Back to Journals » Infection and Drug Resistance » Volume 16
Individualized Pharmaceutical Care for Antifungal Therapy in a Patient with Aspergillus tubingensis Spondylitis After Discontinuation of Rifampicin: A Case Report
Authors Li J , Cai X, Xu Y, Zhang R
Received 17 April 2023
Accepted for publication 28 June 2023
Published 4 July 2023 Volume 2023:16 Pages 4349—4356
DOI https://doi.org/10.2147/IDR.S417604
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jinmeng Li, Xinjun Cai, Yingying Xu, Ruoying Zhang
Department of Pharmacy, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, 310000, People’s Republic of China
Correspondence: Ruoying Zhang, Department of Pharmacy, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, 310000, People’s Republic of China, Email [email protected]
Abstract: Aspergillus tubingensis spondylitis (AS) is a rare spinal infectious disease with severe clinical symptoms and a challenging diagnosis. Treatment of AS is challenging due to its prolonged duration, substantial side effects, and complex drug–drug interactions. However, there is a lack of experience in individualized pharmaceutical care of AS by clinical pharmacists, especially in the presence of rifampicin, which has sustained liver enzyme induction after discontinuation. Our case described an immunocompetent patient infected with Aspergillus tubingensis spondylitis. Clinical pharmacists proposed an individualized treatment regimen for AS, after considering the effects of sustained liver enzyme induction of rifampicin (after discontinuation) on voriconazole, and utilized caspofungin as a bridge-connection scheme. We also paid attention to changes in indicators during treatment and managed adverse reactions. Therapeutic drug monitoring of voriconazole was also used to optimize the dosing regimen. With the individualized pharmaceutical care of clinical pharmacists and the efforts of clinicians, the patient’s incision healed well after 33 days of hospitalization, and she was discharged with significant improvement. Therefore, individualized pharmaceutical care by a clinical pharmacist can help optimize the treatment of Aspergillus tubingensis spondylitis. In clinical practice, drug–drug and drug–diet interactions may affect voriconazole efficacy, and individualized dose adjustment using therapeutic drug monitoring (TDM) is critical to improve efficacy and reduce adverse reactions.
Keywords: individualized pharmaceutical care, Aspergillus tubingensis spondylitis, enzyme-induction effect of rifampin, voriconazole, case report
Introduction
Fungal spondylitis is rare, accounting for about 0.5–1.6% of all spinal infections.1 The main pathogen of fungal spondylitis is Candida, followed by Aspergillus, Coccidioides, and Cryptococcus. Aspergillus spondylitis is rarer, with a mortality rate of 25%.2,3 Aspergillus fumigatus represents the primary cause of Aspergillus spondylitis in general, accounting for 55%, while Aspergillus niger and other Aspergillus niger infections account for only 2.3%.4 Among Aspergillus niger species, cryptic species are frequently recovered in clinical specimens, with Aspergillus tubingensis accounting for about 20% to over 50%.5 Aspergillus tubingensis is more common in patients with immune deficiency and is usually secondary to infections of the lungs, gastrointestinal tract, or brain. Respiratory inhalation of conidia is the most common exogenous mode of transmission of Aspergillus and rarely occurs in patients with normal immune function.6 Here, we highlight a spinal infection caused by Aspergillus tubingensis in a patient with normal immune function.
Voriconazole (VRZ) is the preferred drug therapy for Aspergillus spondylitis. Caspofungin, amphotericin B, micafungin, posaconazole, or itraconazole are recommended for patients with adverse reactions to VRZ.7 VRZ is primarily metabolized by cytochrome P450 (CYP450) isozymes in the liver, primarily CYP2C19, followed by CYP2C9 and CYP3A4. Rifampicin is a potent inducer of liver enzymes that accelerates the metabolism of many drugs, reducing their blood concentrations. Studies found that the induction effect of rifampicin on drug-metabolizing enzymes after discontinuation can continue for 10–14 days. Therefore, in clinical practice, paying attention to the interaction between voriconazole and rifampicin is critical. Moreover, because of the genetic polymorphisms of the CYP2C19 enzyme, VRZ metabolism varies significantly among different populations. TDM of VRZ has become the standard of care to ensure efficacy and avoid adverse effects.8 Lower VRZ exposure has been associated with treatment failure, which may have devastating consequences in individuals who are seriously ill with an invasive infection.
