Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Influence of Comorbidities on Healthcare Expenditures and Perceived Physical and Mental Health Status Among Adults with Multiple Sclerosis: A Propensity Score-Matched US National-Level Study
Authors Bhattacharjee S, Yegezu Z, Kollecas K, Duhrkopf K, Hashemi L, Greene N
Received 17 February 2021
Accepted for publication 30 April 2021
Published 13 May 2021 Volume 2021:13 Pages 377—394
DOI https://doi.org/10.2147/CEOR.S305154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Sandipan Bhattacharjee,1 Zufan Yegezu,2 Kristin Kollecas,3 Kevin Duhrkopf,3 Lobat Hashemi,3 Nupur Greene3
1Health Outcomes Division, College of Pharmacy, The University of Texas at Austin, Austin, TX, USA; 2Department of Pharmacy Practice and Science, College of Pharmacy, The University of Arizona, Tucson, AZ, USA; 3Neurology, Immunology, & Inflammation, Sanofi Genzyme, Cambridge, MA, USA
Correspondence: Sandipan Bhattacharjee
Health Outcomes Division, College of Pharmacy, The University of Texas at Austin, 2409 University Ave., STOP A1930, Austin, TX, 78712-1120, USA
Tel +1-512-471-6924
Fax +1-512-471-8762
Email [email protected]
Objective: To evaluate the effect of comorbidities on healthcare expenditures and perceived physical and mental health status among adults with multiple sclerosis (MS) compared to propensity score-matched non-MS controls.
Methods: A retrospective, cross-sectional, matched cohort study was conducted using Medical Expenditure Panel Survey (2005– 2015) data. The base study sample consisted of adults (age ≥ 18 years) who were alive and had positive total healthcare expenditures during the survey calendar year. Adults with MS were propensity-matched (1:1) to non-MS controls based on age, gender, and race/ethnicity using greedy matching algorithm. Healthcare expenditures consisted of total and subtypes of expenditures. Health status consisted of perceived physical and mental health status. Comorbidities were identified using ICD-9-CM and Clinical Classification System codes. Ordinary least squares regression and multinomial logistic regression were used to analyze the healthcare expenditures and health status variables, respectively.
Results: Final study sample consisted of 541 adults in each MS and non-MS control groups after propensity score matching. After adjusting for potential confounders, individuals with MS had greater total and subtypes of expenditures compared to non-MS controls, and several comorbidities (eg, depression, hypertension) were significantly associated with increased healthcare expenditures. Yearly average total expenditures (expressed in 2018 US$) were significantly (p< 0.001) higher for adults with MS ($29,396) than propensity score-matched non-MS adults ($7875). Moreover, after adjusting for all individual-level factors, adults with MS experienced 363% (p< 0.001) higher total expenditures compared to propensity score-matched non-MS controls. Individuals with MS were more likely to report poorer physical and good mental health status compared to propensity score-matched non-MS controls, and several comorbidities (eg, anxiety, depression) were significant independent predictors of poorer health status. For example, adults with MS were four times more likely (OR: 4.10, 95% CI: 2.42– 6.96) to report fair/poor physical health status compared to excellent/very good physical health status compared with non-MS controls. Adults with MS were 42% (OR: 1.42, 95% CI: 1.01– 1.99) more likely than propensity score-matched non-MS controls to report good rather than very good or excellent mental health status. However, there was no difference between adults with MS and propensity score-matched non-MS controls in terms of reporting fair or poor than very good or excellent mental health status.
Conclusion: Findings from this study indicate substantial economic and health status burdens among adults with MS at the US national-level that are significantly influenced by comorbidities.
Keywords: multiple sclerosis, comorbidities, expenditures, physical health status, mental health status, propensity score matching
Introduction
Multiple Sclerosis (MS) is a disabling central nervous system disease affecting nearly a million individuals in the United States (US), nearly doubling from previous estimates, and approximately 2.3 million individuals globally.1 Individuals with MS suffer from a wide array of physical and psychiatric comorbidities2 and existing literature suggests comorbidities among individuals with MS lead to diagnostic delays,3 elevated risk of relapse,4 higher chances of disability progression,5 as well as reduced health-related quality of life.6 Moreover, presence of comorbidities not only negatively influences the initiation of disease-modifying therapy (DMT) among individuals with MS but also affects the adherence to DMT.7,8 Hence, understanding the comorbidity burden among individuals with MS has the long-term potential to improve the available clinical support, proper allocation of scarce healthcare resources, and quality of life among individuals with MS and their caregivers.
