Back to Journals » Journal of Inflammation Research » Volume 17
Inflammation and Neurodegeneration in Glaucoma: Isolated Eye Disease or a Part of a Systemic Disorder? - Serum Proteomic Analysis
Authors Okruszko MA , Szabłowski M , Zarzecki M , Michnowska-Kobylińska M, Lisowski Ł, Łapińska M, Stachurska Z, Szpakowicz A, Kamiński KA, Konopińska J
Received 24 September 2023
Accepted for publication 23 January 2024
Published 13 February 2024 Volume 2024:17 Pages 1021—1037
DOI https://doi.org/10.2147/JIR.S434989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Michał Andrzej Okruszko,1 Maciej Szabłowski,1 Mateusz Zarzecki,1 Magdalena Michnowska-Kobylińska,1 Łukasz Lisowski,1 Magda Łapińska,2 Zofia Stachurska,2 Anna Szpakowicz,3 Karol Adam Kamiński,2 Joanna Konopińska1
1Department of Ophthalmology, Medical University of Bialystok, Białystok, 15-089, Poland; 2Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Białystok, Białystok, Poland; 3Department of Cardiology, Medical University of Bialystok, Białystok, Poland
Correspondence: Joanna Konopińska, Medical University of Białystok, Kilinskiego 1 STR, Białystok, 15-089, Poland, TelFax + 48857468372, Fax + 48857468372, Email [email protected]
Introduction: Glaucoma is the most common optic neuropathy and the leading cause of irreversible blindness worldwide, which affects 3.54% of the population aged 40– 80 years. Despite numerous published studies, some aspects of glaucoma pathogenesis, serum biomarkers, and their potential link with other diseases remain unclear. Recent articles have proposed that autoimmune, oxidative stress and inflammation may be involved in the pathogenesis of glaucoma.
Methods: We investigated the serum expression of 92 inflammatory and neurotrophic factors in glaucoma patients. The study group consisted of 26 glaucoma patients and 192 healthy subjects based on digital fundography.
Results: Patients with glaucoma had significantly lower serum expression of IL-2Rβ, TWEAK, CX3CL1, CD6, CD5, LAP TGF-beta1, LIF-R, TRAIL, NT-3, and CCL23 and significantly higher expression of IL-22Rα 1.
Conclusion: Our results indicate that patients with glaucoma tend to have lower levels of neuroprotective proteins and higher levels of neuroinflammatory proteins, similar to those observed in psychiatric, neurodegenerative and autoimmune diseases, indicating a potential link between these conditions and glaucoma pathogenesis.
Keywords: glaucoma, inflammation, neurodegeneration, proteomics, Olink
Introduction
Glaucoma is the most common optic neuropathy and the leading cause of irreversible blindness worldwide. On average, it affects 3.54% (2.09–5.82%) of the population aged 40–80 years.1,2 Although there are many advanced treatment options: pharmacological, laser, or surgery, it is estimated that in 2040 glaucoma will distress 111.8 million people.3 Glaucomatous neuropathy is caused by progressive neurodegeneration of the optic nerve and loss of retinal ganglion cells (RGCs) due to apoptosis,4,5 which is observed as a characteristic pattern of morphological changes of the optic nerve head (ONH). It is primarily the consequence of a persistent increase in intraocular pressure (IOP); therefore, glaucoma treatment aims to reduce IOP. However, glaucoma can also develop in patients with normal IOP. Furthermore, glaucomatous optic neuropathy in some patients is known to progress despite implementing medications or surgical treatment. This suggests that some undefined underlying molecular mechanisms lead to glaucoma onset and progression, which is why the search for new therapeutic approaches is continually underway. Therefore, it is crucial to find biomarkers of glaucoma that would enable timely diagnosis and expand the understanding of disease pathogenesis. Recent articles have proposed that autoimmune, oxidative stress and inflammation may be involved in the pathogenesis of glaucoma.6–8 Some studies have found elevated levels of growth factors or cytokines in aqueous humor and serum, suggesting that inflammation involved in their pathophysiology could thus serve as a biomarker for the pathogenesis, development, or severe course of glaucoma.9–11 Khalef et al9 reported increased levels of IL-6, IL-8, transforming growth factor β1, tumour necrosis growth factor α and serum amyloid A. Vidal-Villegas et al6 found increased levels of IL-12, IL-13, and monocyte chemoattractant protein-1 (monocyte chemotactic and activating factor) in aqueous humor glaucomatous patients.
Neuroprotection is a therapeutic approach to preserve neural condition and utility.5 This area is attracting growing attention in glaucoma as it refers to non-IOP-related actions that enable one to prevent or postpone the apoptosis of RGCs. This may be achieved with neurotrophic factors, which regulate the survival, migration, differentiation, proliferation, and maintenance of several classes of neurons. Few studies indicate a possible connection between brain-derived neurotrophic factor, nerve growth factor, ciliary neurotrophic factor, and glaucoma.12–15
This article overviews 92 inflammatory and neurotrophic factors in the Polish Caucasian glaucomatous patient population and in healthy controls. This work aims to detect agents that may serve as serum biomarkers for glaucoma and their link with other general diseases. Although most studies focus on selected biomarkers associated with glaucoma, this study is the first to investigate such a broad spectrum of inflammatory and neurodegeneration serum biomarkers.
Our study was performed on the basis of Bialystok PLUS Study - a cohort study designed to identify novel risk factors for lifestyle diseases, by investigating a broad spectrum of biological, social, emotional, and environmental factors. It is the only study of its kind in Poland and one of the few worldwide. The aim of the study is to invite 10,000 Bialystok residents aged 20–80 to participate in the study. Participants were randomly selected from the Bialystok City Council registry, using stratified sampling based on sex and age strata (10-year intervals) to ensure a representative gender and age distribution. All participants underwent a detailed and comprehensive health evaluation.
Materials and Methods
Bialystok PLUS Study
We have enrolled in a study 218 participants from the Bialystok PLUS study, a prospective population-based cohort providing broad information on the health of the residents of Bialystok, Poland.16 Data were collected from 02/2019 to 02/2021.
The study population was divided into two subgroups based on the findings of the digital fundography. The glaucoma subgroup consisted of 26 subjects with diagnosed glaucomatous optic neuropathy (20 subjects with both eyes affected and 6 with one eye affected). The healthy individuals group consisted of 192 subjects without any pathological findings in fundography.
We have excluded from study population the individuals diagnosed with a history of psoriasis, rheumatoid arthritis, other types of inflammatory arthritis, systemic lupus, inflammatory bowel disease, systemic scleroderma, and fibromyalgia. Moreover, we have excluded from control group all individuals treated for eye diseases.
The clinical characteristics of the study population are available in Table 1.
 |
Table 1 Clinical Characteristics – Continuous Variables |
Laboratory Analyses
Biochemical assessments were conducted utilizing a Cobas c111 machine (Roche, Basel, Switzerland), while blood morphology was examined using a Mythic 18 machine (Cormay, Warsaw, Poland). Proteomic analyses were carried out employing the Olink Inflammation Panel (Olink Proteomics AB, Uppsala, Sweden). Following a minimum 8-hr fasting period, peripheral vein blood samples were collected. Plasma was obtained through centrifugation at 1810 G for 10 min at room temperature. Subsequently, immediate biochemical tests were conducted using an automated biochemical analyzer (Cobas c111 machine, Roche). Blood count analysis was performed on blood collected in an EDTA anticoagulant tube (Mythic 18 machine, Cormay).
For proteomic analysis, plasma samples were stored in a biobank at −80 ◦C, thawed at 4 ◦C, prepared, and sent to the Olink Proteomics laboratory (Uppsala, Sweden) for analysis. Biomarker levels were measured using the proximity extension assay (PEA) multiplexing method, which relies on quantitative PCR. This method provides relative levels of peptides expressed in NPX units (Normalised Protein eXpression) rather than absolute values of biomarker concentrations.
Digital Fundography
All participants underwent nonmydriatic fundography with a 35O digital colour fundus camera (Canon CR-2 PLUS AF, New York, NY, USA). Digital colour images and autofluorescence images were taken for each eye. The image evaluation was performed by trained specialists (ŁL, MMK). This included an evaluation of the optic nerve, macula, and central vessels with their branches visible in field photography. The protocol for detailed evaluation of ONH included its dimensions, shape, colouring, vascularization, tilting, evaluation of neuroretinal rim and cup-to-disc ratio, presence of optic disc haemorrhages and assessment of parapapillary atrophy.