Clinical pharmacists can help improve the efficacy of drug therapy and reduce adverse reactions.9 Therefore, we present a case that clinical pharmacists performed individualized pharmaceutical care to treat Aspergillus tubingensis spondylitis. Clinical pharmacists developed an individualized treatment regimen considering the induction effect of rifampicin on drug-metabolizing enzymes after discontinuation and potential drug interactions with VRZ, and utilized TDM to optimize drug therapy efficacy and minimize adverse reactions.
Case Presentation
A 70-year-old woman (60 kg, 160 cm) was admitted to the hospital with chest and back pain, and limited mobility for five months. Five months ago, the patient felt continuous pain in the chest and back without significant cause. The pain area was fixed and accompanied by fever (the highest temperature was 39 °C). There were no chills or convulsions, chest tightness or wheezing, numb lower limbs, or other symptoms. Four months before admission, thoracic magnetic resonance imaging (MRI) revealed abnormal signals in the appendages of the T9, T11, and T12 vertebrae, consistent with tuberculosis. Therefore, she was treated with isoniazid, rifampicin, ethambutol, and pyrazinamide. However, her symptoms did not improve.
Physical examination on admission was as follows: temperature 37 °C, pulse rate 81 times/min, respiration rate 20 times/min, blood pressure 124/87 mmHg. A focused physical examination revealed kyphosis deformity, tenderness of the spinous process of T11–12 vertebrae, and pain to percussion. The admission diagnosis was bony destruction of the T11-12 vertebrae of unknown cause.
The patient continued to take isoniazid (0.3 g, once a day), rifampicin (0.6 g, once a day), ethambutol (0.75 g, once a day), pyrazinamide (0.5 g, three times a day) for anti-tuberculosis treatment, and tiopronin (200 mg, once a day, intravenous) was given for liver protection. On the second day of hospitalization, computed tomography (CT) of the thoracolumbar vertebrae revealed bony destruction of T11 and T12 vertebral bodies with a slight swelling of the surrounding soft tissues. MRI of the lumbar spine and hip showed presumptive infectious lesions in the T11–12 vertebral bodies and the right appendage of T12, consistent with tuberculosis. Subcutaneous fascial edema was observed in the lumbar region. A T-score of less than −2.5 indicated osteoporosis.
Laboratory tests showed a white blood cell count of 2.5×109/L, low neutrophil counts, uric acid 487 µmol/L, and positive T SPOT-TB testing. Leucogen tablets (20.0 mg, three times a day), calcitonin injection (20 U, twice a week), and calcitriol soft capsules (0.25 µg, twice a day) were added. On the fifth hospital day, a needle biopsy of the affected vertebrae revealed no evidence of tuberculosis.
On the tenth hospital day, the patient was treated with debridement, decompression, instrumentation, and fusion surgery and was given cefazolin (1.0 g every eight hours) to prevent surgical site infection. The day after surgery, body temperature increased to 39 °C, and C-reactive protein (CRP) increased to 129.08 mg/L. Considering the possibility of surgical incision infection, we continued cefazolin. On the thirteenth hospital day, cefazolin was discontinued after the body temperature returned to normal.
On the sixteenth hospital day, the CRP was 21.37 mg/L, and next-generation sequencing of the vertebral body indicated the sequence number of Aspergillus tubingensis was 28. The patient was diagnosed with Aspergillus tubingensis spondylitis. Because the tuberculosis evidence of thoracic vertebral lesions was insufficient, anti-tuberculosis treatment was discontinued. The clinical pharmacist recommended caspofungin (initial loading agent 70 mg, maintaining 50 mg, intravenous injection, once a day) for anti-Aspergillus treatment, and switching it to VRZ after rifampicin discontinuation for 7–14 days, considering the continuous induction effect of rifampicin on drug-metabolizing enzymes.