In recent times, comorbidity burden among individuals with MS has received significant attention, and recognizing the negative influence of comorbidities among individuals with MS, an international initiative was launched by the National Multiple Sclerosis Society (NMSS) to characterize the types and frequencies of comorbidities in this vulnerable population. In a series of articles,2,9–15 the common comorbidities among individuals with MS have been described. While the systematic reviews published from this initiative provided a good understanding of the prevalent comorbidities, these studies were not specific to the US population. Only a handful of studies are available that have examined comorbidity burden among individuals with MS in the US. A study by Capkun et al (2015) using the US Department of Defense (DoD) database showed that rates of mortality and several comorbidities such as infections, cardiovascular diseases, and depression were more common among individuals with MS compared to a matched non-MS cohort.16 Another study using the North American Research Committee on Multiple Sclerosis (NARCOMS) Registry with 16,141 participants (8983 responded) found that the most frequently reported comorbidities were hypercholesterolemia, hypertension, and arthritis.17 Newland et al (2015) conducted a cross-sectional web-based survey among members of the greater Midwest MS society chapter and observed that individuals with MS reported higher rates of depression, arthritis, diabetes, coronary artery disease, migraine headaches, and cancer than the normative population.18 Another study examining the important comorbidities in the US from 1990 to 2001 found that the odds of pressure ulcers, urinary tract infections, and pneumonia/influenza being reported on the death certificate were higher in MS-related deaths than in matched controls.19
Existing studies on MS comorbidity in the US are older,19 lack generalizability,16–18 and do not focus on some of the important issues such as healthcare resource use and expenditures and health status. Moreover, while a couple of existing studies20,21 using the Medical Expenditures Panel Survey (MEPS) data examined the healthcare expenditures among individuals with MS, they did not examine the effect of specific comorbidities on healthcare expenditure burden and also did not use a robust study design (example – propensity score matching). To address the gaps in the existing literature, we examined the prevalence and patterns of comorbidities, healthcare expenditures, and health status (physical and mental) among adults with MS compared to a propensity score-matched non-MS control group using a US nationally representative sample.
Methods
Study Design
Utilizing pooled data from nationally representative Medical Expenditures Panel Survey (MEPS) from 2005 to 2015, a retrospective, cross-sectional, matched cohort study was conducted. As per the recommendation of the MEPS survey designers, pooled data from multiple years of the survey were utilized to obtain adequate sample size.22 MEPS complies with relevant data protection and privacy regulations, details of which can be found elsewhere.23 The University of Arizona Institutional Review Board determined that this study did not require human subject review and approved it as such.
Data Source
The Agency for Healthcare Research and Quality (AHRQ) collects the ongoing MEPS data. Several important information of the US civilian, non-institutionalized individuals as well as their families, their healthcare providers and employers are recorded in MEPS. Health-related data such as the physical and mental health conditions, along with the different types of healthcare service utilization (eg – outpatient services) and treatments (eg – prescription medications) of MEPS participants are collected during the surveys using the original National Health Interview Survey (NHIS) framework. While MEPS consists of several different data files, the household component (MEPS-HC) and the medical conditions (MEPS-MC) were utilized for the purposes of this study. MEPS-HC questionnaire amass data on a wide range of factors such as socio-demographic characteristics, health status, income, as well as healthcare resource use and expenditures. Self-reported medical conditions are recorded in the MEPS-MC files utilizing either International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) codes or Clinical Classification System (CCS) codes that provide comorbidity data. According to an existing study, a median sensitivity of 70% was observed in terms of consistency between the self-reported and healthcare provider reported chronic conditions (eg – hypertension, mental health, diabetes).24
Study Sample
The study sample consisted of adults (i) age ≥18 years; (ii) who were alive during the specific calendar year of survey; and (iii) reported positive total healthcare expenditures. Adults with MS, identified using AHRQ CCS code of “80”, comprised the case group.25 Adults with MS were propensity-matched (1:1) to non-MS controls based on age, gender, and race/ethnicity using a greedy matching algorithm (8:1-digit match). Algorithm for greedy matching technique randomly selects a case (adults with MS) and subsequently selects the control (non-MS adults) whose propensity score is closest to the selected case. Utilizing a similar repetitious process, the greedy matching algorithm continues to match the case and control subjects until all the cases have been matched to appropriate controls. The term “greedy” is used for this algorithm because the closest control is selected to match with a case subject even though this control subject may have the potential to serve as a better match for a later/different case subject.26 Healthcare expenditures consisted of total and subtypes (inpatient, outpatient, emergency room, prescription drugs, home health agency, dental care, and other expenditures) of healthcare expenditures. Health status consisted of perceived physical and mental health status.
Measures
The primary dependent variables included healthcare expenditures and health status. Healthcare expenditures included total as well as subtypes of costs (such as inpatient, outpatient, emergency room, home health agency). Healthcare expenditures for specific service user refer to the MEPS respondents in the study sample who reported using any of the specific services such as inpatient, outpatient, home healthcare, etc. All expenditures were expressed in 2018 US dollar values utilizing the medical care services portion of the consumer price index available in the Bureau of Labor Statistics website.27 Health status consisted of perceived physical and mental health status, both of which were categorized as excellent/very good, good, and fair/poor. During the interview rounds, the perceived physical and mental health statuses of MEPS participants were collected on rating scale ranging from excellent to poor (excellent, very good, good, fair, and poor).
