Back to Journals » International Journal of General Medicine » Volume 16
Inflammatory Markers in Women with Infertility: A Cross-Sectional Study
Authors Duan Y , Zhou Y, Peng Y, Shi X, Peng C
Received 24 January 2023
Accepted for publication 17 March 2023
Published 27 March 2023 Volume 2023:16 Pages 1113—1121
DOI https://doi.org/10.2147/IJGM.S405793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Woon-Man Kung
Yanan Duan,1 Ye Zhou,2 Yiqing Peng,2 Xiuling Shi,2 Cunxu Peng2
1Jining Medical University, Jining, Shandong Province, 272002, People’s Republic of China; 2Department of Gynecology, Affiliated Hospital of Jining Medical University, Jining, Shandong Province, 272029, People’s Republic of China
Correspondence: Cunxu Peng, Affiliated Hospital of Jining Medical University, 89 Guhuai Road, Rencheng District, Jining City, Shandong Province, People’s Republic of China, Tel +86 13385405627, Fax +86 0530-6533898, Email [email protected]
Purpose: Infertility is highly correlated with inflammation. We sought to evaluate the independent relationships between each inflammatory marker in women with infertility.
Patients and Methods: This cross-sectional study included 1028 infertile patients who were hospitalized at Jining Medical University between January 2016 and December 2022. NLR and PLR were the independent and dependent variables measured at baseline, respectively. Age, body mass index (BMI), and menstrual status were covariates. Based on BMI, the study population was split into two groups: Low-BMI and High-BMI.
Results: A stratified analysis revealed that the overweight group had significantly higher levels of WBC, platelet count, lymphocyte count, neutrophil count and NLR. Comparing the overweight group to the normal weight group, the levels were noticeably higher in the overweight group. Significantly positive correlations between NLR and PLR were found in both univariate and multiple regression analyses.
Conclusion: There was a significant positive correlation between NLR and PLR in infertility patients. These results will help in the search for biomarkers of infertility and in the development of infertility prediction models.
Keywords: infertility, inflammation marker, PLR, NLR, lymphocyte count
Introduction
Infertility has been on the rise in recent years, impacting between 8–12% of couples globally who are of reproductive age on an annual basis.1,2 According to the World Health Organization (WHO), infertility is the inability of a sexually active couple to conceive even after a year of trying and no use of contraception.3 It is crucial to understand the etiology of infertility since it has a substantial impact not only on women’s reproductive health but also on couples’ psychological wellbeing and family connections. There is mounting evidence that certain infertility-related illnesses are linked to long-term inflammatory illnesses.4 The inflammatory response is simultaneously influenced by a number of reproductive events, including ovulation, menstrual production, placenta production and implantation, and pregnancy.5–8 It is vital to investigate how the inflammatory response contributes to the etiology of infertility.
The platelet-lymphocyte ratio (PLR) and neutrophil-lymphocyte ratio (NLR) are emerging inflammatory markers. And they are easily assessed from whole blood counts and are an important composite reflection of opposing inflammatory pathways. PLR and NLR may be helpful markers of the body’s systemic immunological and inflammatory condition, according to accumulating evidence.9,10 There are associations between PLR and NLR, and solid tumors and metabolic disorders, respectively, according to a number of earlier research.11–14 On the link between the numerous inflammatory signs in patients with infertility, there is, however, little research. In order to better understand how inflammation contributes to the onset and progression of infertility, the primary goal of this study was to define the relationships between PLR and NLR in women with infertility.
Materials and Methods
Study Design
This cross-sectional study used baseline levels of NLR as independent factors and PLR levels as dependent variables to assess the inflammatory response in infertile patients.
Study Participants
Patients with infertility participated in this study, and information was gathered from the Department of Gynecology at the Affiliated Hospital of Jining Medical University. No identifying information about the participants was accessible, thus data were taken from the hospital’s electronic medical record system in order to maintain patient privacy. Due of the ability to track the study cohort, informed permission was not required. The hospital’s Institutional Review Board gave its approval to the study (approval number: 2022C030). Our research is in line with the Declaration of Helsinki.
