Back to Journals » Infection and Drug Resistance » Volume 16
Infection of Diabetes Foot Caused by Carbapenem-Resistant Proteus penneri Mediated by a Novel Plasmid Containing blaNDM
Authors Wang Z , Wu Y, Chen S, Hou H, Wang Y
Received 2 December 2022
Accepted for publication 11 February 2023
Published 22 February 2023 Volume 2023:16 Pages 1099—1106
DOI https://doi.org/10.2147/IDR.S398914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zerong Wang,1 Yue Wu,1 Shude Chen,1 Heyang Hou,1 Yaowen Wang2
1The Second Clinical Medical College, Lanzhou University, Lanzhou, Gansu, People’s Republic of China; 2Department of Clinical Laboratory, Weifang People’s Hospital, Weifang, Shandong, People’s Republic of China
Correspondence: Yaowen Wang, Department of Clinical Laboratory, Weifang People’s Hospital, No. 151, Guangwen Street, Kuiwen District, Weifang, Shandong, People’s Republic of China, Tel +86 15864599659, Email [email protected]
Objective: A strain of Proteus penneri with carbapenem resistance was found in a patient with a diabetic foot infection. We studied drug resistance, genome, and homology of P. penneri to support clinical prevention and treatment of infection caused by carbapenem-resistant P. penneri (CR-PPE).
Methods: The strains were obtained through bacterial culture from purulence. VITEK 2 compact (GN13) and Kirby–Bauer (K-B) disk diffusion methods were used for antimicrobial susceptibility testing. Ceftriaxone, amikacin, gentamicin, ampicillin, aztreonam, ceftazidime, ciprofloxacin, levofloxacin, cefepime, trimethoprim-sulfamethoxazole, tobramycin, cefotetan, piperacillin-tazobactam, ampicillin-sulbactam, ertapenem, piperacillin, meropenem, cefuroxime, cefazolin, cefoperazone/sulbactam, cefoxitin, and imipenem were used for antimicrobial susceptibility testing. After bacterial genome extraction, sequencing, and sequence assembly, whole-genome sequencing (WGS) was performed to explore the CR-PPE genotype.
Results: CR-PPE was resistant to two carbapenems (imipenem and ertapenem), ceftriaxone, and cefazolin, and was sensitive to aztreonam, piperacillin-tazobactam, and cefotetan. WGS results depict that the resistant phenotype of CR-PPE is consistent with the genotype, without common virulence genes of Enterobacteriaceae bacteria detected (virulence factor database). The carbapenem resistance gene blaNMD-1 is contained in a new plasmid, pWF127-NDM. The transposon Tn125 in pWF127-NDM carrying blaNMD-1 has almost the same structure as Tn125 in the reference plasmid pHFK418-NDM (Accession: MH491967). In addition, through phylogenetic analysis, CR-PPE depicts the closest evolutionary relationship with GCF 024129515.1, which was found in Gallus gallus in the Czech Republic in 2019 (downloaded from National Center for Biotechnology Information database). According to the evolutionary tree, CR-PPE has high homology with the two P. penneri strains found in China.
Conclusion: CR-PPE exhibits strong drug resistance owing to the presence of multiple resistance genes. CR-PPE infection should receive more attention, especially in patients with underlying diseases, such as diabetes and weak immunity.