The Andersen’s Behavioral Model (ABM) of Health Services Use was utilized in this study as the conceptual framework. Using the ABM, the association of predisposing, enabling, need, external environmental, and personal health practices factors with the study outcomes (healthcare expenditures, perceived physical and mental health status) were examined. Age, gender, and race/ethnicity were included as predisposing factors whereas education, marital status, poverty status, and insurance type were a part of the enabling factors. Activities of daily living (ADL) limitations, instrumental activities of daily living (IADL) limitations, functional activities limitations, activities limitations in work/housework, comorbidities and pain were considered to be part of need factor. In terms of the personal health behavior, the current study included body mass index (BMI) and smoking status, while region and metropolitan residence status were part of external environmental factor. MEPS respondents who reported need of help or supervision for any ADL (eg – eating, dressing) or IADL (eg – taking medications, using telephone) were categorized as “yes” for limitations in these activities. If the MEPS respondents reported any limitations in terms of work, housework or school, they were coded as having activities limitations. The pain variable was developed by using the following question: “During past 4 weeks, pain interfered with normal work outside the home and housework”.28 This question for pain assessment was self-reported on a 5-point scale: (i) not at all; (ii) a little bit; (iii) moderately; (iv) quite a bit; and (v) extremely. For the purpose of this study, the pain variable was classified into the following three categories: (i) quite a bit/extreme pain; (ii) a little bit or moderate pain; and (iii) no pain.
Specific comorbidities examined in this study included anemia, anxiety, arthritis, chronic obstructive pulmonary disease (COPD), depression, type 2 diabetes (henceforth referred to as diabetes), cancer, eye problems, gastroesophageal reflux disease (GERD), heart diseases, hyperlipidemia, hypertension, osteoporosis, stroke, and thyroid disease. We identified clusters of comorbidities by grouping diseases according to organ-specific domains.29 Comorbidities were organized into six major clusters: (1) cardio-metabolic (presence of hypertension or heart disease or diabetes), (2) musculoskeletal (arthritis or osteoporosis), (3) psychiatric (anxiety, depression) (4) respiratory (asthma or emphysema), (5) cancers, and (6) other comorbidities (GERD, thyroid disease). Existing studies have used such clustering of chronic conditions, citing evidence of synergistic treatment and management approaches.30,31
Statistical Analyses
Comparison of the distribution of the five ABM factor sets prior and subsequent to propensity score matching was conducted using chi-square tests. Propensity score matching was achieved by utilizing an 8 to 1 greedy matching algorithm to exactly match cases to control (1:1) to obtain the final study sample. Survey procedures in SAS version 9.4 (Cary, NC, USA) were used to accommodate for the complex MEPS design and generate national-level estimates. An a-priori alpha of <0.05 was considered to be statistically significant for all analyses. Healthcare expenditures and health status variables were analyzed using Ordinary Least Squares (OLS) regression and multinomial logistic regression, respectively. We examined the Variance Inflation Factor (VIF) for multicollinearity and a VIF value of <5 was considered to demonstrate that there was no sign of multicollinearity.32
Results
Sample Characteristics
The final study sample consisted of 541 adults with Multiple Sclerosis (MS) and 541 non-MS controls after propensity score matching. Table 1 shows the distribution of patient-level characteristics prior and subsequent to matching between adults with MS and non-MS controls. Several patient-level characteristics were statistically significantly different between the case and control group. After matching, adults with MS were higher likely to be non-white, married, poor, unemployed, have public insurance coverage, and a current smoker compared with matched controls. Balance between the case and control group can be observed from Figures 1 and 2.
 |
Table 1 Distribution of Characteristics Between MS and Non-MS Controls MEPS 2005–2015 |
Comorbidities
Individuals with MS were significantly more likely than propensity score-matched non-MS controls to experience musculoskeletal (46.3% vs 31.0%) and psychiatric comorbidities (41.2% vs 19.0%). Comorbidities that were observed to be significantly different between adults with MS and propensity score-matched non-MS controls, respectively, include anemia (5.5% vs 1.8%), anxiety (17.1% vs 8.5%), arthritis (42.1% vs 30.1%), depression (29.7% vs 13.3%), and osteoporosis (5.7% vs 2.4%). Further details regarding the comorbidities can be found in Table 1.
Unadjusted Incremental Health Expenditures
Table 2 displays the unadjusted incremental health expenditures. Yearly average total expenditures (expressed in 2018 US$) were significantly (p<0.001) higher for adults with MS ($29,396) than propensity score-matched non-MS adults ($7875) (Table 2). Emergency room ($718 vs $226), outpatient ($5754 vs $2749), prescription ($15,533 vs $1620), and home health agency ($1989 vs $218) expenditures were also statistically significantly different between adults with MS compared to propensity score-matched non-MS controls.