The diagnosis of glaucomatous optic neuropathy was based on characteristic optic disc changes observed on stereoscopic photographs, which was proposed as the best reference standard for disease diagnosis according to the European Glaucoma Guidelines.17 To evaluate the damage to ONH, the Disc Damage Likelihood Scale (DDLS) was used.18,19 ONH with grade DDLS ≥6 was classified as glaucomatous. In addition, special attention was drawn to signs of focal damage to the optic nerve disc, such as optic disc hemorrhages, peripapillary atrophy, and the thickness of the retinal nerve fibre layer.20,21 Fundography findings were coded per eye and summed to achieve binary information per patient. (Figures 1-4).
 |
Figure 1 Funduscopic image of glaucomatous optic disc in 74-year female (right eye). |
 |
Figure 2 Funduscopic image of glaucomatous optic disc in 72-year male (right eye). |
 |
Figure 3 Funduscopic image of glaucomatous optic disc in 65-year female (right eye). |
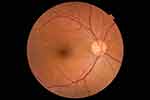 |
Figure 4 Funduscopic image of healthy optic disc in 61-year female (right eye). |
Statistical Analyses
The Shapiro–Wilk test was performed on continuous variables. Subsequently, clinical characteristics were compared with Welch’s t-test for normally distributed continuous variables, Mann–Whitney U-test for non-normally distributed continuous variables, and chi-square tests for categorical variables. Protein expressions are compared with Generalized Linear Model. All models were controlled after fitting. We assumed three models per protein: Model-1 - without covariate correction, Model-2 - with sex, age, and current tobacco smoking status correction and the final Model-3 with sex, age, current tobacco smoking status, diabetes mellitus, and hypertension correction. The statistical significance was established as p < 0.05. The R software was used to perform analyses.22 All results are available in Supplement 1.
Results
Clinical Characteristics of Groups
Glaucoma patients, more frequently had diabetes (p = 0.02) and hypertension (p = 0.02) and also had higher counts of white blood cells (p = 0.046) and granulocytes (p = 0.025) than healthy controls. Detailed data are available in Table 1 and Table 2.
 |
Table 2 Clinical Characteristics – Categorical Variables |
Serum Proteomics
In the final Model-3 patients with glaucoma had significantly lower serum expression of
IL-2Rβ (coefficient = −0.215, p = 0.005), TWEAK (coefficient = −0.146, p = 0.020), CX3CL1 (coefficient = −0.145, p = 0.021), CD6 (coefficient = −0.194, p = 0.025), CD5 (coefficient = −0.137, p = 0.026), LAP TGF-beta1 (coefficient = −0.163, p = 0.026), LIF-R (coefficient = −0.091, p = 0.035), TRAIL (coefficient = −0.128, p = 0.040), NT-3 (coefficient = −0.121, p = 0.044), CCL23 (coefficient = −0.168, p = 0.048) and significantly higher expression of IL-22Rα1 (coefficient = 0.294, p = 0.007).
Detailed data are available in Table 3.
 |
Table 3 Serum Proteomics – Olink Inflammation Panel |
Discussion
Blood Smear Differences
We reported a higher total count of white blood cells and granulocytes in the blood of glaucoma patients compared to healthy individuals. The results confirm peripheral blood smear disturbances that have been widely described in the literature.24–28 Patients with various types of glaucoma tend to have a higher neutrophil count, generally reported to have a higher neutrophil-to-lymphocyte ratio. We have not measured the fractions of granulocytes, although our findings probably depend on the neutrophil count, as they are the main fraction of granulocytes.24–28
Diabetes Mellitus and Hypertension
Our research found a significantly higher occurrence of diabetes in glaucoma than in the control group. Literature suggests that diabetic patients are nearly 1.5 times more likely to develop glaucoma than non-diabetic ones.29 Moreover, the risk of glaucoma increases by 5% each year of diabetes. Diabetes’ impact on glaucoma development has been studied thoroughly. The current theory about the association between these two diseases is that diabetes leading to microvascular damage and vascular dysregulation of the retina and the optic disc is causing glaucomatous damage to the optic nerve head. Diabetes also may disrupt the trabecular meshwork function, thereby elevating IOP.30,31
We have also shown that elevated blood pressure (BP) was significantly more often in the glaucoma group. These results coincide with the literature data – when untreated – hypertension results in a greater risk for open-angle glaucoma.32 Meta-analyses show a risk ratio of 1.69 (95% CI 1.50 to 1.90) in the hypertension group.33
IL-2Rβ
Interleukin 2 receptor subunit beta, also called IL-2Rβ or CD122, is a subunit common to Interleukin 2 receptor (IL-2R) and Interleukin 15 receptor (IL-15R), playing an essential role in the human immune system, taking part in the process of proliferation, activation, differentiation, and survival of T-cells, altogether maintaining the autoimmune homeostasis. Being shared with mentioned cytokine receptors, it belongs to the hematopoietin receptor family, also called type I cytokine receptor superfamily. IL-2Rβ, along with alpha (IL-2Rα/CD25) and gamma (γc/CD132) chains, forms heterotrimeric IL-2R complex (IL-R2αβγc) binding Interleukin 2 (IL-2) with high affinity.34–36
IL-2Rβ is constitutively expressed on the surface of resting or activated natural killer cells (NK), cytotoxic T cells (Tc), regulatory T cells (Treg), B cells, macrophages, monocytes, eosinophils, and dendritic cells (DC).
Furthermore, concentration of subunit correlated negatively with common biomarkers of inflammation: C-reactive protein (CRP), and procalcitonin (PCT), as well as with acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scores, reflecting severity of the disease. Some authors draw attention to IL2RB gene (encoding IL-2Rβ) as a potential biomarker and target for novel sepsis therapy.37–41
The role of expression and active signalling of IL-2Rβ in ophthalmology has been pretty well studied, but mainly in the context of other diseases (dry eye disease, ocular sarcoidosis, ocular malignancies) than glaucoma.42–46
Elevated concentration of soluble form of IL-2R (sIL-2R) or IL-2Rβ (sIL-2Rβ) can be detected in serum of individuals in course of inflammatory or pseudoinflammatory conditions, which corresponds to its fundamental role. In general adult population study, Alende-Castro et al distinguished potential factors influencing serum sIL-2R concentration.47 Individuals over 65 years of age, males, smokers, individuals with atopy, metabolic syndrome, or a history of ischemic heart disease presented higher serum values of the receptor than the standard reference range.
Elevated serum/plasma concentrations of sIL-2R and sIL-2Rβ are observed in the course of many systemic conditions. Cai et al reported increased sIL-2R plasma levels in patients with Diabetes Mellitus type 2 (DM2).48 Furthermore, the authors noted a negative correlation between plasma sIL-2R concentration and high-density lipoprotein (HDL), low-density lipoprotein (LDL) and cholesterol levels. Interestingly, sIL-2R seems to be a valuable biomarker for the assessment of sarcoidosis diagnosis, chronicity, remission, and treatment monitoring.49–53 Furthermore, elevated serum/plasma concentration of sIL-2R/sIL-2Rβ and polymorphisms of IL2RB gene have also been associated with depression, Parkinson’s disease, and several other autoimmune or cardiovascular diseases.54–71
Interestingly, there is a lack of evidence on possible causes of lowered serum sIL-2R and sIL-2Rβ.
Available serum proteomic results made on glaucoma patients are opposite to our findings. Yang et al reported raised mean concentration of sIL-2R in the serum of patients with primary open-angle glaucoma (POAG) and normal pressure glaucoma (NPG), compared to healthy individuals.72 Interestingly, authors did not observe aberrations in IL-2 levels among control and study groups. Contrary to these findings, Huang et al demonstrated comparable serum sIL-2R and IL-2 concentrations in healthy controls and patients with various severity of POAG.73 Whereas in our study, the participants with glaucomatous neuropathy presented lower serum expression of sIL-2Rβ compared to the control group. However, we need to underline one essential difference in our research model. In their studies, Yang et al and Huang et al detect a complex of sIL-2R (containing alpha, beta, and gamma chains), while we measured the concentration of its single beta subunit.72,73 Our approach seems to deliver more detailed and concretized data about the potential role of the immune system in the pathogenesis of glaucoma.
