, and biofilm ultrastructure in the in vitro biofilm model of mature mixed bacterial strains of voice prostheses and validated them on mature biofilms of voice prostheses from patients in vitro. We also explored the possible mechanism by which sodium selenite inhibited and removed the biofilms of the mature mixed strains on voice prostheses. We found that sodium selenite inhibited and removed the mature mixed-bacteria biofilms on voice prostheses, with higher concentrations of sodium selenite corresponding to a more significant inhibitory effect. Sodium selenite may inhibit the synthesis of polysaccharides and proteins in the extracellular matrix of biofilms and destroy the integrity and stability of biofilms to promote bacterial inhibition and removal.
Voice prosthesis biofilms can cause voice flap dysfunction of the voice prosthesis, which is an important factor leading to a shortened service life of voice prostheses. In recent years, scholars have studied the inhibition of voice prosthesis biofilms, with some using antibacterial drugs to inhibit the formation of voice prosthesis biofilms.23–25 However, the use of antibacterial drugs can increase the drug resistance of bacteria and fungi and cannot effectively inhibit and remove biofilms from voice prostheses. Others have inhibited the formation of biofilms by improving the physical and chemical properties of the surface of silicone rubber materials through, for example, biosurfactants, essential oil coatings, chitosan coatings, and metal nanoparticle coatings.26–29 However, surface modifications to voice prosthesis materials have not effectively extended the service life of voice prostheses. Therefore, we still need better methods to inhibit and remove voice prosthesis biofilms.
Sodium selenite has various functions, such as anti-inflammatory, antioxidation, and anticancer functions, and has been used in the treatment and prevention of a variety of clinical diseases, such as cancer, Kaschin–Beck disease in children, and sepsis.10,12,13,30 Therefore, the application of sodium selenite in the treatment and prevention of human diseases is reasonable and safe. Alam et al found that sodium selenite had a strong inhibitory effect on a variety of human microbiota (S. aureus, Streptococcus macularis, Escherichia coli, etc.).14 Later, Narayanan et al studied the inhibition of E. coli biofilms by sodium selenite31 and found that 35 mM sodium selenite could effectively prevent the formation of ureter E. coli biofilms. Since sodium selenite is an inorganic form of selenium, it does not cause drug-resistant mutations in bacteria or fungi, thereby avoiding the formation of drug-resistant strains. Therefore, sodium selenite has good potential to inhibit and remove mature mixed-bacteria biofilms on voice prostheses.
Biofilms are mainly composed of bacterial flora and extracellular matrix. The extracellular matrix can maintain the stability and integrity of biofilm structures, limit the penetration and diffusion of antibacterial drugs in biofilms, and play a major role in biofilm drug resistance.32,33 Proteins and polysaccharides are the main components of the extracellular matrix. The polysaccharides in the extracellular matrix of biofilms can hinder the penetration of antibacterial drugs into biofilms and increase the drug resistance of biofilms.34 Nett and Tan et al applied β-1,3-glucanase to C. albicans biofilms and found that it inhibited biofilm formation and improved sensitivity to antifungal drugs.35,36 Proteins are another important component of the extracellular matrix of biofilms. Proteins are attached to cell-surface polysaccharides, where they help maintain the integrity and stability of biofilms.37 Lynch et al inhibited the expression of glucan-binding protein genes in streptococcal biofilms and reduced the synthesis of glucan proteins. They found that the aggregation of bacterial colonies in the biofilms decreased, and the thickness of the biofilms also decreased significantly.38 Therefore, the polysaccharides and proteins in the extracellular matrix of biofilms play important roles in the drug resistance of biofilms.
Our results showed that the amounts of polysaccharide and protein in the biofilms in the sodium selenite treatment groups were significantly lower than those in the untreated control group. The amount of biofilm and the biofilm formation ability in the sodium selenite treatment groups were also significantly lower than those in the untreated control group. Proteins and polysaccharides in a biofilm may originate from two sources: the proteins and polysaccharides in the extracellular matrix and the proteins and polysaccharides of bacteria in the biofilms. The main source of proteins and polysaccharides in biofilms is the extracellular matrix. We speculate that the mechanism of action of sodium selenite in removing and inhibiting mature mixed-bacteria biofilms on voice prostheses is as follows: Sodium selenite inhibits the synthesis of polysaccharides and proteins in the extracellular matrix of voice prosthesis biofilms, which destroys the integrity and stability of the biofilms, increases the permeation and diffusion of sodium selenite in the biofilms, reduces the resistance of the biofilms to sodium selenite, and thus significantly inhibits and removes mature biofilms on voice prostheses.
No study has explored the inhibition of voice prosthesis biofilms by sodium selenite. We explored the inhibitory and removal effects of sodium selenite on biofilms in an in vitro model of mature voice prosthesis biofilms and verified the effects on mature biofilms from voice prostheses taken from patients. The results showed that sodium selenite significantly inhibited and removed the mixed-bacteria biofilms on voice prostheses. Our study expands the potential of sodium selenite to treat clinical diseases, provides a novel method for inhibition and removal of mature voice prosthesis biofilms, and lays the foundation for the clinical treatment of mature voice prosthesis biofilms by sodium selenite.