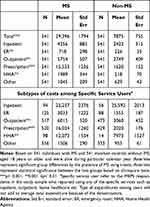 |
Table 2 Total and Subtypes of Costs Among Any Users (2018 US$) |
Unadjusted Total Health Expenditures for Individuals with MS by Comorbidities
Table 3 presents unadjusted total expenditures among adults with MS compared to propensity score-matched non-MS controls in the presence of comorbidities. Individuals with MS and co-occurring arthritis ($33,700 vs $12,345), asthma ($36,147 vs $10,079), cancer ($44,954 vs $22,542), COPD ($31,871 vs $8967), diabetes ($39,579 vs $17,900), eye problems ($36,999 vs $16,092), GERD ($48,536 vs $17,106), heart disease ($40,389 vs $21,148), hyperlipidemia ($34,430 vs $15,393), hypertension ($35,924 vs $12,271), depression ($36,380 vs $17,868), osteoporosis ($37,002 vs $9926), and thyroid disease ($37,196 vs $17,401), had significantly higher total healthcare expenditures than propensity score-matched non-MS controls. Supplementary Tables 1–6 present the differences in subtypes of expenditures among adults with MS and propensity score-matched non-MS controls in the presence of comorbidities. It is noteworthy that the unadjusted health expenditures by comorbidities differed mainly between older adults with MS and propensity score-matched non-MS controls in terms of outpatient (Supplemental Table 3), prescription (Supplemental Table 4), and home healthcare (Supplemental Table 5) expenditures.
 |
Table 3 Unadjusted Total Expenditures by Comorbidities |
Adjusted Incremental Health Expenditures
Table 4 presents the findings from OLS regression analysis of logged healthcare expenditures (total, inpatient, emergency room, outpatient, prescription drugs, home healthcare, and other). After adjusting for all individual-level factors, adults with MS experienced 363% (p<0.001) higher total expenditures compared to propensity score-matched non-MS controls. Similar observations were noted for inpatient, outpatient, and prescription expenditures. Of particular note, prescription expenditures were 1372% (p<0.001) higher among adults with MS compared to propensity score-matched non-MS adults, which probably imply high MS disease-modifying treatment costs in the US. Examination of VIF revealed no sign of multicollinearity in the OLS models (all VIFs were <5).
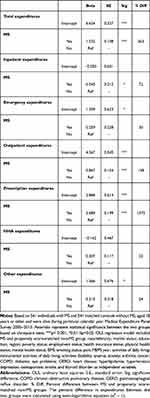 |
Table 4 Intercepts and Parameter Estimates for MS vs Non-MS Groups from Separate OLS Regression Analyses on Logged Healthcare Expenditures (2018 US$) |
Physical and Mental Health Status for Individuals with MS
From Table 5, it can be seen that adults with MS were four times more likely (OR: 4.10, 95% CI: 2.42–6.96) to report fair/poor physical health status compared to excellent/very good physical health status compared with non-MS controls. Presence of depression (OR: 4.81, 95% CI: 2.46–9.42) and anxiety (OR: 2.73, 95% CI: 1.25–5.98) were associated, respectively, with nearly five- and three-fold higher likelihood of reporting fair/poor physical health status compared to excellent/very good physical health status in our study sample. Individuals with MS and co-occurring depression were nearly five times more likely to report fair/poor physical health status than excellent/very good physical health status compared with non-MS controls. Individuals with MS and co-occurring anxiety were nearly three times more likely to report fair/poor physical health status than excellent/very good physical health status compared with non-MS controls. Similar findings were observed for some comorbidities such as heart disease, hyperlipidemia, and COPD.
 |
Table 5 Multinomial Logistic Regression Analysis Physical Health Status |
Table 6 shows the findings from multinomial logistic regression analyses for mental health status. Adults with MS were 42% (OR: 1.42, 95% CI: 1.01–1.99) more likely than propensity score-matched non-MS controls to report good rather than very good or excellent mental health status. However, there was no difference between adults with MS and propensity score-matched non-MS controls in terms of reporting fair or poor than very good or excellent mental health status. Several comorbidities such as cancer, diabetes, hyperlipidemia, hypertension, and depression were significant correlates of fair or poor mental health status. For example, presence of depression was associated with five times more likelihood (OR: 5.01, 95% CI: 2.69–9.31) of report fair/poor mental health status rather than excellent/very good mental health status in our study sample.
 |
Table 6 Multinomial Logistic Regression Analysis Mental Health Status |
Discussion
Findings from this US national-level study indicate that comorbidities significantly influence economic and health status burden among adults with MS. The contribution of this study is significant because it is one of the first steps in a continuum of research that is expected to inform the development of appropriate interventions (such as team-based holistic care approach to MS treatment) to minimize the comorbidity burden among individuals with MS, which has the potential to improve quality of life as well as achieve other improved health outcomes.