Keywords: Proteus penneri, New Delhi metallo-β-lactamase, NDM, carbapenem resistance, diabetes, bacterial infection
Introduction
Over the last decades, Proteus species infections have received special attention because of the emergence of some species resistant to various antimicrobial agents, particularly β-lactams.1 Proteus penneri, first recognized in 1982, is a member of the Enterobacteriaceae family, which is salicin- and esculin-negative and chloramphenicol-resistant.1,2 P. penneri is primarily associated with nosocomial acquired infections and is mainly isolated from urine samples (50%), wound and soft tissue exudates (25%), and blood cultures (15%).3,4 P. penneri, same as P. mirabilis, is naturally resistant to penicillin G, oxacillin, all tested macrolides, lincosamides, streptogramins, glycopeptides, rifampicin, and fusidic acid.1 However, P. penneri is sporadically reported carbapenem-resistant from human and environmental sources.4 Antimicrobial resistance (AMR) has become a pressing issue that poses a significant risk to global public health.5 The increasing spread of carbapenemase-encoding genes has increased the carbapenem-resistant Enterobacteriaceae (CRE) infection threat.6,7
In this retrospective study, a strain of P. penneri with carbapenem resistance was found in patients with diabetic foot infections. Therefore, antimicrobial susceptibility testing and whole-genome sequencing (WGS) were implemented to understand the drug resistance and genome of carbapenemase-resistant P. penneri (CR-PPE). The homology of CR-PPE was analyzed according to the National Center for Biotechnology Information (NCBI) database to provide data support for clinical prevention and treatment of infections caused by CR-PPE.
Materials and Methods
Bacterial Strains
CR-PPE strain was isolated from a hospitalized patient from July 16, 2016, to July 23, 2016, in a tertiary hospital in China. CR-PPE was isolated through bacterial culture from purulence and identified using a Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF-MS) Vitek-MS (Sysmex-bioMerieux, Marcy l’Etoile, France). Escherichia coli (ATCC 25922) was used as a quality control strain.
Antimicrobial Susceptibility Testing
The antimicrobials used included the following: ceftriaxone (CRO), amikacin (AMK), gentamicin (GEN), ampicillin (AMP), aztreonam (ATM), ceftazidime (CAZ), ciprofloxacin (CIP), levofloxacin (LVX), cefepime (FEP), trimethoprim-sulfamethoxazole (SXT), tobramycin (TOB), cefotetan (CTT), piperacillin-tazobactam (TZP), ampicillin-sulbactam (SAM), ertapenem (ETP), and piperacillin (PIP), and were analyzed using the VITEK 2 Compact (GN13) (Sysmex-bioMerieux, Marcy l’Etoile, France). The drugs not included in the GN13 card were tested using the Kirby–Bauer (K-B) diffusion method (Oxoid, Hampshire, UK). The antimicrobial drugs detected using the K-B method included meropenem (MEM), cefuroxime (CXM), cefazolin (CZO), cefoperazone/sulbactam (CSL), cefoxitin (FOX) and imipenem (IPM). The results of susceptibility testing were interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines. E. coli ATCC 25922 was used as the quality control strain.
Bacterial Genome Extraction
Conservation strains were first incubated overnight in an enriched broth and then streaked onto Colombian blood plates (AutoBio, Zhengzhou, China). Species identified with Vitek MS were inoculated overnight in 3 mL of Luria-Bertani (L.B.) broth medium (Oxoid, Hampshire, UK). Bacterial genomic DNA was extracted using an Omega Bio-Tek Bacterial DNA Kit (Doraville, GA, USA), according to the manufacturer’s instructions, and the genomic DNA concentration and quality were checked using a NanoDrop spectrophotometer 2000 (Waltham, MA, USA). The obtained DNA was stored at −80 °C until further analysis.
Sequencing and Sequence Assembly
Bacterial genomic DNA was sequenced from a paired-end library with an average insert size of 350 bp using a NovaSeq sequencer (Illumina, CA, USA). FASTQ format files for each sample set were assembled independently using the de novo assembler SPAdes 3.13.0.8,9 with K-values of 21, 33, 55, 77, and 99, and careful mode to reduce the number of mismatches. Plasmid sequences were assembled using SPAdes.7
WGS Analysis
Antibiotic resistance genes (ARGs) and virulence factor (VF) were identified using NCBI and VF databases with Abricate 0.8 (https://github.com/tseemann/abricate).10 The plasmid type (Inc type) of contigs harboring carbapenemase genes was determined using PlasmidFinder.11 The comparison of different plasmids harboring carbapenemase genes was performed using the BLAST Ring Image Generator.12 The phylogeny was used for single nucleotide polymorphism (SNP) analysis.13 Unrooted maximum-likelihood phylogenetic trees were further generated using MEGAX 10.1.8 with a bootstrap iteration of 1000.14 The phylogenetic tree was visualized with iTOL.15 The strain data for constructing the phylogenetic tree, except for CR-PPE strains in the study, were obtained from NCBI database. There were nine strains of P. penneri in NCBI database (up to Nov 8, 2022) that were used to build the phylogenetic tree and were named after their accession numbers. The sequence data in this study were deposited in the BioProject database under PRJNA901201.