On the twenty-ninth hospital day, caspofungin was discontinued due to stomach discomfort. The treatment regimen was changed to oral VRZ (200 mg every 12 hours). However, the patient complained of blurred vision, hallucinations, and sleeplessness at night after taking two doses of VRZ tablets. The clinical pharmacist suggested that VRZ tablets be adjusted to 200 mg daily and instructed the patient to stop eating grapefruit on the thirtieth hospital day.
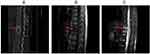
On the thirty-third hospital day, the trough concentration of VRZ was 0.82 mg/L (effective range: 1.0–5.0 µg/mL); therefore, the clinical pharmacist suggested it should be adjusted to 150 mg every 12 hours, but this regimen was not accepted. The patient was discharged in stable condition, on VRZ tablets (200 mg every 12 hours), calcitriol soft capsules (0.25 µg twice a day), and leucogen tablets (20 mg three times per day), and the patient was instructed present for complete blood count, erythrocyte sedimentation, liver and kidney function tests, electrolytes, CRP, and VRZ levels regularly. The patient was followed up and measured VRZ concentrations at eleven days, three months, and six months after discharge. The VRZ dose was adjusted based on monitoring results. Liver functions remained normal during VRZ treatment. The timeline of the treatment process is displayed in Table 1. The dosing regimen and trough concentration changes of VRZ during the treatment are presented in Figure 1. Radiographic images, including CT scans and MRIs, are shown in Figure 2.
 |
Table 1 The Timeline of the Overall Treatment Process |
 |
Figure 1 The timeline of VRZ treatment process. The dots represent VRZ concentrations in mg/L. |
Discussion
Aspergillus tubingensis spondylitis (AS) is a rare opportunistic fungal infection that can be easily misdiagnosed, posing treatment challenges. Older adults (≥ 50 years) often have underlying diseases and organ dysfunction, independent risk factors for mortality of spinal fungal infection.10 VRZ is recommended as the first-line treatment against Aspergillus osteomyelitis. However, drug–drug and diet–drug interactions alter VRZ levels; VRZ is extensively metabolized through CYP450 enzymes, which VRZ inhibits. VRZ presents a nonlinear pharmacokinetic profile with significant inter-individual and intraindividual variability in adults; therefore, it is recommended to fully evaluate the patient’s previous medication history, liver and kidney function, drug–drug and diet–drug interactions, and perform TDM.
Medication monitoring is the application of professional knowledge by pharmacists to provide pharmaceutical services related to drug use. This expertise is essential for rational medication prescription. A recent study showed that medication monitoring optimized pharmacotherapy in polymedicated patients and contributed to health promotion, reducing medication spending and expenses related to emergency care and hospitalizations.11 However, the use of clinical pharmacists in the care of older adults with Aspergillus spondylitis is never studied. In the present report, clinical pharmacists determined all actual and potential drug-related problems. They optimized anti-Aspergillus therapy by reviewing the previous medication history, liver and kidney function, and drug–drug and diet–drug interactions.
The Choice of Antifungals
The patient worked in recycling waste before the onset of the disease and did not wear masks and gloves during work; this may have been the cause of Aspergillus tubingensis infection. An MRI of the thoracic spine showed abnormal signals in the attachments of T11, T12, and T9 vertebrae, consistent with no effect of anti-tuberculosis therapy for more than 4 months. The patient was diagnosed with AS on the thirteenth day of hospital admission. To select antifungal drugs, we rely on the following analysis:
- Effectiveness: According to the Practice Guidelines for Diagnosis and Management of Aspergillus IDSA (2016),7 VRZ is recommended as the first choice to treat Aspergillus osteomyelitis. Other antifungal drugs are recommended for patients with adverse reactions, including caspofungin, amphotericin B, micafungin, posaconazole, and itraconazole.