Comorbidities
Musculoskeletal and psychiatric comorbidities were most commonly reported among individuals with MS compared to non-MS controls. Specifically, anemia, arthritis, osteoporosis, anxiety, and depression were more commonly reported among individuals with MS. With respect to arthritis and depression, our study is consistent with existing literature that found depression and arthritis to be higher among individuals with MS than the rest of the population.18 According to a European cross-sectional database analysis, those with MS showed a higher prevalence of any mental health (40.3% in MS vs 17.9% in non-MS) and physical (64.7% in MS vs 47.3% in non-MS) comorbidities compared to non-MS controls.33 Another study examining comorbidity incident rates among MS patients before and after diagnosis found a significantly increased incidence rates of osteoporosis and fractures after diagnosis of MS.34 Anxiety and depression are the most commonly cited psychiatric comorbidities found in MS patients.35–46 Thus, our study findings are consistent with these existing studies.
Expenditures
Previous studies about the economic impact of MS on affected individuals showed increased mean annual costs in inpatient services, radiology services, emergency room, office visit, and therapies compared to non-MS controls.20,21,47 However, none of these studies examined the influence of comorbidities on healthcare expenditures among adults with MS. According to a retrospective discharge-level cohort study using hospital data, researchers found a significantly higher mean adjusted cost and incremental cost per hospitalization day in hospitalized MS patients with cardiac conditions compared to MS patients without cardiac conditions.48 However, the study by Franklin et al48 only examined the cardiac comorbidities and their study sample was limited to hospitalized individuals with MS. The current study provides national-level cost information related to a comprehensive list of comorbidities among community-dwelling adults with MS patients and thus more generalizable.
Our study found that several co-occurring chronic conditions contributed to incremental costs differently across different types of expenditures. Total expenditures were higher among individuals with MS when they concurrently had arthritis, asthma, cancer, COPD, diabetes, eye problems, GERD, heart disease, hyperlipidemia, hypertension, depression, osteoporosis, or thyroid disease in comparison to non-MS matched controls. Co-occurring GERD and osteoporosis added the greatest total expenditure among individuals with MS. Asthma and COPD added the third and fourth greatest cost total expenditure among individuals with MS, respectively. Total expenditures were the greatest among individuals with MS when they concurrently had the following comorbidity categories: 1. Other, 2. Cardio-metabolic, or 3. Respiratory compared to non-MS matched controls. Individuals with co-occurring MS and arthritis or any other disease within the musculoskeletal category have significantly higher emergency room expenditures compared with non-MS controls. Individuals with co-occurring MS and asthma or COPD or any other disease within the respiratory category have the greatest outpatient expenditures compared with non-MS controls. While co-occurring osteoporosis and thyroid disease added the greatest prescription expenditures compared with non-MS controls, individuals with a comorbid disease within the respiratory or other category showed the greatest overall prescription expenditures.
Individuals with co-occurring hyperlipidemia have significantly greater home healthcare and other expenditures compared with non-MS controls. Individuals with co-occurring MS and hypertension or hyperlipidemia or any other disease within the cardio-metabolic category have the greatest home healthcare expenditures compared with non-MS controls. Co-occurring osteoporosis or any other disease within the musculoskeletal category added the second greatest home healthcare expenditures compared with non-MS controls. Total expenditures were higher among individuals with MS when they concurrently had arthritis, asthma, cancer, COPD, diabetes, eye problems, GERD, heart disease, hyperlipidemia, hypertension, depression, osteoporosis, or thyroid disease in comparison to matched controls. Co-occurring GERD and osteoporosis added the greatest total expenditure among individuals with MS.