Between January 2016 and December 2022, 1028 female patients of reproductive age who were diagnosed with infertility at the Department of Gynecology, Affiliated Hospital of Jining Medical University. The pursuing inclusion standards were applied: (1) infertility as determined by auxiliary tests and preoperative clinical presentation; (2) inability to conceive following a year of regular, unprotected sexual activity. With these criteria, people who: (1) used antibiotics within three months of the procedure; (2) had haematological disorders, malignancies, autoimmune diseases, metabolic diseases, hypersplenism, or active infections; (3) used glucocorticoids, long-term immunomodulatory drugs, or anti-inflammatory drugs; (4) were younger than 18 years old; and (5) were pregnant or nursing were excluded from the study. Last but not least, 320 instances in all were included in the study. The study population was divided into the Low-BMI group (BMI < 24.00 kg/m2) and the High-BMI group (BMI ≥ 24.00 kg/m2) based on BMI values.
Variables
Retrospective data collection was done on patient information such as age, BMI, menstrual status, and regular blood indicators. BMI is calculated by dividing weight (kg) by the square of height (m). The regularity of menstruation, the amount of menstruation and the absence of dysmenorrhoea are obtained by asking the patient during history taking. The criteria for regular menstruation are a menstrual cycle of 21 to 35 days and a period of 4 to 6 days. Those who meet the criteria are considered regular and those who do not are considered irregular. Menstrual flow is judged to be less than 20mL, 20–80mL is normal and over 80mL is too much. During hospitalization, the following routine blood indices were obtained. After an 8-hour fast, peripheral venous blood was collected and processed in the laboratory. Blood indicators were usually obtained 2 days before the procedure. All measurements were taken in our hospital by laboratory technicians and inspectors. Platelet counts, lymphocyte count and neutrophil count were measured using the Sysmex XN2000 blood cell analyzer. In order to calculate PLR, the lymphocyte count was divided by the platelet count, NLR by the neutrophil count respectively. All data were collected using the same blood sample.
The study included the following kinds of covariates: (1) demographic information; (2) previously reported factors affecting the study’s variables; and (3) variables chosen based on clinical knowledge. Consequently, a fully adjusted model was produced using the following variables: Menstrual status (at baseline) is an example of a categorical variable. Continuous variables include age, body mass index (BMI), and standard blood indicators are examples of continuous variables (obtained at baseline).
Statistical Analyses
There are two ways to portray continuous variables: those with a normal distribution are shown as the average standard deviation (SD). Frequencies or percentages are used to express categorical variables. To examine group differences, we utilized the 2 test (for categorical variables) and one-way analysis of variance (for a normal distribution) (quartiles). There were two stages to the data analysis. Three models were created in Step 1 using univariate and multivariate linear regression analyses: Model I (no covariates were adjusted) and Model II (social demographics were the only covariates modified). In Step 2, the nonlinearities of NLR and PLR were resolved using a generalized additive model and smooth curve fitting (punitive spline approach). All analyses were performed using the R statistical package (http://www.R-project.org R Foundation). Statistical significance was set at P values <0.05 (double-sided).
Results
Baseline Characteristics of the Study Participants
The quantitative data collection includes 320 people who met the inclusion criteria (Figure 1). Table 1 displays the baseline characteristics of the patients chosen. Based on BMI values, the study population was separated into two groups: Low-BMI (BMI 24.00 kg/m2) and High-BMI (BMI 24.00 kg/m2). The average age of all participants was 35.76±4.69 years, with roughly 50.63% having multiple UL. Mean white blood cell count, platelet count, lymphocyte count, neutrophil count, PLR and NLR were 5.44±1.65*109/L, 271.27±62.97*109/L, 1.96±0.54*109/L, 3.84±1.60*109/L, 147.49±49.13 and 2.12±1.19. Meanwhile, the mean white blood cell count, platelet count, lymphocyte count and neutrophil count were all higher in the high BMI group than in the low BMI group. In addition, there were statistical differences in BMI, WBC, platelet count, lymphocyte count and neutrophil count between the high BMI and low BMI groups. However, there were no statistically significant differences in PLR and NLR between the two groups.