Results and Discussion
CR-PPE was isolated from the purulence of an elderly man with serious basic diseases. The exact isolation time was July 17, 2016, during hand and foot surgery. The clinical characteristics of patients are summarized in Table 1.
 |
Table 1 The Characteristics of Patient |
Analysis of Medical Record Characteristics
The patient had diabetes for four years and took metformin at home, but the blood glucose level was unstable The patient had a history of left-limb paralysis caused by cerebral thrombosis 20 years prior. The patient presented with a left foot infection for 20 days. Because the treatment effect in the local hospital was poor, he visited a tertiary hospital in East China for further therapy. On admission, a dry gangrenous wound approximately 3×3 cm in size was observed on the left dorsum of the foot, with the bottom of the wound reaching the bone. The surrounding skin was dark, and the local epidermis could be seen to fall off. An ulcer approximately 2×1 cm in size was observed on the dorsum of the right foot. Pulsation of the right dorsalis pedis artery was weakened, and the skin temperature of the right foot was reduced. After admission, the patient received a dressing change and antibacterial and symptomatic treatment, such as hypoglycemic therapy, invigorating blood circulation, and relieving swelling. After treatment, the blood glucose level improved, and the right foot wound healed, but infection control was poor. The dry gangrene wound on the left dorsum of the foot did not improve. Tissue debridement of wound skin and subcutaneous or amputation were recommended by doctors. The patient and his family refused surgery and asked for conservative treatment, changing the dressing at home after discharge. An integrated view of the medical records is depicted in Figure 1.
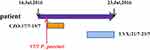 |
Figure 1 Integrated view of the medical record. Abbreviations: CZO, Cefazolin; LVX, Levofloxacin. Notes: The date marked by arrow was the date of isolation of the P. penneri strains. |
On July 17, 2016, CR-PPE was isolated through bacterial culture of the purulence collected, while CZO sodium pentahydrate antibacterial (2 g/24 h) (CLSI recommends 1 g/12 h) was administered on the same day for three days. The infection did not improve after three days. The wound was characterized by necrotic substances and exudation with a peculiar smell. LVX lactate (0.2 g/24 h) (CLSI recommend 0.75 g/24 h) antibacterial treatment was administered on July 21, 2016, until the discharge on July 23. At discharge, the infection was poorly controlled. The size of the dry gangrenous wound (3×3 cm) on the left dorsum of the foot did not decrease, and exudation and smell persisted.
Antimicrobial Susceptibility Phenotype and Genotype
CR-PPE is resistant to two carbapenems (IPM and ETP), GEN, CRO, CZO, CXM, and FOX and sensitive to ATM, TZP, and CTT. Tet(B), sul2, blaOXA-1, and other resistance genes have been detected to exist in CR-PPE. The resistant phenotype of CR-PPE was consistent with the genotype, and no virulence gene was detected. For plasmid prediction, Col3M_1 is forecasted. The characteristics of patient are summarized in Table 1. Specific antimicrobial susceptibility testing results and predicted resistance genes are displayed in Table 2.
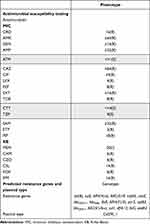 |
Table 2 Outcomes of Antimicrobial Susceptibility Testing, Predicted Resistance Genes and Plasmid Type of CR-PPE |
Shaded parts represent sensitive results of antimicrobial susceptibility testing.