Health Status
Comorbidities in MS can have a variety of effects on health outcomes and clinical decision-making. Existing evidence suggests comorbidities in MS affect treatment decision-making. For example, the more comorbidities that are present among individuals with MS, the less likely healthcare providers are to recommend initiating MS disease-modifying therapies.7 Individuals with MS were significantly more likely to report fair/poor physical and mental health status compared to excellent/very good physical health status compared with non-MS controls. Additionally, the current study found that comorbidities such as the presence of depression negatively affects both physical and mental health status. This is consistent with existing literature that found comorbidities in MS to worsen baseline functional status.49 Depression has been shown to have a greater impact on functional status50,51 and is greatly impacted by modifiable factors52 like diet and exercise. With respect to physical and mental functioning, certain disease combinations have been found to have synergistic, negative additive, or positive effects.53
Co-occurring diabetes, cardiovascular disease, and/or chronic respiratory disease have been found to be synergistically linked with worsening physical functioning in combination.53 Presence of co-occurring depression, anxiety, heart disease, hyperlipidemia, and COPD were associated with higher likelihood of reporting of fair/poor physical health status compared to excellent/very good physical health status. Our findings of co-occurring depression worsening physical health status are consistent with existing literature about the significant impact of depression on functional status among individuals with MS.50,51 Surprisingly, presence of comorbid diabetes or thyroid disease did not show significant differences in physical health status. This conflicts with existing literature that observed diabetes and thyroid disease being synergistic and the most “favorable” or positive chronic diseases with respect to physical functioning, assuming self-perceived physical health status was worse across all chronic diseases.53 Findings from the present study observed that co-occurring heart disease is associated with higher likelihood of reporting fair/poor physical health status, indicating heart disease to have a negative effect on physical health status. This is inconsistent with existing literature that found cardiovascular disease to have a relatively neutral effect on physical functioning. Association of co-occurring COPD with increased likelihood of reporting fair/poor physical health status was consistent with existing literature that found chronic respiratory disease and arthritis to have a relatively negative or unfavorable effect on physical functioning.53
Individuals with co-occurring diabetes, hyperlipidemia, cancer, depression and anxiety were more likely to report fair/poor mental health status compared to excellent/very good mental health status. However, presence of co-occurring thyroid disease did not show significant differences in mental health status. This conflicts with existing literature that observed thyroid disease, across all chronic diseases, having a negative effect with respect to worsening mental functioning.29,53,54 Despite thyroid disease not being found to be a significant predictor of lower mental health status in our study, the negative effect of thyroid disease has been shown to be partly due to gender being a negative effect on mental functions.53 It has been shown that women and those younger in age with a chronic disease generally have a lower mental health status than men and older individuals.53 Although the effect of gender and age on mental functioning was not explored in our study, individuals with MS were more likely to be women and younger in age (18–64 age group) compared to non-MS controls before matching. Future considerations may explore whether age and gender have a negative effect on mental functioning specifically in the context of MS. Similar to that of physical health status, mental health status was determined based on self-perceived health status in our study rather than objective measurement of health status which were used in the existing literature.
Although our findings are not consistent with the health status trends among certain comorbid chronic diseases with respect to their positive, negative, or neutral effects, the nature of these relationships in the context of MS still remains unclear. Despite the clinical manifestation of MS being unique from other chronic diseases, our findings will add to the knowledge base of this disease state and encourage future considerations in areas where information is limited.
While work has been done on the effects of comorbid chronic disease and quality of life, there is still little known about the impacts of comorbidities among individuals with MS. As the aging population and the prevalence of MS grows in the US, preventative measures can be improved upon as the knowledge base about the effects of certain comorbid conditions in MS become clearer. Our study provides a nationwide representation of the prevalence, economic, and health status burden among individuals with MS compared to propensity score-matched non-MS controls.
Strengths of this study are as follows: (i) use of a nationally representative sample of community-dwelling adults and (ii) use of a broad set of individual-level variables including pain. However, limitations of this study are as follows: (i) potential of recall bias as MEPS uses self-reported data; (ii) sequence of comorbidities cannot be obtained; (iii) severity and types of MS (such as Relapsing-Remitting MS) information unavailable; and (iv) inability of establishing cause–effect relationships. However, in order to minimize recall bias, interviews are conducted at regular intervals of 4–5 months by MEPS.24 And lastly, availability of only 3-digit ICD-9-CM codes in the publicly available MEPS dataset may have limited the identification of some specific comorbidities.
Conclusions
Comorbidities among individuals with MS are important considerations as they negatively impact those living with the disease. Despite the importance of understanding comorbidity burden among individuals with MS, only a few studies have been conducted to examine the comorbidity burden among those in the US. Findings from this study indicate substantial economic and health status burden among adults with MS at the US national-level that are significantly influenced by comorbidities.
Acknowledgments
The abstract of this paper accepted for presentation (but not presented due to COVID-19 pandemic) at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Annual International Meeting. Orlando, FL, US. May 2020 as a poster presentation/conference talk with interim findings. Citation for the published abstract is as follows:
Bhattacharjee S, Yegezu Z, Kollecas K, Duhrkopf K, Greene N, Hashemi L. Influence of Comorbidities on Healthcare Expenditures and Perceived Physical and Mental Health Status Among Adults with Multiple Sclerosis: A Propensity Score-Matched US National-Level Study. Abstract published in Value in Health, 2020, 23 (Supplement 1), S283.
Funding
This study was funded by Sanofi Genzyme.
Disclosure
Z Yegezu, K Kollecas, K Duhrkopf served as a contact between Sanofi Genzyme and University of Arizona. K Kollecas, K Duhrkopf, and N Greene are employees and shareholders of Sanofi Genzyme. L Hashemi was an employee of Sanofi Genzyme at the time this article was written. The authors report no other conflicts of interest in this work.