Upon activation, natural killer (NK) cells could undergo surface receptor consumption through processes such as receptor internalization, degradation, or regulation of receptor gene expression. We speculate that decreased sIL-2Rβ serum levels could potentially correspond with greater subunit utilization by activated NK and T cells, underlining the systemic nature of the disease.74
IL-22Rα1
Interleukin 22 Receptor Subunit Alpha 1 (IL-22Rα1) is a long-chain transmembrane protein, representative of the type II cytokine family, highly specific for Interleukin-22 (IL-22), Interleukin-20 (IL-20), and Interleukin-24 (IL-24). Unlike IL-22, alpha 1 subunit secretion is highly tissue-specific and expressed only by certain nonimmune epithelial cells, fibroblasts, hepatocytes, and pancreatic stellate cells, but also tissues of the digestive and respiratory systems. Interestingly, IL-22Rα1 expression was also discovered in the ocular surface of mice and trabecular meshwork cells.75,76 IL-22 receptor (IL-22R) consists of IL-22Rα1 and IL-10Rβ subunits. A high-affinity initial binding of IL-22 to IL-22Rα1 is followed by recruitment of IL-10Rβ to stabilise the ligand/receptor complex. The heterodimeric receptor mediates IL-22-activated downstream signalling through JAK/TYK2/STAT3, NF-κB, MAPK, and Akt pathways, playing an essential role in epithelial integrity and homeostasis, stimulation of tissue repair and resistance against extracellular pathogens.77–79
In systemic disorders, the increased expression of IL-22Rα1 constitutes an essential part of the neuroprotective cascade induced by IL-22 during many liver conditions. IL-22Rα1 mediated signalling attenuates fibrosis caused by increased activity of hepatic stellate cells, inhibits cell apoptosis, and promotes mitosis of the hepatocytes.80
On the other hand, IL-22Rα1 overexpression may become a valuable marker and potential treatment target for certain rheumatoid diseases.81 Interestingly, overexpression of IL22RA1 was also associated with psoriasis, and therefore inhibition of Il-22Rα1 signalling, both on genetic and proteomic levels, may constitute a novel therapy for the disease.82,83
Regarding genetics, IL2RA1, the gene encoding IL-22Rα1, seems to be an important target for future diagnostic and therapeutic processes of malignant conditions. Zhang et al identified 11 types of cancer associated with upregulated transcript levels of IL22RA1.84 The authors noted that patients’ poor survival was linked to greater gene expression. IL22RA1 polymorphisms were also linked with chronic rhinosinusitis occurrence and severity.85,86
Concerning ocular conditions, increased expression of Il-22Rα1 by retinal glial Müller cells was reported in the posterior segment of mice globes with experimentally induced autoimmune uveitis. IL-22-induced signalling inhibits pro-inflammatory and promotes anti-inflammatory pathways, improving the survival of retinal ganglion cells (RGC) and enhancing the neurotrophic and neuroprotective effects of upregulated glial cell-derived neurotrophic factor and nerve growth factor.87 Contrary to these findings, Lindborg et al suggested that IL-22 secreted by neurons (RGCs or other neuronal cells) may restrict axon development through paracrine signalling via immune or glial-expressed IL-22Rα1.88 IL-22Rα1 was previously described as a potentially important factor in pathogenesis of DR and fungal keratitis, but there is a lack of evidence on its role in glaucoma pathogenesis.79,89,90
Our study shows the increased serum expression of IL-22Rα1, which is indirectly consistent with the research conducted by Zhao et al.91 The overexpression of IL22RA1 in human Tenon’s capsule fibroblasts of individuals with glaucoma is related to intensified cell migration, proliferation, fibrosis, and autophagy, modulated by NR_003923 – long noncoding RNA (lncRNA). Summarized effects of increased activity of IL22RA1 and NR_003923 were associated with glaucoma progression. The above findings may be a valuable link to understanding the true pathophysiology of glaucoma, developing novel therapeutic targets, and improving glaucoma surgery outcomes.89,92
TWEAK and TRAIL
Tumor necrosis factor-related weak inducer of apoptosis (TWEAK) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) are members of the tumor necrosis factor superfamily (TNFSF). TNFSF members are involved in inflammatory and autoimmune pathways, including nervous system diseases.
TWEAK is a cytokine regulating multiple processes such as cell survival and proliferation, angiogenesis, and induction of other cytokines. It is expressed in multiple tissues, on immune cells and endothelial cells, and it arises as soluble TWEAK (sTWEAK) after cleavage. Membrane-bound as well as soluble forms of TWEAK act via receptor-fibroblast growth factor-inducible protein 14 (Fn14), activating the ERK, PI3K/Akt, and NF-κB signalling pathways.93–95
Interestingly, in rodents, the Fn14 expression in excitatory thalamocortical neurons of the dorsal lateral geniculate nucleus is induced by the vision expression signals from the retina. The lack of Fn14 provides physiological deficits and changes in synaptic morphology.96 Actions mediated by TWEAK-Fn14 seem to be important in microglial-regulated postsynaptic remodelling in the dorsal lateral geniculate nucleus.97
Considering eye diseases, the TWEAK-Fn14 pathway role was reported to be important in retinal pathologies – mainly neovascularization.98–100 Current evidence shows only local effects of TWEAK overexpression, which is difficult to match with lowered serum TWEAK expression that we report.
Serum/plasma levels of sTWEAK were reported in multiple inflammatory and autoimmune diseases. Maarouf et al reported statistically higher sTWEAK serum levels in patients with multiple sclerosis than in healthy controls, especially during relapses of the disease.101
Karadag et al reported significantly higher plasma concentration of sTWEAK in patients with bipolar depression than in healthy controls, but not between patients with major depression and healthy controls or bipolar depression patients.102 Contrary to these findings, Schmidt et al and Melin et al reported decreased sTWEAK in patients with depression compared to healthy controls, although research conducted by Melin et al was conducted on patients with depression and type 1 diabetes.103,104
In our results, we report lowered sTWEAK in glaucoma patients compared to the controls, which could be evidence of a link between glaucoma and depression, especially since this type of link has been previously established by Gubin et al who reported that the severity of depression is positively related to RGCs loss.105
Multiple publications report lowered sTWEAK as a biomarker of atherosclerosis-associated diseases, which could be a possible link with the atherosclerotic theory of glaucoma.106–110
TRAIL is a cytokine that plays an important role in regulating innate and adaptive immune systems. Similarly to TRAIL and other TNFSFs, it can be expressed as a trans-membrane protein (ie on epithelial, mucosal cells, neurons, and oligodendrocytes) and as a soluble form (sTRAIL). TRAIL could act via multiple receptors – DR4 and DR5 to induce caspase-dependent apoptosis, DcR1, DcR2, and osteoprotegerin to activate the NFκB pathway.93,111,112
TRAIL also acts as a suppressor of autoimmunity via inducing Th1 cells apoptosis, which changes the ratio of Th1/Th2 cells because Th2 cells are more resistant to TRAIL-induced apoptosis. Moreover, it promotes the growth of Treg cells.113,114 Regarding our results, the lowered serum sTRAIL expression in glaucoma patients seems to be difficult to interpret because, in the available evidence, patients with glaucoma tend to have lower concentrations of the circulating Th1 cells and increased concentrations of Tregs – what theoretically should be supported with TRAIL overstimulation.115,116 Although our results are based only on circulating sTRAIL, thus we cannot evaluate trans-membrane TRAIL expression on cells. Unfortunately, we did not assess circulating levels of Th1, Th2, and Treg cells to compare with the available evidence.
In the context of eye diseases, TRAIL seems to be important in ophthalmic oncology – also as an additional treatment to traditional medications. It also acts as an antiangiogenic cytokine, which might be crucial in neovascular retinal diseases such as proliferative diabetic retinopathy (PDR). Interestingly, patients with PDR have decreased expression of sTRAIL in the conjunctival sac fluid. On the other hand, TRAIL might also have a dark side – mutations in DR4 receptor genes and low serum levels of DcR1 receptor provide a higher risk for age-related macular degeneration development.111
Serum/plasma proteomic research also shows a possible connection between sTRAIL concentration and autoimmune and cardiometabolic diseases. Moreno et al's findings have not shown differences in serum sTRAIL concentration between patients with MS and healthy individuals, but they have noticed significant sTRAIL decrement during MS relapses.117 They suggested the sTRAIL concentration decrement could relate to enhanced survival of pathogenic T lymphocytes.
Interestingly, Cheng et al reported that serum sTRAIL concentrations positively correlate with total cholesterol and LDL, while Mori et al have found a negative correlation between serum sTRAIL and coronary artery disease severity.118,119
Regarding our results, lowered sTRAIL serum expression might be a supporting argument for the autoimmune, neuroinflammatory, and atherosclerotic pathways in glaucoma development.
CX3CL1
C-X3-C motif chemokine ligand 1 (CX3CL1), known as fractalkine, is a chemokine mainly expressed by neurons. It has an interesting profile of action because it could act pro- and anti-inflammatory. Moreover, it is a chemoattractant for immune cells. It is expressed by microglia, natural killer cells, dendritic cells, monocytes, T cells, and macrophages. Fractalkine is probably involved in pathways of neuroinflammatory diseases.120 Animal models showed that fractalkine signalling is important in glaucoma RGCs protection and reduction of inflammatory cytokines in diabetic retinopathy.121,122 Interestingly, inhibition of fractalkine signalling via blockade of the fractalkine receptor – CX3CR1 improved the recovery of neurological function in the rat model of spinal cord injury.123
Begum et al reported increased serum expression of fractalkine in athletes with sport-related traumatic brain injury, hypothesizing that fractalkine increases microglia activity in response to neurotoxicity and protects them from apoptosis.124 In clinical studies on patients with Alzheimer’s, serum expression of fractalkine was decreased and correlated with the level of cognitive impairment.125
In the context of our study, the decreased serum expression of fractalkine in glaucoma patients could be interpreted as reduced neuroprotective abilities and a possible link with Alzheimer’s disease.
CD5 and CD6
Cluster of differentiation 5 (CD5) is a transmembrane protein expressed mainly on the surface of T-cells and B-1a cells. Expression of CD5 is upregulated during T-cells’ strong activation. Due to this, CD5 remains a good immunohistochemical marker for T-cells, although less sensitive than the currently used CD3. For this reason, CD5 should be considered an indirect marker of T lymphocyte activity, including autoimmune activity, consistent with the autoimmune theory of glaucoma development.126
It is suggested that CD5 negatively regulates TCR signalling from the onset of T-cell activation and therefore plays a key role in regulating cell survival and apoptosis. It could even inhibit the development of autoimmune reactions or cancer.127
In addition, circulating, soluble forms of CD5 (sCD5) could be found in healthy individuals’ serum in the pico/nanomolar range due to proteolytic cleavage following lymphocyte activation. Furthermore, elevated levels of soluble CD5 (sCD5) have been detected in the serum of patients with various autoimmune and inflammatory conditions (Sjogren syndrome, septic syndromes, atopic dermatitis, and various rheumatoid diseases).128–133 However, the exact importance of the release of sCD5 following lymphocyte activation remains unknown, and it needs further research.133
Due to those mentioned above, we could speculate if sCD5 and CD5 expression levels inhibit autoimmunity and inflammation development. Our study shows a decreased serum expression of CD5 in glaucoma patients, supporting this notion.