WGS Analysis and Plasmid Profile Analysis of pWF127-NDM
The results of WGS analysis demonstrated that the drug resistance phenotype of CR-PPE was consistent with the genotype; thus, CR-PPE demonstrated multidrug resistance (MDR) to common clinical drugs. Moreover, WGS analysis indicated that the strain was blaNDM positive, and sequencing confirmed it to be the blaNMD-1 type. No virulence genes were detected. No virulence gene was detected in this bacterium, indicating that CR-PPE is a low-virulence strain and generally does not cause serious infection or even death. The clinical manifestations also confirmed the above findings.
However, blaNDM does not belong to the plasmid Col3M_1. Therefore, we speculated that the plasmid containing blaNDM is a new type of plasmid, pWF127-NDM. By predicting the sequence of second-generation sequencing, the plasmid pWF127-NDM was 78, 874 bp in size, with 104 open reading frames. The partial structures of Tn6588, Tn125, and Tn6727 were located in the exogenous insertion region of the plasmid. blaNDM-1, whose cluster is arranged sequentially as ISAba125, blaNDM-1, and bleMBL elements, was embedded in the composite transposon Tn125. The specific plasmid structure is illustrated in Figure 2. There have been few reports of CR-PPE infection cases in China.16 However, the resistance genes and plasmids of CR-PPE infection cases have not been studied. To the best of our knowledge, pWF127-NDM is the first report of a carbapenem-resistant plasmid containing blaNDM-1 in P. penneri in China. The bladNDM-1 originates from an unknown environmental bacterial ancestor and is integrated into the chromosome of Acinetobacter. The Tn125 transposon carrying blaNDM-1 is likely to be subsequently constructed by this Acinetobacter, which is then transferred to various host plasmids and then horizontally transferred to Enterobacteriaceae, such as Proteus.17
 |
Figure 2 Plasmid map of the plasmid pWF127-NDM harboring blaNDM-1. |
Environment of Carbapenem Resistance Gene
We found that the multidrug-resistance-encoding region of pWF127-NDM contained Tn125, Tn6588, and Tn6806. blaNDM-1, which is located on Tn125, is situated in the second variable area of the class I integron. The upstream region of blaNDM-1 is bleMBL, a component of Tn125, and transposase tnpA is carried by ISCR1. ISAba125 and aphA6 are downstream of blaNDM-1. The specific gene structure is displayed in Figure 3. Tn125 promoted blaNDM-1 expression and enhanced the hydrolysis activity of the strain toward carbapenems. The bleMBL gene upstream of blaNDM-1 could stabilize blaNDM-1.18 In addition, consistent with its ability to transpose, ISAba125 was described in association with increased resistance levels to β-lactams in Enterobacteriaceae.15 Compared with the reference plasmid pHFK418-NDM (Accession: MH491967), there is an approximately 60k fragment deletion in the plasmid skeleton region, while other skeleton regions are the same. The differences in the exogenous insertion region were mainly concentrated in the Tn6588 and Tn125 regions. Only two genes, trpF and dsbD, in Tn125 of pWF127-NDM swap positions compared to pHFK418-NDM. The other parts of Tn125 were the same. blaNDM-1 was transmitted by the plasmid. In the conjugation experiments, the plasmid pHFK418-NDM from P. mirabilis HFK418 was transferred to E. coli, thereby generating blaNDM-1 positive E. coli.19 Therefore, we speculate that blaNDM-1 in this CR-PPE may be transmitted from other Enterobacteriaceae-resistant plasmids. Moreover, the plasmid pWF127-NDM may spread blaNDM-1 to other bacteria, leading to carbapenem resistance. CR-PPE infection, spread of transmission of drug-resistant plasmids, and carbapenem resistance should be given extensive attention.