References
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi:10.1212/WNL.0000000000007035
2. Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263–281. doi:10.1177/1352458514564491
3. Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2):117–124. doi:10.1212/01.wnl.0000333252.78173.5f
4. Tettey P, Siejka D, Simpson S, et al. Frequency of comorbidities and their association with clinical disability and relapse in multiple sclerosis. Neuroepidemiology. 2016;46(2):106–113. doi:10.1159/000442203
5. Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–1047. doi:10.1212/WNL.0b013e3181d6b125
6. Berrigan LI, Fisk JD, Patten SB, et al. Health-related quality of life in multiple sclerosis: direct and indirect effects of comorbidity. Neurology. 2016;86:1417–1424. doi:10.1212/WNL.0000000000002564
7. Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology. 2016;86(14):1287–1295. doi:10.1212/WNL.0000000000002543
8. Tarrants M, Oleen-Burkey M, Castelli-Haley J, Lage MJ. The impact of comorbid depression on adherence to therapy for multiple sclerosis. Mult Scler Int. 2011;2011:271321. doi:10.1155/2011/271321
9. Culpepper WJ. The incidence and prevalence of comorbidity in multiple sclerosis. Mult Scler. 2015;21(3):261–262. doi:10.1177/1352458515574151
10. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler. 2015;21(3):282–293. doi:10.1177/1352458514564490
11. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler. 2015;21(3):294–304. doi:10.1177/1352458514564489
12. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305–317. doi:10.1177/1352458514564487
13. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015;21(3):318–331. doi:10.1177/1352458514564485
14. Marrie RA, Reider N, Stuve O, et al. The incidence and prevalence of comorbid gastrointestinal, musculoskeletal, ocular, pulmonary, and renal disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):332–341. doi:10.1177/1352458514564488
15. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler. 2015;21(3):342–349. doi:10.1177/1352458514564486
16. Capkun G, Dahlke F, Lahoz R, et al. Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult Scler Relat Disord. 2015;4(6):546–554. doi:10.1016/j.msard.2015.08.005
17. Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler. 2008;14(8):1091–1098. doi:10.1177/1352458508092263
18. Newland P, Jensen MP, Budhathoki C, Lorenz R. Secondary health conditions in individuals with multiple sclerosis: a cross-sectional web-based survey analysis. J Neurosci Nurs. 2015;47(3):124–130. doi:10.1097/JNN.0000000000000130
19. Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26(2):102–107. doi:10.1159/000090444
20. Earla JR, Thornton JD, Hutton GJ, Aparasu RR. Marginal health care expenditure burden among U.S. civilian noninstitutionalized individuals with multiple sclerosis: 2010–2015. J Manag Care Spec Pharm. 2020;26(6):741–749. doi:10.18553/jmcp.2020.26.6.741
21. Campbell JD, Ghushchyan V, Brett McQueen R, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: national US estimates. Mult Scler Relat Disord. 2014;3(2):227–236. doi:10.1016/j.msard.2013.09.004
22. Sommers J. An examination of state estimates using multiple years of data from the medical expenditure panel survey, household component. 2006: agency for healthcare research and quality working paper no. 06004; 2006. Available from: https://meps.ahrq.gov/data_files/publications/workingpapers/wp_06004.pdf.
23. Agency for Healthcare Research and Quality (AHRQ). Medical expenditure panel survey: medical care provider participants’ corner - confidentiality/HIPAA; 2021. Available from: https://meps.ahrq.gov/communication/participants/confidentialitymcp.shtml.
24. Machlin S, Cohen J, Elixhauser A, Beauregard K, Steiner C. Sensitivity of household reported medical conditions in the medical expenditure panel survey. Med Care. 2009;47(6):618–625. doi:10.1097/MLR.0b013e318195fa79
25. Agency for Healthcare Research and Quality (AHRQ). Healthcare cost and utilization project – HCUP A federal-state-industry partnership in health data (Clinical Classifications Software (CCS) 2014); 2014. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/CCSUsersGuide.pdf.
26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
27. Burea of Labor Statistics (BLS); 2021. Available from:https://www.bls.gov/cpi/factsheets/.
28. MEPS HC-155. 2012 full year consolidated data file. MEPS data documentation: pain definition 2012; 2012. Available from: http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h155/h155doc.pdf.
29. Fortin M, Dubois MF, Hudon C, Soubhi H, Almirall J. Multimorbidity and quality of life: a closer look. Health Qual Life Outcomes. 2007;5:52. doi:10.1186/1477-7525-5-52
30. Vyas A, Pan X, Sambamoorthi U. Chronic condition clusters and polypharmacy among adults. Int J Family Med. 2012;2012:193168. doi:10.1155/2012/193168
31. Meduru P, Helmer D, Rajan M, Tseng CL, Pogach L, Sambamoorthi U. Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J Gen Intern Med. 2007;22(Suppl 3):408–418. doi:10.1007/s11606-007-0310-5
32. James GWD, Hastie T, Tibshirani R. An Introduction to Statistical Learning: With Applications in R.
33. Simpson RJ, McLean G, Guthrie B, Mair F, Mercer SW. Physical and mental health comorbidity is common in people with multiple sclerosis: nationally representative cross-sectional population database analysis. BMC Neurol. 2014;14:128. doi:10.1186/1471-2377-14-128
34. Persson R, Yood MU, Wagner M, et al. Increased risk of comorbidities in patients before and after multiple sclerosis diagnosis and initiation of treatment: a study using the US department of defense database. Neurology. 2019;92(15).