In our research, we have also found a decreased soluble CD6 serum expression. Literature has no direct association between CD6 expression and glaucoma or neurodegeneration.
We speculate that decreased CD6 expression in the glaucoma group could suggest inhibited T-cell activation because of counterregulatory, anti-inflammatory mechanisms, similar to CD5.
LAP TGF-beta1
Transforming growth factor-beta 1 (TGF-β1) is the multifunctional polypeptide cytokine that takes part in the regulation of cell proliferation, growth, differentiation, and apoptosis, especially in bones but not only TGF-B1 is secreted as a latent form as a dimer consisting of latency-associated peptide (LAP) and TGF-β1.
Smag proteins inhibit TGF-β1 signalling. This counter-regulation plays an important role in eg inflammatory bowel disease, fibrosis, and cancer (especially colorectal cancer).134
The association between increased expression of proteins from the transforming growth factor beta family and glaucoma and DR is well established. Few studies show elevated TGF1 alpha and beta concentrations in aqueous humour and vitreous fluid. TGF-β1 plays a crucial role, eg in scar formation after glaucoma surgery.106
TGF-β1 is also associated with the pathogenesis of pseudoexfoliation glaucoma since it activates TGF-βpathway.135
In neuroinflammation and neurodegeneration, TGF-β1 is linked with possible Alzheimer’s disease pathogenesis. TGF1 beta is involved in neuronal development and synaptic plasticity as a trophic factor. Impaired TGF-β1 signalling results in increased Amyloid β deposition and enhanced neurodegeneration.136
Chronic inflammation and ageing-associated processes reduce TGF-β1/Smad signalling, resulting in cytotoxic activation of microglia and further neurodegeneration.
High levels of TGF-β1 have been associated with increased production of extracellular matrix components, such as fibronectin and collagen, which can lead to excessive deposition and remodelling of the extracellular matrix in the trabecular meshwork and optic nerve head.
In our glaucoma study group, we have found that a decreased expression of LAP-TGFβ-1 seems reasonable – higher free TGFB1 expression in glaucoma patients results in a decreased propeptide expression due to increased dissociation of TGFB1.
Soluble LIF-R
Leukemia Inhibitory Factor Receptor Complex (LIF-R) is a receptor for Leukemia Inhibitory Factor (LIF) and other cytokines such as Oncostatin M, Cardiotrophin-1, Ciliary Neurotrophic Factor, Cardiotrophin-Like cytokine. LIF-R expressed on cell membrane provides a signalling pathway for mentioned cytokines, but interestingly soluble LIF-R (sLIF-R) plays a different role. sLIF-R binds circulating LIF and inhibits signal transduction from LIF to membrane-bound LIF-R. It is hypothesized that sLIF-R prevents the generalized action of LIF, allowing LIF to bind only to local receptors.137
How sLIF-R interacts with other ligands for membrane-bound LIF needs to be clarified. Available evidence reports only interaction between Oncostatin M and soluble gp130 unit, which is part of the LIF-R complex. High concentrations of soluble gp130 could attenuate LIF and Oncostatin M mediated proteoglycan resorption in cartilage tissue.138
LIF and other LIF-R receptor ligands were reported as neuroprotective proteins for retinal cells in animal models.139–141
Referring to our results, decreased sLIF-R serum expression in glaucoma patients could result in generalized and stronger action of LIF and probably other LIF-R ligands, which could result in intensified neuroprotection. However, we have not noticed a significant difference in LIF and Oncostatin M expression between glaucoma patients and healthy individuals. Unfortunately, expressions of other ligands were not investigated in our research. sLIF-R serum expression was not examined for diseases caused by inflammation or neurodegeneration. Thus, possible connections with glaucomatous optic nerve degeneration are difficult to be assessed.
NT-3
Neurotrophin-3 (NT3) is a member of the neurotrophin family, which are proteins essential for appropriate nervous system functioning. They play an important role in neuron development, survival, and synaptic plasticity.142 Interestingly, NT-3 is not a tissue-specific protein, and its highest NT-3 RNA expression was reported in female reproductive system tissues.143
The available literature referring to the involvement of neurotrophins in glaucoma pathophysiology mainly focuses on the failure of axonal transport in RGCs, which potentially results in disturbed retrograde transport of neurotrophins from target cell to RGC soma, and anterograde transport of neurotrophin receptors from RGCs soma to axon termini.144
Neurotrophins interact with tropomyosin receptor kinase (Trk) receptors A, B, and C with different binding affinities. The most selective Trk receptor for NT-3 is TrkC. All neurotrophins could also influence processes of survival/apoptosis via p75 - “death receptor”. Furthermore, p75 receptor expression regulates Trk activities by determining when NT-3 binds with TrkA (which typically has a lower affinity to bind NT-3 than TrkC).145 Interestingly, TrkA and TrkC could trigger neuron death in NT-3 deficient environment, and adding NT-3 could prevent that process.146
Referring to The Human Protein Atlas and the transcriptome atlas of the adult human retina, TrkC receptor RNA is expressed in Rod photoreceptors, Muller glia, bipolar cells, horizontal cells, and RGCs.147,148
An optic nerve transection model showed that expression ratios between Trk receptors mRNA in RGCs change over time after cell damage.149 Results from another study show that a change in Trk mRNA expression does not have to be accompanied by a change in protein expression. TrkC mRNA and protein expression decline in the described IOP-induced injury model but TrkB mRNA declines without change in TrkB protein expression after injury.150 Ogłodek et al reported that NT-3 serum concentrations were lower in patients with depression than in healthy controls and were inversely related to the severity of symptoms, which is further supported by the results by Gubin et al, mentioned above.105,151
Concluding both publications, we could hypothesise that NT-3 may be a link between glaucoma and depression.
Our findings of reduced serum expression of NT-3 in glaucoma patients could be a new piece in the glaucoma pathophysiology puzzle, in addition to pointing to a probable link with depression.
CCL23
CC-chemokine ligand 23 (CCL23) cytokine, which mechanisms of action are poorly described due to the lack of this cytokine in rodent models. In research on human diseases, CCL23 was described mainly in the context of oncological and haematological diseases.152 Shih et al reported that CCL23 suppresses the production and release of polymorphonuclear leukocytes and monocytes, which might explain why glaucoma patients in our research had increased granulocyte count compared to the healthy controls.53
In the context of inflammation and neuroinflammation-driven pathology in diseases, current proteomic reports showed only increased levels of CCL23 in patients. Dallas Heart Study Report showed elevated plasma levels of CCL23 were associated with coronary artery calcium.153 At the same time, Faura et al found that higher plasma levels of CCL23 predict the progression of mild cognitive impairment to Alzheimer’s disease.154 Therefore, based on current evidence, lowered serum expression of CCL23 could be only linked with increased granulocyte count, which the main subgroup – neutrophils were reported to be increased in all types of glaucoma, as we described above.
Limitations
The main limitation of our study is using quite a limited evaluation of the optic nerve head based only on fundography, which is an imperfect diagnostic method. Advanced imaging solutions would make the diagnosis of glaucoma more reliable; however, several studies have shown that DDLS is strongly correlated with the degree of glaucomatous visual field damage and other diagnostic modalities. Moreover, according to the literature, the DDLS system has a good interobserver agreement (Cohen’s kappa 0.902),19 and research suggests that DDLS is a more accurate method of detecting and monitoring glaucomatous disc changes compared to other scales.155,156 Therefore, DDLS is recommended for glaucoma detection schemes and referral guidelines. Another limitation is the relatively small study group; however, the number of glaucoma subjects reflects the general population.
Conclusion
In summary, our research indicates that patients with glaucomatous neuropathy tend to have lower levels of neuroprotective proteins and higher levels of neuroinflammatory proteins. However, identifying a single serum biomarker as a reliable tool for diagnosing and monitoring glaucomatous neuropathy seems to be impossible at this stage of knowledge. Future, more comprehensive research should focus on identifying a certain risk phenotype associated with glaucoma, including specific pro-inflammatory and neuroprotective factors. Additionally, similar proteomic changes have been observed in psychiatric, neurodegenerative, and autoimmune diseases, indicating a potential link between these conditions and glaucoma pathogenesis. Further studies are needed to explore this possible connection and develop new therapeutic strategies for glaucoma and possibly related neurodegenerative disorders. Proteomics appears to be a promising avenue in searching for new glaucoma medications. Interestingly, in this context, only the proteome of retinal cells has been studied so far.157 Therefore, our work provides potentially new insights from the perspective of the peripheral proteome.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shpak AA, Guekht AB, Druzhkova TA, Troshina AA, Gulyaeva NV. Glial cell line-derived neurotrophic factor (GDNF) in patients with primary open-angle glaucoma and age-related cataract. Mol Vis. 2022;28:39–47.
2. Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021;105(3):493–510. doi:10.1016/j.mcna.2021.01.004
3. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
4. He S, Stankowska DL, Ellis DZ, Krishnamoorthy RR, Yorio T. Targets of Neuroprotection in Glaucoma. J Ocul Pharmacol Ther. 2018;34(1–2):85–106. doi:10.1089/jop.2017.0041
5. Kuo CY, Liu CJL. Neuroprotection in Glaucoma: basic Aspects and Clinical Relevance. J Personalized Me. 2022;12(11):1884. doi:10.3390/jpm12111884
6. Vidal-Villegas B, Burgos-Blasco B, Santiago Alvarez JL, et al. Proinflammatory Cytokine Profile Differences between Primary Open-Angle and Pseudoexfoliative Glaucoma. Ophthalmic Res. 2021;65(1):111–120. doi:10.1159/000519816
7. Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Decreased total antioxidants status in the plasma of patients with pseudoexfoliation glaucoma. Mol Vis. 2011;17:2769–2775.
8. Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45(4):367–376. doi:10.1016/j.freeradbiomed.2008.04.026
9. Khalef N, Labib H, Helmy H, El Hamid MA, Moemen L, Fahmy I. Levels of cytokines in the aqueous humor of eyes with primary open angle glaucoma, pseudoexfoliation glaucoma and cataract. Electron Physician. 2017;9(2):3833–3837. doi:10.19082/3833
10. Chono I, Miyazaki D, Miyake H, et al. High interleukin-8 level in aqueous humor is associated with poor prognosis in eyes with open angle glaucoma and neovascular glaucoma. Sci Rep. 2018;8(1):14533. doi:10.1038/s41598-018-32725-3
11. Yin Z, Gao Y, Tang Y, Tian X, Zheng Y, Han Q. Aqueous humor cytokine levels are associated with the severity of visual field defects in patients with primary open-angle glaucoma. BMC Ophthalmology. 2023;23(1):141. doi:10.1186/s12886-023-02875-8
12. Schuettauf F, Vorwerk C, Naskar R, et al. Adeno-associated viruses containing bFGF or BDNF are neuroprotective against excitotoxicity. Curr Eye Res. 2004;29(6):379–386. doi:10.1080/02713680490517872
13. Lambiase A, Aloe L, Centofanti M, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106(32):13469–13474. doi:10.1073/pnas.0906678106
14. Ji JZ, Elyaman W, Yip HK, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19(2):265–272. doi:10.1111/j.0953-816x.2003.03107.x
15. Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002;110(3):555–567. doi:10.1016/s0306-4522(01)00557-7
16. Chlabicz M, Jamiołkowski J, Łaguna W, et al. A Similar Lifetime CV Risk and a Similar Cardiometabolic Profile in the Moderate and High Cardiovascular Risk Populations: a Population-Based Study. J Clin Med. 2021;10(8):1584. doi:10.3390/jcm10081584
17. Spaeth GL. European glaucoma society terminology and guidelines for glaucoma. Br J Ophthalmol. 2021;105(Suppl 1):1–169. doi:10.1136/bjophthalmol-2021-egsguidelines
18. Cheng KKW, Tatham AJ. Spotlight on the Disc-Damage Likelihood Scale (DDLS). Clin Ophthalmol. 2021;15:4059–4071. doi:10.2147/OPTH.S284618
19. Bochmann F, Howell JP, Meier C, Becht C, Thiel MA. The disc damage likelihood scale (DDLS): interobserver agreement of a new grading system to assess glaucomatous optic disc damage. Klin Monbl Augenheilkd. 2009;226(4):280–283. doi:10.1055/s-0028-1109288
20. Susanna R, Vessani RM. New findings in the evaluation of the optic disc in glaucoma diagnosis. Curr Opin Ophthalmol. 2007;18(2):122–128. doi:10.1097/ICU.0b013e328040bfe0
21. Fingeret M, Medeiros FA, Susanna R, Weinreb RN. Five rules to evaluate the optic disc and retinal nerve fiber layer for glaucoma. Optometry. 2005;76(11):661–668. doi:10.1016/j.optm.2005.08.029
22. R Core Team. R: a language and environment for statistical computing. 2022. Available from: https://www.R-project.org/.
23. American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: standards of Medical Care in Diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S17–S38. doi:10.2337/dc22-S002
24. Li S, Cao W, Han J, Tang B, Sun X. The diagnostic value of white blood cell, neutrophil, neutrophil-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio in patients with primary angle closure glaucoma. Oncotarget. 2017;8(40):68984–68995. doi:10.18632/oncotarget.16571
25. Ozgonul C, Sertoglu E, Mumcuoglu T, Kucukevcilioglu M. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Novel Biomarkers of Primary Open-Angle Glaucoma. J Glaucoma. 2016;25(10):e815–e820. doi:10.1097/IJG.0000000000000392
26. Zhang A, Ning L, Han J, et al. Neutrophil-To-Lymphocyte Ratio as a Potential Biomarker of Neovascular Glaucoma. Ocul Immunol Inflamm. 2021;29(2):417–424. doi:10.1080/09273948.2019.1677916
27. Atalay K, Kaldirim Erdogan H, Kirgiz A, Asik Nacaroglu S. Predictive role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in normal-tension glaucoma. Med Hypotheses. 2017;103:54–56. doi:10.1016/j.mehy.2017.04.001
28. Kurtul BE, Ozer PA, Kabatas EU. Elevated neutrophil-to-lymphocyte ratio in pseudoexfoliation syndrome. Eye (Lond). 2016;30(8):1045–1048. doi:10.1038/eye.2016.89
29. Zhao YX, Chen XW. Diabetes and risk of glaucoma: systematic review and a Meta-analysis of prospective cohort studies. Int J Ophthalmol. 2017;10(9):1430–1435. doi:10.18240/ijo.2017.09.16
30. Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a Risk Factor for Glaucomatous Optic Neuropathy. Ophthalmologica. 2005;219(1):1–10. doi:10.1159/000081775
31. Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. doi:10.4239/wjd.v6.i1.92
32. Lee JS, Kim YJ, Kim S, et al. Increased Risks of Open-Angle Glaucoma in Untreated Hypertension. Am J Ophthalmol. 2023;252:111–120. doi:10.1016/j.ajo.2023.03.032
33. Nislawati R, Taufik Fadillah Zainal A, Ismail A, Waspodo N, Kasim F, Gunawan AMAK. Role of hypertension as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. BMJ Open Ophth. 2021;6(1):e000798. doi:10.1136/bmjophth-2021-000798
34. Graßhoff H, Comdühr S, Monne LR, et al. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.648408
35. Witkowska AM. On the Role of sIL-2R Measurements in Rheumatoid Arthritis and Cancers. Mediators of Inflammation Nan/Nan/Nan. 2005;121–130. doi:10.1155/MI.2005.121
36. LaPorte KM, Hernandez R, Savio AS, Malek TR. Robust IL-2-dependent antitumor immunotherapy requires targeting the high-affinity IL-2R on tumor-specific CD8+ T cells. J Immunother Cancer. 2023;11(6):e006611. doi:10.1136/jitc-2022-006611
37. Liang G, Li J, Pu S, He Z. Screening of Sepsis Biomarkers Based on Bioinformatics Data Analysis. J Healthcare Engineering. 2022;2022:e6788569. doi:10.1155/2022/6788569
38. Lu J, Li Q, Wu Z, et al. Two gene set variation indexes as potential diagnostic tool for sepsis. Am J Transl Res. 2020;12(6):2749.
39. Lou W, Yan J, Wang W. Downregulation of miR-497-5p Improves Sepsis-Induced Acute Lung Injury by Targeting IL2RB. Biomed Res Int. 2021;2021:6624702. doi:10.1155/2021/6624702
40. Yang Y, Zhang Y, Li S, et al. A Robust and Generalizable Immune-Related Signature for Sepsis Diagnostics. IEEE/ACM Trans Comput Biol Bioinform. 2022;19(6):3246–3254. doi:10.1109/TCBB.2021.3107874
41. Zhou J, Zhang Y, Zhuang Q. IL2RB affects Th1/Th2 and Th17 responses of peripheral blood mononuclear cells from septic patients. Allergologia et Immunopathologia. 2023;51(3):1–7. doi:10.15586/aei.v51i3.757
42. Ghosh SP, Yu Z, Kelegere Y, et al. Evidence for IL-2 signaling in corneal epithelium. Invest Ophthalmol Visual Sci. 2022;63(7).