 |
Table 1 Baseline Characteristics of the Study Population |
 |
Figure 1 Inclusion and exclusion criteria process. |
Univariate Analysis of PLR
Table 2 shows the results of the univariate analysis. Age, BMI, menstrual status and WBC were not correlated with PLR in the study population. Secondly, Platelet Count (β=0.43, 95% CI=0.36, 0.50), Neutrophil Count (β=4.09, 95% CI=0.65, 7.52), NLR (β=19.49, 95% CI=15.46, 23.53) was positively correlated with PLR in the whole study population. Lymphocyte Count (β=−62.83, 95% CI=−70.39, −55.28) was significantly negatively correlated with PLR. Separate analyses of the study population by BMI yielded similar results to the total study population.
 |
Table 2 Univariate Analysis of PLR |
Results of Unadjusted and Adjusted Linear Regression
In this work, two models were developed to examine the separate effects of NLR and PLR (Table 3). In the unadjusted model, NLR was positively associated with PLR (β = 19.49, 95% CI = 15.46, 23.53). After correction for age (model I), NLR remained positively associated with PLR (β=19.24, 95% CI=15.18, 23.30). Subsequently, independent BMI subgroup analyses were performed on the study population, with results for the low BMI group (β=23.24, 95% CI=17.11, 29.36) and the high BMI group (β=15.83, 95% CI=10.56, 21.11) largely similar to those for the whole study population.
 |
Table 3 Relationship Between NLR and PLR in Different Models |
Relationship Between NLR and PLR
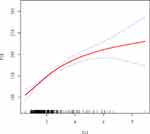
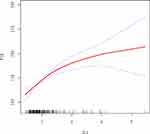
In this study, we investigated the relationship between PLR and NLR (Figure 2). After adjusting for potential confounders such as age, the results of the smoothed fitted curves and generalised additive models showed that PLR increased as NLR increased. Based on the techniques described above, separate smoothed-fit curves were created for low and high BMI patients with infertility, and there was no significant difference in the relationship between NLR and PLR compared to the overall population studied. (Figures 3 and 4) Threshold effects were further investigated based on the curve fits, as shown in Table 4. Curiously, NLR was significantly positively correlated with PLR when NLR < 2.69 (β = 33.38, 95% CI = 23.93, 42.82), whereas NLR was positively correlated with PLR when NLR > 2.69 (β = 9.89, 95% CI = 2.95, 16.83).
 |
Table 4 Threshold Effect Analysis of the Relationship Between NLR and PLR |
Discussion
In this work, saturation threshold analysis, multiple regression analysis and univariate analysis were all used to thoroughly analyse the relationship between PLR and NLR. The results of the study showed that PLR was positively associated with NLR. The relationship was fairly consistent with the entire study population based on BMI-based subgroup analyses. Notably, NLR was significantly positively correlated with PLR when NLR was less than 2.69, but positively correlated with PLR when NLR was greater than 2.69. Therefore, we consider the positive correlation between PLR and NLR to be stable.
The third major disease affecting human life and health today, after cancer and cardiovascular disease, infertility has emerged as a major medical and social issue affecting the reproductive health and development of all humans.2 Tubal factors, ovulation issues, endometriosis, and unexplained infertility are typical reasons of female infertility.3 Of these, polycystic ovarian syndrome (PCOS) is linked to the majority of ovulatory diseases of infertility.15 C-reactive protein (CRP), IL-18, TNF-α, IL-6, white blood cell count (WBC), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1α (MIP-1α) are all noticeably elevated, according to a prior review.16 Numerous studies17,18 have demonstrated that inflammation is linked to PCOS progression and is favorably correlated with markers such PLR, NLR, and SII. The severity of endometriosis adhesions was shown to favorably correlate with PLR, CA125, and co-markers by Guo C et al19 PLR and NLR may be employed as possible blood indicators for endometriosis, according to a number of studies.20–22 Patients who have been given the diagnosis of unexplained infertility or premature ovarian failure also display an imbalanced adaptive immune system and continue to experience chronic inflammation.23 This generally agrees with what we discovered. The increased risk of infertility and subfertility is caused by obesity.3,24,25 As a result, we separated the study population into two groups based on BMI: Low-BMI group and High-BMI group. The results of the subgroup analysis revealed that the overweight group had significantly higher levels of WBC, platelet count, lymphocyte count, neutrophil count and NLR. The connection between PLR and NLR did not exhibit any notable abnormalities in the univariate or multivariate analysis of the two groups.