35. Barzola-Castro N, Cepeda-Escalante RE, Jimenez-Zambrano JA, Burgos-Campuzano AD, Acuña-Chong MG. Do anxiety and depression levels interfere with cognitive performance in Ecuadorian multiple sclerosis patients? - A case-control study. Multiple Scleros J. 2019;25:130.
36. Scherder R, Kant N, Wolf ET, Pijnenburg B, Scherder EJ. Psychiatric and physical comorbidities and pain in patients with multiple sclerosis. J Pain Res. 2018;11:325–334. doi:10.2147/JPR.S146717
37. Whitehouse CE, Fisk JD, Bernstein CN, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology. 2019;92:e406–e417. doi:10.1212/WNL.0000000000006854
38. Marrie RA, Walld R, Bolton JM, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28(3):333–342. doi:10.1017/S2045796017000579
39. Leavitt V, Tosto G, Riley C. Cognitive signatures of depression and anxiety in multiple sclerosis. Neurology. 2019;92(15).
40. Latas M, Vucinic latas D, Spasic Stojakovic M. Anxiety disorders and medical illness comorbidity and treatment implications. Curr Opin Psychiatry. 2019;32(5):429–434. doi:10.1097/YCO.0000000000000527
41. Hoang H, Laursen B, Stenager EN, Stenager E. Psychiatric co-morbidity in multiple sclerosis: the risk of depression and anxiety before and after MS diagnosis. Mult Scler. 2016;22(3):347–353. doi:10.1177/1352458515588973
42. Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology. 2015;85(22):1972–1979. doi:10.1212/WNL.0000000000002174
43. Simpson RJ, McLean G, Guthrie B, Mair F, Mercer SW. Physical and mental health comorbidity is common in people with multiple sclerosis: nationally representative cross-sectional population database analysis. BMC Neurol. 2014;14(1).
44. Zhang T, Zullo AR, Shireman TI. Effects of comorbidities on functional decline in nursing home residents with multiple sclerosis. J Am Geriatr Soc. 2018;66:S138. doi:10.1111/jgs.15264
45. Tauil CB, Grippe TC, Dias RM, et al. Suicidal ideation, anxiety, and depression in patients with multiple sclerosis. Arq Neuropsiquiatr. 2018;76(5):296–301. doi:10.1590/0004-282x20180036
46. Janssens AC, Buljevac D, van Doorn PA, et al. Prediction of anxiety and distress following diagnosis of multiple sclerosis: a two-year longitudinal study. Mult Scler. 2006;12(6):794–801. doi:10.1177/1352458506070935
47. Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16(9):703–712. doi:10.18553/jmcp.2010.16.9.703
48. Franklin MA, Happe LE, Dillman R, Marshall LZ. Frequency and economic impact of comorbid cardiac conditions with multiple sclerosis. J Manag Care Special Pharm. 2014;20(8):795–799. doi:10.18553/jmcp.2014.20.8.795
49. Alsallom F, Woodson S, Briggs F, Serra A, Abboud H. Impact of multiple comorbidities on eds at presentation in patients with multiple sclerosis: a cross-sectional study. Neurology. 2018;90(15).
50. Gill S, Santo J, Blair M, Morrow SA. Depressive symptoms are associated with more negative functional outcomes than anxiety symptoms in persons with multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2019;31(1):37–42. doi:10.1176/appi.neuropsych.18010011
51. Golan D, Doniger GM, Wissemann K, et al. The impact of subjective cognitive fatigue and depression on cognitive function in patients with multiple sclerosis. Multiple Scleros J. 2017;24:196–204. doi:10.1177/1352458517695470
52. Gascoyne CR, Simpson S, Chen J, van der Mei I, Marck CH. Modifiable factors associated with depression and anxiety in multiple sclerosis. Acta Neurol Scand. 2019;140(3):204–211. doi:10.1111/ane.13132
53. Rijken M, van Kerkhof M, Dekker J, Schellevis FG. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Qual Life Res. 2005;14(1):45–55. doi:10.1007/s11136-004-0616-2
54. Haggerty JJ, Prange AJ. Borderline hypothyroidism and depression. Annu Rev Med. 1995;46:37–46. doi:10.1146/annurev.med.46.1.37
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