43. Barak V, Kalickman I, Pe’er J. sIL-2R- an Immuno-biomarker for Prediction of Metastases in Uveal Melanoma. Anticancer Res. 2022;42(3):1447–1453. doi:10.21873/anticanres.15615
44. Cénit MC, Márquez A, Cordero-Coma M, et al. Evaluation of the IL2/IL21, IL2RA and IL2RB genetic variants influence on the endogenous non-anterior uveitis genetic predisposition. BMC Med. Genet. 2013;14(1):52. doi:10.1186/1471-2350-14-52
45. Suzuki K, Namba K, Mizuuchi K, et al. Validation of systemic parameters for the diagnosis of ocular sarcoidosis. Jpn J Ophthalmol. 2021;65(2):191–198. doi:10.1007/s10384-020-00793-6
46. Gundlach E, Hoffmann MM, Prasse A, Heinzelmann S, Ness T. Interleukin-2 Receptor and Angiotensin-Converting Enzyme as Markers for Ocular Sarcoidosis. PLoS One. 2016;11(1):e0147258. doi:10.1371/journal.pone.0147258
47. Alende-Castro V, Alonso-Sampedro M, Fernández-Merino C, et al. Factors influencing serum concentrations of soluble interleukin-2 receptor: a general adult population study. All Life. 2023;16(1):2169958. doi:10.1080/26895293.2023.2169958
48. Cai B, Zhang J, Zhang M, et al. Micro-inflammation characterized by disturbed Treg/Teff balance with increasing sIL-2R in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2013;121(4):214–219. doi:10.1055/s-0033-1333687
49. Zhou Y, Zhang Y, Zhao M, Li Q, Li H. sIL-2R levels predict the spontaneous remission in sarcoidosis. Respir Med. 2020;171. doi:10.1016/j.rmed.2020.106115
50. Vorselaars ADM, van CHM, Zanen P, et al. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. 2015;109(2):279–285. doi:10.1016/j.rmed.2014.11.009
51. Gupta S, Parmar M, Padappayil RP, Bansal A, Daouk S. Role of Serum Soluble Interleukin-2 Receptor Level in the Diagnosis of Sarcoidosis: a Systematic Review and Meta-Analysis. Int J Med. 2022;22277713. doi:10.1101/2022.07.16.22277713
52. Schimmelpennink M, Quanjel M, Vorselaars A, et al. Value of serum soluble interleukin-2 receptor as a diagnostic and predictive biomarker in sarcoidosis. Expert Rev Respir Med. 2020;14(7):749–756. doi:10.1080/17476348.2020.1751614
53. Kobayashi Y, Sato T, Nagai T, et al. Association of high serum soluble interleukin 2 receptor levels with risk of adverse events in cardiac sarcoidosis. ESC Heart Failure. 2021;8(6):5282–5292. doi:10.1002/ehf2.13614
54. Al-Hakeim HK, Al-Rammahi DA, Al-Dujaili AH. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J Affective Disorders. 2015;182:106–114. doi:10.1016/j.jad.2015.04.044
55. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5083 controls. Brain Behav Immun. 2020;87:901–909. doi:10.1016/j.bbi.2020.02.010
56. Maes M, Carvalho AF. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol Neurobiol. 2018;55(12):8885–8903. doi:10.1007/s12035-018-1016-x
57. Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-Motor Symptoms in Patients with Parkinson’s Disease – correlations with Inflammatory Cytokines in Serum. PLoS One. 2012;7(10):e47387. doi:10.1371/journal.pone.0047387
58. Ramanzini LG, Camargo LFM, Silveira JOF, Bochi GV. Inflammatory markers and depression in Parkinson’s disease: a systematic review. Neurol Sci. 2022;43(12):6707–6717. doi:10.1007/s10072-022-06363-7
59. Nies YH, Yahaya MF, Lim WL, Teoh SL. Microarray-based analysis of differential gene expression profile in rotenone-induced Parkinson’s disease zebrafish model. CNS Neurol Disord Drug Targets. 2023. doi:10.2174/1871527322666230608122552
60. Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi:10.1056/NEJMoa0906312
61. Brunner PM, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7(1):8707. doi:10.1038/s41598-017-09207-z
62. Sun H, Liu C, Zhang X, et al. Using bioinformatics analysis to screen abnormal methylated differentially expressed hub genes of Kawasaki disease and construct diagnostic model. Heliyon. 2022;8(11):e11905. doi:10.1016/j.heliyon.2022.e11905
63. Liao Y, Ke B, Long X, Xu J, Wu Y. Upregulated Expression of IL2RB Causes Disorder of Immune Microenvironment in Patients with Kawasaki Disease. Biomed Res. Int. 2022;2022:e2114699. doi:10.1155/2022/2114699
64. Hinks A, Cobb J, Marion MC, et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet. 2013;45(6):664–669. doi:10.1038/ng.2614
65. Zheng Y, Cai B, Ren C, et al. Identification of immune related cells and crucial genes in the peripheral blood of ankylosing spondylitis by integrated bioinformatics analysis. PeerJ. 2021;9:e12125. doi:10.7717/peerj.12125
66. Akiyama M, Sasaki T, Kaneko Y, et al. Serum soluble interleukin-2 receptor is a useful biomarker for disease activity but not for differential diagnosis in IgG4-related disease and primary Sjögren’s syndrome adults from a defined population. Clin Exp Rheumatol. 2018;36 Suppl 112(3):157–164.
67. Keindl M, Davies R, Bergum B, et al. Impaired activation of STAT5 upon IL-2 stimulation in Tregs and elevated sIL-2R in Sjögren’s syndrome. Arthritis Res Therapy. 2022;24(1):101. doi:10.1186/s13075-022-02769-y
68. Hamamoto K, Inaba M, Yamada S, et al. Increased soluble IL-2 receptor levels in serum from a patient with painless thyroiditis. Thyroid Research. 2013;6(1):12. doi:10.1186/1756-6614-6-12
69. Bouzid D, Amouri A, Fourati H, et al. Polymorphisms in the IL2RA and IL2RB Genes in Inflammatory Bowel Disease Risk. Genetic Testing and Molecular Biomarkers. 2013;17(11):833–839. doi:10.1089/gtmb.2013.0291
70. Pan Y, Zhang J, Li J, Zhao W. Identification and Validation of Immune Markers in Coronary Heart Disease. Comput Math Methods Med. 2022;2022:2877679. doi:10.1155/2022/2877679
71. Goncharova IA, Bragina E, Zhalsanova I, Freidin MB, Nazarenko MS. Putative regulatory functions of SNPs associated with bronchial asthma, arterial hypertension and their comorbid phenotype. Vavilovskii Zhurnal Genet Selektsii. 2021;25(8):855–863. doi:10.18699/VJ21.099
72. Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001;131(4):421–426. doi:10.1016/S0002-9394(00)00862-X
73. Huang P, Qi Y, Xu YS, et al. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma. 2010;19(5):324–330. doi:10.1097/IJG.0b013e3181b4cac7
74. Fernandez IZ, Baxter RM, Garcia-Perez JE, et al. A novel human IL2RB mutation results in T and NK cell–driven immune dysregulation. J Exp Med. 2019;216(6):1255–1267. doi:10.1084/jem.20182015
75. Ji YW, Mittal SK, Hwang HS, et al. Lacrimal gland–derived IL-22 regulates IL-17-mediated ocular mucosal inflammation. Mucosal Immunol. 2017;10(5):1202–1210. doi:10.1038/mi.2016.119
76. Keller KE. Analysis of interleukin-20 receptor complexes in trabecular meshwork cells and effects of cytokine signaling in anterior segment perfusion culture. Mol Vis. 2019;25:266–282.