- Drug–drug interactions: Aspergillus spondylitis was diagnosed definitely, and the anti-tuberculosis regimen was discontinued. However, rifampicin is a potent inducer of CYP3A4, CYP2C, CYP1A2, and CYP2D6. After rifampicin discontinuation, the induction effect on drug metabolic enzyme in vivo lasts 7–10 days or longer.12–14 VRZ is metabolized by CYP2C19, CYP3A4, and CYP2C9 metabolic enzymes, and it also can inhibit these enzymes. When VRZ is combined with rifampicin, the maximum concentration (Cmax) and area under the curve (AUC) levels of VRZ cannot return to the level without rifampicin, even at double doses. Moreover, combining VRZ and rifampicin can cause the latter to rise to toxic levels.15 Therefore, it is not advisable to use VRZ within 7–10 days after discontinuation of rifampicin, and a bridging scheme should be considered. Previous studies found that caspofungin is not affected by the P450 enzyme. Stone et al found that when rifampicin reaches a steady state, there was no significant change in the AUC or Cmax of caspofungin on the first or fourteenth day after combination with rifampicin.16 The killing curves of caspofungin are concentration-dependent, and its clinical efficacy was correlated with AUC and Cmax. For older adults, total caspofungin exposure increases by about 28%; however, dose adjustment is not recommended.
- Availability of drugs: In the hospital, anti-Aspergillus drugs such as VRZ, amphotericin B and caspofungin were available.
- Safety of drugs: Amphotericin B is associated with adverse reactions such as nephrotoxicity, chills, hyperthermia, and severe hypokalemia. Caspofungin is not associated with severe adverse effects and only with mild adverse reactions such as nausea, vomiting, and headache.17
- Patient status: This patient’s liver and kidney functions were normal.
Under these circumstances, clinical pharmacists recommend caspofungin as the transitional therapy, and change to the VRZ regimen after rifampicin has been discontinued for 7–14 days. The clinician accepted the suggestion and revised the treatment plan. After three days of caspofungin administration, the patient’s lower back pain was significantly relieved, CRP was markedly reduced, and no serious adverse events occurred.
Identification and Management of Adverse Reactions with VRZ
VRZ is a triazole with in vitro and in vivo activity against various fungal pathogens, including Aspergillus, while adverse events, including hallucinations, neurotoxicity, hepatotoxicity, visual impairment, and renal toxicity, limit its use. Studies found that the adverse reaction ratio of mental abnormities and neurotoxicity of VRZ is about twice as high as other antifungal drugs.18,19 VRZ is easily transmissible through the blood-brain barrier, and its concentration in brain tissue is 2–3 times that in serum, so there is a significant correlation between adverse psychiatric reactions and VRZ levels in the brain. These effects can be ameliorated or eliminated shortly after drug discontinuation.
Notably, the probability of visual toxicity is six-fold higher for VRZ than for other antifungals.18 Studies showed that the incidence of visual impairment in patients > 65 years was 49.49% among the Chinese population.19,20 The top three clinical manifestations of visual impairment were chromatism (45.45%), blurred vision (32.32%), and flash sensation (10.10%); 93.94% had visual impairment within the first week of the initial medication, and 34.34% had appeared on the first day of administration. Visual impairment caused by VRZ usually does not destroy the essential structure of the eye, so it can be recovered after discontinuation or dose reduction.19
In the present case study, the patient had blurry bilateral vision, hallucinations, and sleeplessness after taking two doses of VRZ. The following day, the pharmacist consultation noted that the patient had recently eaten a substantial number of grapefruit. Studies showed that the furanocoumarin derivatives from grapefruit strongly inhibited CYP450 in the intestine and liver, reducing P450 activity by 85.3%.21,22 The order of P450 enzyme inhibition was CYP2C19 > CYP2D6 >CYP2C9 > CYP3A4 > CYP2A6 > CYP2E1 > CYP2B6. Enzyme activity and the pharmacodynamic effects of the affected drug return to baseline levels within three days of discontinuing grapefruit.23 VRZ is primarily metabolized by CYP2C19 and secondly metabolized by CYP3A4 and CYP2C9. Therefore, grapefruit can significantly increase the VRZ concentration and should be banned during treatment.24 Due to the COVID-19 epidemic, laboratory personnel were isolated and could not conduct tests; therefore, the clinical pharmacist instructed the patient to stop eating grapefruit. VRZ levels were measured 5 days later, and the dose was adjusted accordingly. Symptoms did not recur after adjusting the VRZ regimen.