Our study has certain limitations, such as the small number of in vitro voice prostheses from patients tested for verification. In the future, we will conduct validation on more voice prosthesis biofilms from patients in vitro.
Conclusion
Sodium selenite can effectively remove and inhibit mature mixed-bacteria biofilms on voice prostheses, reducing their biofilm formation ability and metabolic activity. Sodium selenite applied in vivo to prevent biofilms on voice prostheses is promising and has clinical potential in the future. Our results can provide novel ideas for the treatment of mature voice prosthesis biofilms.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Peking Union Medical College Hospital (ethics approval no. JS2084) and conducted in accordance with the Declaration of Helsinki, and all patients signed informed consent forms.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences under Grant number XDA1601040201 and the Nature Science Foundation of Beijing under Grant number 7192171.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Kemps GJF, Krebbers I, Pilz W, Vanbelle S, Baijens LWJ. Affective symptoms and swallow-specific quality of life in total laryngectomy patients. Head Neck. 2020;42(11):3179–3187. doi:10.1002/hed.26365
2. Scott AJ, McGuire JK, Manning K, Leach L, Fagan JJ. Quality of life after total laryngectomy: evaluating the effect of socioeconomic status. J Laryngol Otol. 2019;133(2):129–134. doi:10.1017/s0022215119000215
3. Sharpe G, Camoes Costa V, Doubé W, Sita J, McCarthy C, Carding P. Communication changes with laryngectomy and impact on quality of life: a review. Qual Life Res. 2019;28(4):863–877. doi:10.1007/s11136-018-2033-y
4. Wulff NB, Højager A, Wessel I, Dalton SO, Homøe P. Health-related quality of life following total laryngectomy: a systematic review. Laryngoscope. 2021;131(4):820–831. doi:10.1002/lary.29027
5. Lorenz KJ. Stimmrehabilitation nach totaler Laryngektomie: Ein chronologischer, medizinhistorischer Überblick. [Voice rehabilitation after total laryngectomy: a chronological review of medical history]. HNO. 2015;63(10):663–4, 666–80. German. doi:10.1007/s00106-015-0043-4
6. Tawfik GM, Makram OM, Zayan AH, et al. Voice rehabilitation by voice prostheses after total laryngectomy: a systematic review and network meta-analysis for 11,918 patients. J Speech Lang Hear Res. 2021;64(7):2668–2681. doi:10.1044/2021_jslhr-20-00597
7. Sayed SI, Datta S, Deore N, Kazi RA, Jagade MV. Prevention of voice prosthesis biofilms: current scenario and future trends in prolonging prosthesis lifetime. J Indian Med Assoc. 2012;110(3):175–8, 180.
8. Tsikopoulos A, Petinaki E, Festas C, et al. In vitro inhibition of biofilm formation on silicon rubber voice prosthesis: α systematic review and meta-analysis. ORL J Otorhinolaryngol Relat Spec. 2022;84(1):10–29. doi:10.1159/000516345
9. Oliveira CR, Viana ET, Gonçalves TF, Mateus-Silva JR, Vieira RP. Therapeutic use of intravenous selenium in respiratory and immunological diseases: evidence based on reviews focused on clinical trials. Adv Respir Med. 2022;90(2):134–142. doi:10.5603/ARM.a2022.0018
10. Jayachandran P, Knox SJ, Garcia-Cremades M, Savić RM. Clinical pharmacokinetics of oral sodium selenite and dosing implications in the treatment of patients with metastatic cancer. Drugs R D. 2021;21(2):169–178. doi:10.1007/s40268-021-00340-9
11. Chen W, An J, Guo J, et al. Sodium selenite attenuates lung adenocarcinoma progression by repressing SOX2-mediated stemness. Cancer Chemother Pharmacol. 2018;81(5):885–895. doi:10.1007/s00280-018-3561-4
12. Bloos F, Trips E, Nierhaus A, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266–1276. doi:10.1001/jamainternmed.2016.2514
13. Chelkeba L, Ahmadi A, Abdollahi M, et al. The effect of high-dose parenteral sodium selenite in critically ill patients following sepsis: a clinical and mechanistic study. Indian J Crit Care Med. 2017;21(5):287–293. doi:10.4103/ijccm.IJCCM_343_16
14. Alam MF, Safhi MM, Moni SS, Jabeen A. In vitro antibacterial spectrum of sodium selenite against selected human pathogenic bacterial strains. Scientifica. 2016;2016:9176273. doi:10.1155/2016/9176273
15. Ticac B, Ticac R, Rukavina T, et al. Microbial colonization of tracheoesophageal voice prostheses (Provox2) following total laryngectomy. Eur Arch Otorhinolaryngol. 2010;267(10):1579–1586. doi:10.1007/s00405-010-1253-8