77. Mossner S, Kuchner M, Fazel Modares N, et al. Synthetic interleukin 22 (IL-22) signaling reveals biological activity of homodimeric IL-10 receptor 2 and functional cross-talk with the IL-6 receptor gp130. J Biol Chem. 2020;295(35):12378–12397. doi:10.1074/jbc.RA120.013927
78. Lécart S, Morel F, Noraz N, et al. IL‐22, in contrast to IL‐10, does not induce Ig production, due to absence of a functional IL‐22 receptor on activated human B cells. Int Immunol. 2002;14(11):1351–1356. doi:10.1093/intimm/dxf096
79. Mallela LS, Sharma P, Rao TSR, Roy S. Recombinant IL-22 promotes protection in a murine model of Aspergillus flavus keratitis and mediates host immune responses in human corneal epithelial cells. Cellular Microbiology. 2021;23(9):e13367. doi:10.1111/cmi.13367
80. Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28(S1):56–60. doi:10.1111/jgh.12032
81. Mitra A, Raychaudhuri SK, Raychaudhuri SP. Functional role of IL-22 in psoriatic arthritis. Arthritis Res Therapy. 2012;14(2):R65. doi:10.1186/ar3781
82. Lifshiz Zimon R, Lerman G, Elharrar E, et al. Ultrasound targeting of Q-starch/miR-197 complexes for topical treatment of psoriasis. J Control Release. 2018;284:103–111. doi:10.1016/j.jconrel.2018.05.040
83. Desmet E, Van Gele M, Grine L, Remaut K, Lambert J. Towards the development of a RNAi-based topical treatment for psoriasis: proof-of-concept in a 3D psoriasis skin model. Exp Dermatol. 2018;27(5):463–469. doi:10.1111/exd.13414
84. Zhang S, Yang G. IL22RA1/JAK/STAT Signaling Acts As a Cancer Target Through Pan-Cancer Analysis. Front Immunol. 2022;13:915246. doi:10.3389/fimmu.2022.915246
85. Dinarte VRP, Silva WA, Baccarin ARD, Tamashiro E, Valera FC, Anselmo-Lima WT. Association of interleukin 22 receptor subunit alpha 1 gene polymorphisms with chronic rhinosinusitis. Br J Otorhinolaryngol. 2021;87(5):505–511. doi:10.1016/j.bjorl.2019.10.006
86. Endam LM, Bossé Y, Filali-Mouhim A, et al. Polymorphisms in the interleukin-22 receptor alpha-1 gene are associated with severe chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;140(5):741–747. doi:10.1016/j.otohns.2008.12.058
87. Mattapallil MJ, Kielczewski JL, Zárate-Bladés CR, et al. Interleukin 22 ameliorates neuropathology and protects from central nervous system autoimmunity. J Autoimmun. 2019;102:65–76. doi:10.1016/j.jaut.2019.04.017
88. Lindborg JA, Tran NM, Chenette DM, et al. Optic nerve regeneration screen identifies multiple genes restricting adult neural repair. Cell Rep. 2021;34(9):108777. doi:10.1016/j.celrep.2021.108777
89. Li J, Liu W, Wang Y, et al. Salidroside Inhibits Ganglion Cell Apoptosis by Suppressing the Müller Cell Inflammatory Response in Diabetic Retinopathy. Curr Eye Res. 2023;1(2):1–12. doi:10.1080/02713683.2023.2204208
90. Wang Y, Yu H, Li J, et al. Th22 cells induce Müller cell activation via the Act1/TRAF6 pathway in diabetic retinopathy. Cell Tissue Res. 2022;390(3):367–383. doi:10.1007/s00441-022-03689-8
91. Zhao Y, Zhang F, Pan Z, Luo H, Liu K, Duan X. LncRNA NR_003923 promotes cell proliferation, migration, fibrosis, and autophagy via the miR-760/miR-215-3p/IL22RA1 axis in human Tenon’s capsule fibroblasts. Cell Death Dis. 2019;10(8):1–13. doi:10.1038/s41419-019-1829-1
92. Yu S, Tam ALC, Campbell R, Renwick N. Emerging Evidence of Noncoding RNAs in Bleb Scarring after Glaucoma Filtration Surgery. Cells. 2022;11(8):1301. doi:10.3390/cells11081301
93. Sonar S, Lal G. Role of Tumor Necrosis Factor Superfamily in Neuroinflammation and Autoimmunity. Front Immunol. 2015; 6. doi:10.3389/fimmu.2015.00364
94. Xu WD, Zhao Y, Liu Y. Role of the TWEAK/Fn14 pathway in autoimmune diseases. Immunol Res. 2016;64(1):44–50. doi:10.1007/s12026-015-8761-y
95. Vendrell J. TWEAK: a New Player in Obesity and Diabetes. Front Immunol. 2013;4:88. doi:10.3389/fimmu.2013.00488
96. Cheadle L, Tzeng CP, Kalish BT, et al. Visual Experience-Dependent Expression of Fn14 Is Required for Retinogeniculate Refinement. Neuron. 2018;99(3):525–539.e10. doi:10.1016/j.neuron.2018.06.036
97. Cheadle L, Rivera SA, Phelps JS, et al. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron. 2020;108(3):451–468.e9. doi:10.1016/j.neuron.2020.08.002
98. Ebihara N, Nakayama M, Tokura T, Iwatsu M, Ushio H, Murakami A. Proinflammatory Effect of TWEAK/Fn14 Interaction in Human Retinal Pigment Epithelial Cells. Curr Eye Res. 2009;34(10):836–844. doi:10.3109/02713680903122037
99. Ameri H, Liu H, Liu R, et al. TWEAK/Fn14 Pathway Is a Novel Mediator of Retinal Neovascularization. Invest Ophthalmol Vis Sci. 2014;55(2):801. doi:10.1167/iovs.13-12812
100. El-Asrar AM A, De Hertogh G, Nawaz MI, et al. The Tumor Necrosis Factor Superfamily Members TWEAK, TNFSF15 and Fibroblast Growth Factor-Inducible Protein 14 Are Upregulated in Proliferative Diabetic Retinopathy. Ophthalmic Res. 2015;53(3):122–130. doi:10.1159/000369300
101. Maarouf A, Stephan D, Ranjeva MP, et al. High levels of serum soluble TWEAK are associated with neuroinflammation during multiple sclerosis. J Transl Med. 2019;17(1):51. doi:10.1186/s12967-019-1789-3
102. Karadag H, Saygili G, Yuksel R, Baris Usta M, Topcuoglu C, Erzin G. SERUM TNF- RELATED WEAK INDUCER OF APOPTOSIS (TWEAK), TNF- RELATED APOPTOSIS-INDUCING LIGAND (TRAIL) LEVELS IN PATIENTS WITH BIPOLAR DEPRESSION, MAJOR DEPRESSION AND A HEALTHY CONTROL GROUP. Psychiatria Danubina. 2021;33(br 3):314–319. doi:10.24869/psyd.2021.314
103. Schmidt FM, Koch J, Nowak C, et al. Ligands and receptors of the TNF superfamily are decreased in major depression and during early antidepressant therapy. J Psychiatr Res. 2019;119:116–121. doi:10.1016/j.jpsychires.2019.09.010
104. Melin EO, Dereke J, Hillman M. Low levels of soluble TWEAK, indicating on-going inflammation, were associated with depression in type 1 diabetes: a cross-sectional study. BMC Psychiatry. 2020;20(1):574. doi:10.1186/s12888-020-02977-3
105. Gubin D, Neroev V, Malishevskaya T, et al. Depression scores are associated with retinal ganglion cells loss. J Affect Disord. 2023;333:290–296. doi:10.1016/j.jad.2023.04.039
106. Wang R, Chen B, Wei H, et al. Collecting and deactivating TGF-β1 hydrogel for anti-scarring therapy in post-glaucoma filtration surgery. Mater Today Bio. 2022;14:100260. doi:10.1016/j.mtbio.2022.100260
107. Jelić-Ivanović Z, Bujišić N, Spasić S, Bogavac-Stanojević N, Spasojević-Kalimanovska V, Kotur-Stevuljević J. Circulating sTWEAK improves the prediction of coronary artery disease. Clin. Biochem. 2009;42(13):1381–1386. doi:10.1016/j.clinbiochem.2009.06.001
108. Urbonaviciene G, Martin-Ventura JL, Lindholt JS, et al. Impact of soluble TWEAK and CD163/TWEAK ratio on long-term cardiovascular mortality in patients with peripheral arterial disease. Atherosclerosis. 2011;219(2):892–899. doi:10.1016/j.atherosclerosis.2011.09.016
109. Fernández-Laso V, Sastre C, Valdivielso JM, et al. Soluble TWEAK levels predict the presence of carotid atherosclerotic plaques in subjects free from clinical cardiovascular diseases. Atherosclerosis. 2015;239(2):358–363. doi:10.1016/j.atherosclerosis.2015.01.040
110. Turkmen K, Tonbul HZ, Erdur FM, et al. Soluble TWEAK independently predicts atherosclerosis in renal transplant patients. BMC Nephrol. 2013;14(1):144. doi:10.1186/1471-2369-14-144
111. Perri P, Zauli G, Gonelli A, et al. TNF-Related Apoptosis Inducing Ligand in Ocular Cancers and Ocular Diabetic Complications. Biomed Res. Int. 2015;2015:e424019. doi:10.1155/2015/424019
112. Tisato V, Gonelli A, Voltan R, Secchiero P, Zauli G. Clinical perspectives of TRAIL: insights into central nervous system disorders. Cell Mol Life Sci. 2016;73(10):2017–2027. doi:10.1007/s00018-016-2164-7
113. Ikeda T, Hirata S, Fukushima S, et al. Dual Effects of TRAIL in Suppression of Autoimmunity: the Inhibition of Th1 Cells and the Promotion of Regulatory T Cells. J Immunol. 2010;185(9):5259–5267. doi:10.4049/jimmunol.0902797
114. Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10(2):203–210. doi:10.1038/sj.cdd.4401138
115. Guo C, Wu N, Niu X, Wu Y, Chen D, Guo W. Comparison of T Helper Cell Patterns in Primary Open-Angle Glaucoma and Normal-Pressure Glaucoma. Med Sci Monit. 2018;24:1988–1996. doi:10.12659/MSM.904923
116. Bell K, Holz A, Ludwig K, Pfeiffer N, Grus FH. Elevated Regulatory T Cell Levels in Glaucoma Patients in Comparison to Healthy Controls. Curr Eye Res. 2017;42(4):562–567. doi:10.1080/02713683.2016.1205629
117. Moreno M, Sáenz-Cuesta M, Castilló J, et al. Circulating levels of soluble apoptosis-related molecules in patients with multiple sclerosis. J Neuroimmunol. 2013;263(1):152–154. doi:10.1016/j.jneuroim.2013.07.013
118. Mori K, Ikari Y, Jono S, et al. Association of serum TRAIL level with coronary artery disease. Thrombosis Research. 2010;125(4):322–325. doi:10.1016/j.thromres.2009.11.024
119. Cheng W, Liu F, Wang Z, et al. Soluble TRAIL Concentration in Serum Is Elevated in People with Hypercholesterolemia. PLoS One. 2015;10(12):e0144015. doi:10.1371/journal.pone.0144015
120. Angelopoulou E, Paudel YN, Shaikh MF, Piperi C. Fractalkine (CX3CL1) signaling and neuroinflammation in Parkinson’s disease: potential clinical and therapeutic implications. Pharmacol Res. 2020;158:104930. doi:10.1016/j.phrs.2020.104930
121. Breen KT, Anderson SR, Steele MR, Calkins DJ, Bosco A, Vetter ML. Loss of Fractalkine Signaling Exacerbates Axon Transport Dysfunction in a Chronic Model of Glaucoma. Front Neurosci. 2016;10. doi:10.3389/fnins.2016.00526
122. Jiang M, Xie H, Zhang C, et al. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J Cell Mol Med. 2022;26(4):1229–1244. doi:10.1111/jcmm.17179
123. Chen G, Zhou Z, Sha W, et al. A novel CX3CR1 inhibitor AZD8797 facilitates early recovery of rat acute spinal cord injury by inhibiting inflammation and apoptosis. Int J Mol Med. 2020;45(5):1373–1384. doi:10.3892/ijmm.2020.4509
124. Begum G, Reddy R, Yakoub KM, Belli A, Davies DJ, Di Pietro V. Differential Expression of Circulating Inflammatory Proteins Following Sport-Related Traumatic Brain Injury. Int J Mol Sci. 2020;21(4):1216. doi:10.3390/ijms21041216