Dose Adjustment Under VRZ Monitoring
The pharmacokinetic characteristics of VRZ vary across populations. CYP2C19 primarily metabolizes VRZ, while most Asians are poor metabolizers of CYP2C19. The average blood concentration of VRZ in elderly patients (≥ 65 years old) is 80–90% higher than in younger patients. The maximal concentration and total drug exposure were 83% and 113% higher in women than men, respectively.8
Chen et al showed that TDM for VRZ is recommended for patients with CYP2C19 mutations, concomitant drugs potentially influencing VRZ pharmacokinetics, hepatic dysfunction, adverse events, poor clinical responses, or life-threatening fungal infections.25 The steady-state trough concentration of VRZ is associated with therapeutic efficacy and adverse effects, and VRZ has a narrow therapeutic window. Therefore, routine TDM of VRZ is beneficial.8,26
The patient was an older woman with Aspergillus spondylitis who had experienced neurotoxicity, suspected adverse reactions on the first day of VRZ administration, and indications for monitoring the blood concentration of VRZ. The 2018 Guideline of Individualized Medication of VRZ in the Chinese population recommended that the trough concentration of VRZ be maintained between 0.5 µg/mL and below 5.0 µg/mL for Chinese patients.25 The Clinical Practice Guideline for the TDM of VRZ proposed that a trough concentration of ≥1.0 µg/mL is strongly recommended to improve efficacy. A trough concentration of ≥2.0 µg/mL is suggested for invasive aspergillosis. Moreover, to decrease the adverse effects of VRZ, trough concentration < 4.0 µg/mL is strongly recommended for Asians.27 Considering these factors, the target value of VRZ steady-state trough blood concentration was set at 1.0–4.0 µg/mL according to the patient’s Asian ethnicity, advanced age, the severity of infection, and guideline recommendations.
On the thirty-third hospital day, the patient’s trough VRZ level was 0.82 µg/mL, below the target. Possible explanations for this abnormally low trough concentration are as follows: (1) The patient has no previous history of liver disease, the liver function was normal, and there was no interaction between VRZ and other drugs (ie, leucogen, calcitonin, or calcitriol). The duration of the enzyme induction effect after rifampicin discontinuation is generally 7–10 days, while it had been discontinued for 17 days. Therefore, we can rule out that the decrease in VRZ level was caused by abnormal liver function or drug interaction. (2) Dietary factors: enzyme activity inhibition and the pharmacodynamic effects of the affected drug generally return to baseline levels within three days of discontinuing grapefruit, and the VRZ concentration had not reached a steady state. (3) Because the patient had a severe adverse event after taking two doses of VRZ, on the second day of administration, VRZ was reduced from 200 mg once every 12 hours to 200 mg once daily. VRZ for patients weighing ≥ 40 kg should be 200 mg once every 12 hours. Our patient’s weight was 60 kg; therefore, we might infer that the abnormally low trough was related to an inadequate dose.
A Chinese guideline recommended that if the patients’ steady-state trough concentration of VRZ is below the lower limit of target concentration, a maintenance dosage of VRZ should be increased by 50%, followed by dosage adjustment based on the serum level.23 The clinical pharmacist suggested adjusting the regimen to 150 mg once every 12 hours. The steady-state trough concentration should be achieved 4–7 days after adjusting the VRZ dosing regimen, followed by dosage adjustment based on the monitoring. While the attending physician believed that the VRZ concentration was too low and may reduce clinical efficacy, VRZ tablets 200 mg, once every 12 hours, were temporarily administered orally; the VRZ trough concentration was remeasured five days later. Subsequently, they asked to be discharged, so the repetitive monitoring of VRZ trough concentration was not carried out on schedule.