 |
Figure 3 Genetic environment of blaNDM-1. Adapted from Wang P, Jiang X, Mu K, et al. DANMEL: a manually curated reference database for analyzing mobile genetic elements associated with bacterial drug resistance. mLife. 2022; 1: 460-64. © 2022 The Authors. mLife published by John Wiley & Sons Australia, Ltd. on behalf of Institute of Microbiology, Chinese Academy of Sciences.20 |
Homology Analysis
The phylogenetic tree consisted of P. penneri from this study and nine P. penneri strains. The P. penneri of this study has the closest evolutionary relationship with GCF 024129515.1, which was found in Gallus gallus in the Czech Republic in 2019. Second, P. penneri is also closely related to GCF 019375415.1 and GCF 022369495.1, both from China, in terms of evolutionary relationships. Therefore, we speculated that homologous transmission of P. penneri may be more common. Meanwhile, among the eight P. penneri strains in a clinical observation study biochemically, the strains were homologous except for a few reactions.21 In addition, from the phylogenetic tree, the strains were isolated from humans and animals, such as Gallus gallus, swine, and Manis javanica. Several strains have been identified since 2014. A specific evolutionary tree is illustrated in Figure 4.
 |
Figure 4 Evolutionary tree of 10 strains of Proteus penneri. |
Limitation
The conclusion of this study was based on only one patient, and further research is required.
Conclusion
Proteus penneri has been overlooked and understudied, and there are few reports on the isolation of P. penneri from patients with infection.22,23 In this retrospective study, a strain of CR-PPE was found in the gangrenous purulence of a patient with diabetes. CR-PPE is resistant to two carbapenems (IPM and ETP) and is sensitive to ATM, TZP, and CTT. The major carbapenem resistance mechanism of this strain was determined by the plasmid pWF127-NDM, which contains blaNDM-1 located on Tn125. This plasmid has never been reported in P. penneri. CR-PPE has the closest evolutionary relationship with GCF 024129515.1, which was found in Gallus gallus in the Czech Republic in 2019. Second, according to the evolutionary tree, CR-PPE has high homology with the two P. penneri found in China. P. penneri usually infects the urinary tract, blood, and abdominal wounds.4,24 In hospitals, P. penneri infections often occur because of multiple-drug resistance, especially carbapenem resistance, with the potential to spread drug-resistant plasmids of nosocomial pathogens. Therefore, patients with underlying diseases, such as diabetes and weak immunity, should be carefully monitored for CR-PPE infection, as well as those undergoing abdominal or urogenital surgery.
Ethics Approval and Informed Consent
The study obtained the informed consent of the patient, who signed and agreed to participate in the study. Written informed consent was obtained from all patients. The study was approved by the Medical Research Ethics Committee of Weifang People’s Hospital (approval number: KYLL2022101-1).
Consent for Publication
All details of any content and images can be published, and every author has shown the article content to be published. Patient agreed to publish the details of the case and signed the written informed consent for the case details to be published.
Author Contributions
All authors made substantial contributions to the development and execution of the study, including the design, data acquisition, analysis, and interpretation. The authors participated in the drafting, revision, and critical review of the article and gave their final approval for publication. They are accountable for all aspects of the work and have agreed on the submission to the specified journal.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stock I. Natural antibiotic susceptibility of Proteus spp., with special reference to P. mirabilis and P. penneri strains. J Chemother. 2003;15(1):12–26. doi:10.1179/joc.2003.15.1.12