125. Liu C, Cui G, Zhu M, Kang X, Guo H. Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol. 2014;7(12):8342–8355.
126. Wakefield D, Wildner G. Is glaucoma an autoimmune disease? Clin Transl Immunology. 2020;9(10):e1180. doi:10.1002/cti2.1180
127. Voisinne G, Gonzalez de Peredo A, Roncagalli R. CD5, an Undercover Regulator of TCR Signaling. Front Immunol. 2018;9:2900. doi:10.3389/fimmu.2018.02900
128. Calvo J, Places L, Espinosa G, et al. Identification of a natural soluble form of human CD5: circulating CD5. Tissue Antigens. 1999;54(2):128–137. doi:10.1034/j.1399-0039.1999.540203.x
129. Ramos-Casals M, Font J, García-Carrasco M, et al. High circulating levels of soluble scavenger receptors (sCD5 and sCD6) in patients with primary Sjögren’s syndrome. Rheumatology. 2001;40(9):1056–1059. doi:10.1093/rheumatology/40.9.1056
130. Aibar J, Martínez-Florensa M, Castro P, et al. Pattern of soluble CD5 and CD6 lymphocyte receptors in critically ill patients with septic syndromes. J Crit Care. 2015;30(5):914–919. doi:10.1016/j.jcrc.2015.04.120
131. Jamin C, Magadur G, Lamour A, et al. Cell-free CD5 in patients with rheumatic diseases. Immunol Lett. 1992;31(1):79–83. doi:10.1016/0165-2478(92)90014-f
132. Noh GW, Lee KY. Circulating soluble CD5 in atopic dermatitis. Mol Cells. 1998;8(5):618–622.
133. Andrés M V-D, Casadó-Llombart S, Català C, Leyton-Pereira A, Lozano F, Aranda F. Soluble CD5 and CD6: lymphocytic Class I Scavenger Receptors as Immunotherapeutic Agents. Cells. 2020;9(12):2589. doi:10.3390/cells9122589
134. Stolfi C, Troncone E, Marafini I, Monteleone G. Role of TGF-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules. 2021;11(1):17. doi:10.3390/biom11010017
135. Roodnat AW, Callaghan B, Doyle C, et al. Genome-Wide RNA Sequencing of Human Trabecular Meshwork Cells Treated with TGF-β1: relevance to Pseudoexfoliation Glaucoma. Biomolecules. 2022;12(11):1693. doi:10.3390/biom12111693
136. Estrada LD, Oliveira-Cruz L, Cabrera D. Transforming Growth Factor Beta Type I Role in Neurodegeneration: implications for Alzheimer´s Disease. Curr Protein Pept Sci. 2014;19(12):1180–1188.
137. Jorgensen MM, de la Puente P. Leukemia Inhibitory Factor: an Important Cytokine in Pathologies and Cancer. Biomolecules. 2022;12(2):217. doi:10.3390/biom12020217
138. Hui W, Bell M, Carroll G. SOLUBLE GLYCOPROTEIN 130 (gp130) ATTENUATES OSM- AND LIF-INDUCED CARTILAGE PROTEOGLYCAN CATABOLISM. Cytokine. 2000;12(2):151–155. doi:10.1006/cyto.1999.0550
139. Chollangi S, Wang J, Martin A, Quinn J, Ash JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis. 2009;34(3):535–544. doi:10.1016/j.nbd.2009.03.012
140. Yang JY, Lu B, Feng Q, et al. Retinal Protection by Sustained Nanoparticle Delivery of Oncostatin M and Ciliary Neurotrophic Factor Into Rodent Models of Retinal Degeneration. Trans Vision Sci Technol. 2021;10(9):6. doi:10.1167/tvst.10.9.6
141. Hu Q, Huang C, Wang Y, Wu R. Expression of leukemia inhibitory factor in the rat retina following acute ocular hypertension. Molecular Medicine Reports. 2015;12(5):6577–6583. doi:10.3892/mmr.2015.4287
142. Benito-Gutiérrez E, Garcia-Fernàndez J, Comella JX. Origin and evolution of the Trk family of neurotrophic receptors. Mol Cell Neurosci. 2006;31(2):179–192. doi:10.1016/j.mcn.2005.09.007
143. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
144. Claes M, De Groef L, Moons L. Target-Derived Neurotrophic Factor Deprivation Puts Retinal Ganglion Cells on Death Row: cold Hard Evidence and Caveats. Int J Mol Sci. 2019;20(17):4314. doi:10.3390/ijms20174314
145. Gupta A, Galletti JG, Yu Z, Burgess K, de Paiva CS AB. C’s of Trk Receptors and Their Ligands in Ocular Repair. Int J Mol Sci. 2022;23(22):14069. doi:10.3390/ijms232214069
146. Nikoletopoulou V, Lickert H, Frade JM, et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467(7311):59–63. doi:10.1038/nature09336
147. Karlsson M, Zhang C, Méar L, et al. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31):eabh2169. doi:10.1126/sciadv.abh2169
148. Lukowski SW, Lo CY, Sharov AA, et al. A single-cell transcriptome atlas of the adult human retina. EMBO J. 2019;38(18):e100811. doi:10.15252/embj.2018100811
149. Cui Q, Tang LS, Hu B, So KF, Yip HK. Expression of trkA, trkB, and trkC in Injured and Regenerating Retinal Ganglion Cells of Adult Rats. Invest Ophthalmol Visual Sci. 2002;43(6):1954–1964.
150. Guo Y, Johnson E, Cepurna W, Jia L, Dyck J, Morrison JC. Does elevated intraocular pressure reduce retinal TRKB-mediated survival signaling in experimental glaucoma? Exp Eye Res. 2009;89(6):921–933. doi:10.1016/j.exer.2009.08.003
151. Ogłodek EA, Just MJ, Szromek AR, Araszkiewicz A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol Rep. 2016;68(5):945–951. doi:10.1016/j.pharep.2016.04.003
152. Karan D. CCL23 in Balancing the Act of Endoplasmic Reticulum Stress and Antitumor Immunity in Hepatocellular Carcinoma. Front Oncol. 2021;11. doi:10.3389/fonc.2021.727583
153. Castillo L, Rohatgi A, Ayers CR, et al. Associations of Four Circulating Chemokines with Multiple Atherosclerosis Phenotypes in a Large Population-Based Sample: results from the Dallas Heart Study. J Interferon Cytokine Res. 2010;30(5):339–347. doi:10.1089/jir.2009.0045
154. Faura J, Bustamante A, Penalba A, et al. CCL23: a Chemokine Associated with Progression from Mild Cognitive Impairment to Alzheimer’s Disease. J Alzheimers Dis. 2020;73(4):1585–1595. doi:10.3233/JAD-190753
155. Spaeth GL, Lopes JF, Junk AK, Grigorian AP, Henderer J. Systems for staging the amount of optic nerve damage in glaucoma: a critical review and new material. Surv Ophthalmol. 2006;51(4):293–315. doi:10.1016/j.survophthal.2006.04.008
156. Bayer A, Harasymowycz P, Henderer JD, Steinmann WG, Spaeth GL. Validity of a new disk grading scale for estimating glaucomatous damage: correlation with visual field damage. Am J Ophthalmol. 2002;133(6):758–763. doi:10.1016/s0002-9394(02)01422-8
157. Sulak R, Liu X, Smedowski A. The concept of gene therapy for glaucoma: the dream that has not come true yet. Neural Regeneration Res. 2024;19(1):92–99. doi:10.4103/1673-5374.375319
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