Eleven days after discharge, the patient’s condition was stable, and the VRZ trough was 5.95 mg/L. Therefore, the clinical pharmacist suggested that VRZ be adjusted to 150 mg every twelve hours, and the doctor accepted the suggestion. The trough was 3.85 mg/L three months after discharge. CT and MRI of the spine revealed that the paravertebral soft tissue swelling was improved. Six months after discharge, the VRZ trough was 3.10 mg/L, and CT and MRI revealed slight swelling of paravertebral soft tissue, which was a significant improvement. The complete blood counts, erythrocyte sedimentation rates, CRPs, and renal and hepatic function tests of this patient remained normal throughout the follow-up.
Conclusion
To our knowledge, this is the first case report of clinical pharmacists provide individualized pharmaceutical care for the treatment of AS in an immunocompetent patient, after considering the effects of sustained liver enzyme induction of rifampicin (after discontinuation) on voriconazole. Individualized pharmaceutical care by a clinical pharmacist can help optimize the treatment of AS. Drug–drug interactions and diet may affect VRZ efficacy. Individualized dose adjustment using TDM is critical to improve efficacy and reduce adverse reactions.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author Ruoying Zhang on reasonable request.
Ethics Approval and Consent to Participate
This study was supported by the Ethics Committee of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine (Ethics Committee Approval of Biomedical Research Involving Humans, Approval No.: 2022JS018) and was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Consent for Publication
Written and informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Acknowledgments
We thank all members of orthopedics department of Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine for their help in the collection of clinical data.
Funding
This study was supported by funding from Hangzhou biomedicine and health industry development project (No. 2021WJCY320) and Zhejiang Pharmaceutical Society Hospital pharmacy project (No. 2020ZYY08).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saeed K, Esposito S, Ascione T, et al. International Society of Antimicrobial Chemotherapy (ISAC) Bone and Skin & Soft Tissue Infections Working Group. Hot topics on vertebral osteomyelitis from the international society of antimicrobial chemotherapy. Int J Antimicrob Agents. 2019;54(2):125–133. doi:10.1016/j.ijantimicag.2019.06.013
2. Liu BF, Yang G, Gao YZ. Research progress of fungal spondylitis. Chin J Spine Spinal Cord. 2021;31(10):951–955. doi:10.3969/j.issn.1004-406X.2021.10.12
3. Gamaletsou MN, Rammaert B, Bueno MA, et al. Aspergillus osteomyelitis: epidemiology, clinical manifestations, management, and outcome. J Infect. 2014;68(5):478–493. doi:10.1016/j.jinf.2013.12.008
4. Gabrielli E, Fothergill AW, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect. 2014;20(6):559–565. doi:10.1111/1469-0691.12389
5. Born T, Aruanno M, Kampouri E, et al. Aspergillus tubingensis endocarditis: a case report and review of the literature. Mycopathologia. 2022;187(2–3):249–258. doi:10.1007/s11046-022-00621-0
6. Comacle P, Le Govic Y, Hoche-Delchet C, et al. Spondylodiscitis due to aspergillus terreus in an immunocompetent host: case report and literature review. Mycopathologia. 2016;181(7–8):575–581. doi:10.1007/s11046-016-0007-6
7. Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi:10.1093/cid/ciw326
8. He GL, Zhang J. Voriconazole therapeutic drug monitoring: is it necessary ? Chin J Infect Chemother. 2014;14(1):77–81.