2. Hickman FW, Steigerwalt AG, Farmer JJ, et al. Identification of Proteus penneri sp. nov., formerly known as Proteus vulgaris indole negative or as Proteus vulgaris biogroup 1. J Clin Microbiol. 1982;15(6):1097–1102. doi:10.1128/jcm.15.6.1097-1102.1982
3. Hamilton AL, Kamm MA, Ng SC, et al. Proteus spp. as Putative Gastrointestinal Pathogens. Clin Microbiol Rev. 2018;31(3):e00085–17. doi:10.1128/CMR.00085-17
4. Cantón R, Sánchez-Moreno MP, Morosini Reilly MI. Proteus penneri [Proteus penneri]. Enferm Infecc Microbiol Clin. 2006;24(1):8–13. doi:10.1157/13094272
5. Li Y, Wang Q, Peng K, et al. Emergence of Carbapenem- and Tigecycline-Resistant Proteus cibarius of Animal Origin. Front Microbiol. 2020;11:1940. doi:10.3389/fmicb.2020.01940
6. Du X, He F, Shi Q, et al. The Rapid Emergence of Tigecycline Resistance in blaKPC-2 Harboring Klebsiella pneumoniae, as Mediated in Vivo by Mutation in tetA During Tigecycline Treatment. Front Microbiol. 2018;9:648. doi:10.3389/fmicb.2018.00648
7. Forsberg KJ, Patel S, Wencewicz TA, et al. The Tetracycline Destructases: a Novel Family of Tetracycline-Inactivating Enzymes. Chem Biol. 2015;22(7):888–897. doi:10.1016/j.chembiol.2015.05.017
8. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
9. Castanheira M, Doyle TB, Deshpande LM, et al. Activity of ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam against carbapenemase-negative carbapenem-resistant Enterobacterales isolates from US hospitals. Int J Antimicrob Agents. 2021;58(5):106439. doi:10.1016/j.ijantimicag.2021.106439
10. Feldgarden M, Brover V, Haft DH, et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob Agents Chemother. 2019;63(11):e00483–19. doi:10.1128/AAC.00483-19
11. Carattoli A, Zankari E, García-Fernández A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58(7):3895–3903. doi:10.1128/AAC.02412-14
12. Alikhan NF, Petty NK, Ben Zakour NL, et al. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi:10.1186/1471-2164-12-402
13. Kaas RS, Leekitcharoenphon P, Aarestrup FM, et al. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One. 2014;9(8):e104984. doi:10.1371/journal.pone.0104984
14. Kumar S, Stecher G, Li M, et al. MEGA X: molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi:10.1093/molbev/msy096
15. Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–W259. doi:10.1093/nar/gkz239
16. Li X, Du Z, Tang Z, et al. Distribution and drug sensitivity of pathogenic bacteria in diabetic foot ulcer patients with necrotizing fasciitis at a diabetic foot center in China. BMC Infect Dis. 2022;22(1):396. doi:10.1186/s12879-022-07382-7
17. Bontron S, Nordmann P, Poirel L. Transposition of Tn125 Encoding the NDM-1 Carbapenemase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60(12):7245–7251. doi:10.1128/AAC.01755-16
18. Jorgensen SCJ, Rybak MJ. Meropenem and Vaborbactam: stepping up the Battle against Carbapenem-resistant Enterobacteriaceae. Pharmacotherapy. 2018;38(4):444–461. doi:10.1002/phar.2092
19. Dong D, Li M, Liu Z, et al. Characterization of a NDM-1- Encoding Plasmid pHFK418-NDM From a Clinical Proteus mirabilis Isolate Harboring Two Novel Transposons, Tn6624 and Tn6625. Front Microbiol. 2019;10:2030. doi:10.3389/fmicb.2019.02030
20. Wang P, Jiang X, Mu K, et al. DANMEL: a manually curated reference database for analyzing mobile genetic elements associated with bacterial drug resistance. mLife. 2022; 1: 460–464. doi: 10.1002/mlf2.12046
21. Kishore J. Isolation, identification & characterization of Proteus penneri--A missed rare pathogen. Indian J Med Res. 2012;135(3):341–345.
22. Tsai WC, Chu SH, Lee SY, et al. The effect of different brands of MacConkey and xylose lysine deoxycholate agar on the isolation of Aeromonas hydrophila. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1987;20(3):257–261.
23. Kim BN, Kim NJ, Kim MN, et al. Bacteraemia due to tribe Proteeae: a review of 132 cases during a decade (1991-2000). Scand J Infect Dis. 2003;35(2):98–103. doi:10.1080/0036554021000027015
24. Krajden S, Fuksa M, Petrea C, et al. Expanded clinical spectrum of infections caused by Proteus penneri. J Clin Microbiol. 1987;25(3):578–579. doi:10.1128/jcm.25.3.578-579.1987
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