The mechanism might work like this. Through enhanced NF-kB -p65 phosphorylation, increased expression of the redox family of NADPH oxidases (NOX), and the formation of superoxide (O2), which is then reduced to hydrogen peroxide (H2O2) by the superoxide dismutase, inflammation causes oxidative stress (SOD). Following free movement from the organelle to the cytoplasm, ROS species (O2− and H2O2) stimulate NF-kB -p65 phosphorylation, which raises the release of proinflammatory cytokines like TNF-α and IL-6 and spreads inflammation.
A significant risk factor for the emergence of infertility is inflammation. After correcting for other factors, our results demonstrate that PLR is favorably linked with NLR in infertile patients. Interestingly, the super-rearranged group had significantly greater levels of WBC, platelet count, lymphocyte count, neutrophil count and NLR than the regular group. This implies that the inflammatory response may be crucial to the development and spread of disease. Dynamic monitoring of PLR and NLR may help to determine the progression of infertility and provide a feasible direction for clinical treatment.26
This study’s advantage is that non-linearity is examined in an observational study. Additionally, it has undergone statistical adjustment to significantly lessen impacting influences. Additionally, this study’s extensive data set was successful in minimizing population selection bias.
This study does have certain restrictions, though. First, patients with infertility who had been identified in southwest Shandong, China, made up the study’s participants. Therefore, additional multicenter investigations are required to enhance this study’s generalizability and extrapolation. Second, obesity is a significant factor that affects infertility; however, we just grouped obesity and did not thoroughly examine the connection between obesity and the inflammatory response. Finally, due to distinct pathophysiology, women under the age of 18 were excluded from this investigation.
Conclusion
To our knowledge, this is the first clinical study to examine the relationship between PLR, an indicator of inflammation, and NLR in infertility patients. In addition, this study grouped infertility patients by different BMIs. In women with infertility, this study found a stable positive correlation between PLR and NLR. The results of this study will contribute to the development of future infertility prediction models and provide a new possible attempt to diagnose infertility.
Abbreviations
BMI, body mass index; CI, confidence interval; WBC, white blood cell count; PLR, Platelet-Lymphocyte Ratio; NLR, Neutrophil-Lymphocyte Ratio; PCOS, polycystic ovary syndrome; TNF, tumor necrosis factor; CRP, C-reactive protein; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; NOX, NADPH oxidases; SOD, superoxide dismutase.
Data Sharing Statement
The data used to support the findings of this study have been included in this article.
Ethics Approval
This study was approved by the Jining Medical University’s Affiliated Hospital institutional review board (approval number: 2022C030).
Consent to Participate
The requirement for informed consent was waived given the retrospective nature of this study.
Consent to Publish
Identifying information was removed to protect patient confidentiality.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the TCM Science & Technology Development Plan Project of Shandong Province [grant number 2019-0482].
Disclosure
The authors have no conflict of interests to report.