16. Bertl K, Zatorska B, Leonhard M, Matejka M, Schneider-Stickler B. Anaerobic and microaerophilic pathogens in the biofilm formation on voice prostheses: a pilot study. Laryngoscope. 2012;122(5):1035–1039. doi:10.1002/lary.23193
17. Buijssen KJ, van der Laan BF, van der Mei HC, et al. Composition and architecture of biofilms on used voice prostheses. Head Neck. 2012;34(6):863–871. doi:10.1002/hed.21833
18. Leonhard M, Schneider-Stickler B. Voice prostheses, microbial colonization and biofilm formation. Adv Exp Med Biol. 2015;830:123–136. doi:10.1007/978-3-319-11038-7_8
19. Leonhard M, Zatorska B, Moser D, Tan Y, Schneider-Stickler B. Evaluation of combined growth media for in vitro cultivation of oropharyngeal biofilms on prosthetic silicone. J Mater Sci Mater Med. 2018;29(4):45. doi:10.1007/s10856-018-6051-7
20. Leonhard M, Tobudic S, Moser D, Zatorska B, Bigenzahn W, Schneider-Stickler B. Growth kinetics of candida biofilm on medical polymers: a long-term in vitro study. Laryngoscope. 2013;123(3):732–737. doi:10.1002/lary.23662
21. Tan Y, Leonhard M, Moser D, Ma S, Schneider-Stickler B. Inhibition of mixed fungal and bacterial biofilms on silicone by carboxymethyl chitosan. Colloids Surf B Biointerfaces. 2016;148:193–199. doi:10.1016/j.colsurfb.2016.08.061
22. Pierce CG, Uppuluri P, Tristan AR, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494–1500. doi:10.1038/nport.2008.141
23. Pentland DR, Stevens S, Williams L, et al. Precision Antifungal Treatment Significantly Extends Voice Prosthesis Lifespan in Patients Following Total Laryngectomy. Front Microbiol. 2020;11:975. doi:10.3389/fmicb.2020.00975
24. Ciofu O, Rojo-Molinero E, Macià MD, Oliver A. Antibiotic treatment of biofilm infections. Apmis. 2017;125(4):304–319. doi:10.1111/apm.12673
25. Algburi A, Comito N, Kashtanov D, Dicks LMT, Chikindas ML. Control of biofilm formation: antibiotics and beyond. Appl Environ Microbiol. 2017;83(3). doi:10.1128/aem.02508-16
26. Sahal G, Woerdenbag HJ, Hinrichs WLJ, et al. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J Ethnopharmacol. 2020;246:112188. doi:10.1016/j.jep.2019.112188
27. Tan Y, Leonhard M, Moser D, Ma S, Schneider-Stickler B. Long-term antibiofilm activity of carboxymethyl chitosan on mixed biofilm on silicone. Laryngoscope. 2016;126(12):E404–e408. doi:10.1002/lary.26096
28. Gross M, Ashqar F, Sionov RV, et al. Sustained release varnish containing chlorhexidine for prevention of Streptococcus mutans biofilm formation on voice prosthesis surface: an in vitro study. Int Microbiol. 2022;25(1):177–187. doi:10.1007/s10123-021-00205-w
29. Caciandone M, Niculescu AG, Roșu AR, et al. PEG-functionalized magnetite nanoparticles for modulation of microbial biofilms on voice prosthesis. Antibiotics. 2021;11(1). doi:10.3390/antibiotics11010039
30. Jirong Y, Huiyun P, Zhongzhe Y, et al. Sodium selenite for treatment of Kashin-Beck disease in children: a systematic review of randomised controlled trials. Osteoarthritis Cartilage. 2012;20(7):605–613. doi:10.1016/j.joca.2012.02.012
31. Narayanan A, Nair MS, Muyyarikkandy MS, Amalaradjou MA. Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite. Int J Mol Sci. 2018;19(6):Jun. doi:10.3390/ijms19061703
32. Han F, Yuan ZT, Liang XL, Xiong YK, Yang M, Ma GQ. 细菌生物被膜耐药机制及天然药物干预的研究进展. [Research advances in drug resistance mechanisms of bacterial biofilm and natural drug intervention]. Zhongguo Zhong Yao Za Zhi. 2021;46(14):3560–3565. Chinese. doi:10.19540/j.cnki.cjcmm.20210318.601
33. Pereira R. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol. 2021;131(1):11–22. doi:10.1111/jam.14949
34. Singh S, Datta S, Narayanan KB, Rajnish KN. Bacterial exo-polysaccharides in biofilms: role in antimicrobial resistance and treatments. J Genet Eng Biotechnol. 2021;19(1):140. doi:10.1186/s43141-021-00242-y
35. Nett J, Lincoln L, Marchillo K, et al. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51(2):510–520. doi:10.1128/aac.01056-06
36. Tan Y, Ma S, Leonhard M, Moser D, Schneider-Stickler B. β-1,3-glucanase disrupts biofilm formation and increases antifungal susceptibility of Candida albicans DAY185. Int J Biol Macromol. 2018;108:942–946. doi:10.1016/j.ijbiomac.2017.11.003
37. Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem. 2015;7(4):493–512. doi:10.4155/fmc.15.6
38. Lynch DJ, Fountain TL, Mazurkiewicz JE, Banas JA. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol Lett. 2007;268(2):158–165. doi:10.1111/j.1574-6968.2006.00576.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.