9. Mohmoud SH. Patient Assessment in Clinical Pharmacy: A Comprehensive Guide. Beijing: Chemical Industry Press; 2022.
10. Xu ZY, Lü CL, Lu GB, et al. Prognostic factors of Candida and Aspergillus spine infection: a meta-analysis. J Spinal Surg. 2021;19(2):120–125. doi:10.3969/j.issn.1672-2957.2021.02.010
11. Bezerra HS, Brasileiro Costa AL, Pinto RS, et al. Economic impact of pharmaceutical services on polymedicated patients: a systematic review. Res Social Adm Pharm. 2022;18:3492–3500. doi:10.1016/j.sapharm.2022.03.005
12. Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet. 2001;40(5):327–341. doi:10.2165/00003088-200140050-00002
13. Lu H, Shi QZ, Li L, et al. The study on drug interactions based on therapeutic drug monitoring of voriconazole. J Chin Antibiotics. 2019;44(7):864–867. doi:10.13461/j.cnki.cja.006676
14. Lin ZQ, Chen TT, Wu SF, et al. How long can the effect of rifampicin on the plasma concentration of voriconazole last ? ADR J. 2020;22(10):573–576. doi:10.3760/cma.j.cn114015-20200414-00405
15. Meng XM, Zhang L. Voriconazole related drug interactions and countermeasures. Chin J New Drugs Clin Rem. 2009;28(6):415–420.
16. Stone JA, Migoya EM, Hickey L, et al. Potential for interactions between caspofungin and nelfinavir or rifampin. Antimicrob Agents Chemother. 2004;48(11):4306–4314. doi:10.1128/AAC.48.11.4306-4314.2004
17. Gilbert DN, Chambers HF, Saag MS, et al. The Sanford Guide to Antimicrobial Therapy 2020.
18. Xing YM, Chen L, Feng Y, et al. Meta-analysis of the safety of voriconazole in definitive, empirical, and prophylactic therapies for invasive fungal infections. BMC Infect Dis. 2017;17:798. doi:10.1186/s12879-017-2913-8
19. Ren XL, Zhang X, Zhan YQ, et al. Safety study of voriconazole clinical application: datas based on 10-year spontaneous reports in Beijing. Chin J Hosp Pharm. 2022;42:437–442. doi:10.13286/j.1001-5213.2022.04.18
20. Ren XL, Zhang X, Zhan YQ, et al. Analysis of 99 cases of voriconazole-induced visual impairment. Chin J Clin Pharmacol. 2022;38(6):589–591.
21. Liu Y, Zhang S, Jiang T, et al. Mechanistic study of bergamottin-induced inactivation of CYP2C9. Food Chem Toxicol. 2021;153:112278. doi:10.1016/j.fct.2021.112278
22. Hidaka M, Fujita K, Ogikubo T, et al. Potent inhibition by star fruit of human cytochrome P450 3A (CYP3A) activity. Drug Metab Dispos. 2004;32(6):581–583. doi:10.1124/dmd.32.6.581
23. Zhang LH, Deng M. Effect of grapefruit juice on the pharmacokinetics. Pharm J Chin PLA. 2013;29(5):471–475. doi:10.3969/j.issn.1008-9926.2013.05.022
24. Sugar AM, Liu XP. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med Mycol. 2000;38:209–212. doi:10.1080/mmy.38.3.209.212
25. Chen K, Zhang X, Ke X, et al. Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese Pharmacological Society. Ther Drug Monit. 2018;40(6):663–674. doi:10.1097/FTD.0000000000000561
26. van Wanrooy MJ, Rodgers MG, Span LF, et al. Voriconazole therapeutic drug monitoring practices in intensive care. Ther Drug Monit. 2016;38(3):313–318. doi:10.1097/FTD.0000000000000284
27. Takesue Y, Hanai Y, Oda K, et al. Japanese Antimicrobial Therapeutic Drug Monitoring Guideline Committee. Clinical practice guideline for the therapeutic drug monitoring of voriconazole in Non-Asian and Asian adult patients: consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Clin Ther. 2022;44(12):1604–1623. doi:10.1016/j.clinthera.2022.10.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.