References
1. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi:10.1016/j.clinbiochem
2. Farquhar CM, Bhattacharya S, Repping S, et al. Female subfertility. Nat Rev Dis Primers. 2019;5(1):7. doi:10.1038/s41572-018-0058-8
3. Carson SA, Kallen AN. Diagnosis and management of infertility: a review. JAMA. 2021;326(1):65–76. doi:10.1001/jama.2021.4788
4. Fabozzi G, Verdone G, Allori M, et al. Personalized nutrition in the management of female infertility: new insights on chronic low-grade inflammation. Nutrients. 2022;14(9):1918. doi:10.3390/nu14091918
5. Akopians AL, Pisarska MD, Wang ET. The role of inflammatory pathways in implantation failure: chronic endometritis and hydrosalpinges. Semin Reprod Med. 2015;33:298–304. doi:10.1055/s-0035-1554916
6. Petraglia F, Arcuri F, de Ziegler D, et al. Inflammation: a link between endometriosis and preterm birth. Fertil Steril. 2012;98:36–40. doi:10.1016/j.fertnstert.2012.04.051
7. Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin Rev Allergy Immunol. 2017;53:40–53. doi:10.1007/s12016-016-8596-x
8. Sotiros A, Thornhill D, Post MD, et al. Inflammatory cytokines, placental pathology, and neurological outcomes in infants born to preterm preeclamptic mothers. PLoS One. 2021;16:e0260094. doi:10.1371/journal.pone.0260094
9. Qin Z, Li H, Wang L, et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: a population-based study. Front Immunol. 2022;13:863640. doi:10.3389/fimmu.2022.863640
10. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. doi:10.3390/molecules25235725
11. Duan Y, Peng Y, Shi X, et al. Correlation between platelet-lymphocyte ratio and neutrophil-lymphocyte ratio in patients with uterine leiomyoma: a cross-sectional study. J Oncol. 2022;2022:3257887. doi:10.1155/2022/3257887
12. Ma W, Cui C, Feng S, et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with newly diagnosed moyamoya disease: a cross-sectional study. Front Neurol. 2021;12:631454. doi:10.3389/fneur.2021.631454
13. Tang Y, Peng B, Liu J, et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front Immunol. 2022;13:975400. doi:10.3389/fimmu.2022.975400
14. Ma L, Zeng A, Chen B, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: a meta-analysis. Int Immunopharmacol. 2019;76:105949. doi:10.1016/j.intimp
15. Glendining KA, Campbell RE. Recent advances in emerging PCOS therapies. Curr Opin Pharmacol. 2023;68:102345. doi:10.1016/j.coph.2022.102345
16. Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22:3789. doi:10.3390/ijms22073789
17. Vitale SG, Fulghesu AM, Mikuš M, et al. The translational role of miRNA in polycystic ovary syndrome: from bench to bedside-A systematic literature review. Biomedicines. 2022;10(8):1816. doi:10.3390/biomedicines10081816
18. Taşkömür AT, Erten Ö. Relationship of inflammatory and metabolic parameters in adolescents with PCOS: BMI matched case-control study. Arch Endocrinol Metab. 2022;2:2359–3997000000497. doi:10.20945/2359-3997000000497
19. Guo C, Zhang C. Platelet-to-lymphocyte ratio and CA125 level as a combined biomarker for diagnosing endometriosis and predicting pelvic adhesion severity. Front Oncol. 2022;12:896152. doi:10.3389/fonc.2022.896152
20. Jing X, Li C, Sun J, et al. Systemic inflammatory response markers associated with infertility and endometrioma or uterine leiomyoma in endometriosis. Ther Clin Risk Manag. 2020;16:403–412. doi:10.2147/TCRM.S232849
21. Mikuš M, Goldštajn MŠ, Brlečić I, et al. CTLA4-Linked autoimmunity in the pathogenesis of endometriosis and related infertility: a systematic review. Int J Mol Sci. 2022;23(18):10902. doi:10.3390/ijms231810902
22. Chen T, Wei JL, Leng T, et al. The diagnostic value of the combination of hemoglobin, CA199, CA125, and HE4 in endometriosis. J Clin Lab Anal. 2021;35(9):e23947. doi:10.1002/jcla.23947
23. Shahbazi M, Ehsani M, Mohammadnia-Afrouzi M, et al. Female unexplained infertility: a disease with imbalanced adaptive immunity. J Hum Reprod Sci. 2019;12:274–282. doi:10.4103/jhrs.JHRS_30_19
24. Mikuš M, Matak L, Vujić G, et al. The short form endometriosis health profile questionnaire (EHP-5): psychometric validity assessment of a Croatian version. Arch Gynecol Obstet. 2023;307(1):87–92. doi:10.1007/s00404-022-06691-1
25. Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;105(8):e2695–709. doi:10.1210/clinem/dgaa285
26. Mikuš M, Vitale SG, Ćorić M, et al. State of the art, new treatment strategies, and emerging drugs for non-hormonal treatment of endometriosis: a systematic review of randomized control trials. Gynecol Endocrinol. 2022;38(11):911–917. doi:10.1080/09513590
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.